Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals

You are here
- Volume 11, Issue 1
- Systematic review of global clinical practice guidelines for neonatal hyperbilirubinemia
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Meng Zhang 1 , 2 ,
- http://orcid.org/0000-0003-4884-4248 Jun Tang 1 , 2 ,
- Yang He 1 , 2 ,
- Wenxing Li 1 , 2 ,
- Zhong Chen 1 , 2 ,
- http://orcid.org/0000-0002-0408-1288 Tao Xiong 1 , 2 ,
- Yi Qu 1 , 2 ,
- Youping Li 3 ,
- Dezhi Mu 1 , 2
- 1 Department of Pediatrics , Sichuan University West China Second University Hospital , Chengdu , China
- 2 Key Laboratory of Obstetrics & Gynecologic and Pediatric Diseases and Birth Defects of the Ministry of Education , Sichuan University , Chengdu , China
- 3 Chinese Evidence-Based Medicine Center , Sichuan University West China Hospital , Chengdu , China
- Correspondence to Professor Jun Tang; tj1234753{at}sina.com
Objective Hyperbilirubinemia is one of the most common clinical symptoms in newborns. To improve patient outcomes, evidence-based and implementable guidelines are required. However, clinical guidelines may vary in quality, criteria and recommendations among regions and countries. In this study, we aimed to systematically assess the quality of guidelines using the Appraisal of Guidelines for Research & Evaluation (AGREE)-II instrument and summarise the specific recommendations for neonatal hyperbilirubinemia in order to provide suggestions for future guideline development.
Design Systematic review.
Interventions We searched the PubMed, Embase, Medline and guideline databases for relevant articles on 10 April 2020. The studies were screened by two independent reviewers according to our inclusion criteria. Two reviewers independently extracted the descriptive data. Four appraisers assessed the guidelines using the AGREE-II instrument.
Results Our systematic review appraised 12 clinical practice guidelines for the diagnosis and management of neonatal hyperbilirubinemia. The 12 guidelines achieved an average score of 36%–89%. The guidelines received the highest scores for clarity of presentation and lowest scores for rigour of development. Most recommendations for diagnosis were relatively consistent, but recommendations regarding risk factors, the initiating threshold of treatment and pharmacotherapy varied.
Conclusions Our study revealed that current guidelines vary in the quality of the developing process and are inconsistent with regards to recommendations. Future guidelines should afford more attention to the quality of methodologies in guideline development, and more qualified evidence is needed to standardise the initiating threshold of treatment for neonatal hyperbilirubinemia.
- neonatology
- developmental neurology & neurodisability
- protocols & guidelines
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-040182
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
This study is the first English systematic appraisal of guidelines targeted to neonates with hyperbilirubinemia.
The strengths also included the use of the validated Appraisal of Guidelines for Research & Evaluation (AGREE) II instrument and four independent reviewers to minimise subjective bias.
A Chinese-language guideline by the Chinese Pediatric Society was appraised.
The AGREE-II was used to evaluate guidelines with less attention on detailed recommendations.
We only assessed guidelines through the reported literature without the use of additional methods such as contacting guideline developers.
INTRODUCTION
Neonatal hyperbilirubinemia, characterised by the elevation of total serum bilirubin (TSB), is one of the most common clinical conditions affecting newborns, particularly preterm infants. Hyperbilirubinemia affects approximately 60% of full-term and 80% of preterm neonates. 1 Approximately 10% of newborns are likely to develop clinically significant hyperbilirubinemia requiring close monitoring and treatment. 2 In the early period (0–6 days), neonatal hyperbilirubinemia accounted for 1309.3 deaths per 100 000 livebirths and was the seventh most common cause of neonatal deaths. 3 Effective and timely treatment with phototherapy or exchange transfusion can reduce the occurrence of neurological dysfunction in neonates with hyperbilirubinemia.
Clinical practice guidelines are in place to aid clinical, policy-related and system-related decisions. 4 Guidelines have also been developed to bridge the gap between research and clinical practice. 5 Therefore, guidelines have become increasingly popular in recent years. 6 Although several organisations from different regions have developed clinical practice guidelines, these guidelines may vary widely in quality. 7 8 Moreover, the criteria for diagnosis and treatment in published guidelines vary among regions and countries. 9
The Appraisal of Guidelines for Research & Evaluation (AGREE) instrument is used to assess methodological rigour and transparency of a guideline. 10 In this study, we aimed to systematically review and assess the quality of guidelines on neonatal hyperbilirubinemia using the AGREE-II instrument in order to provide suggestions for future guideline development.
Selection criteria
We included clinical practice guidelines produced by local, regional, national or international groups or affiliated governmental organisations for the diagnosis and management of hyperbilirubinemia in newborn infants. The guidelines were included if they met the following criteria: (1) published in English or Chinese language, (2) based on systematic evidence synthesis and containing specific statements to guide decisions regarding hyperbilirubinemia, (3) include recommendations for the diagnosis and/or treatment of neonatal hyperbilirubinemia and (4) published between 2000 and 2020, and only the most recent editions of updated guidelines were considered.
Search strategy
A systematic literature search was performed on 10 April 2020. We searched for relevant studies in the PubMed, Embase and Medline databases. In addition, we searched the Guidelines International Network, National Health Service Evidence website, National Institute for Health and Care Excellence (NICE) website, Scottish Intercollegiate Guidelines Network website, Turning Research Into Practice Database and Wan fang Database. The titles and abstracts of the searched citations were screened by two independent reviewers (MZ and YH). Any discrepancies between the reviewers were resolved by discussion. The detailed search strategy for PubMed is shown in the online supplemental material .
Supplemental material
Guideline characteristics.
Two independent reviewers (MZ and YH) extracted the general characteristics of the included guidelines: country, founding organisation, year of publication or updating status, method of evidence identification and funding.
Appraisal of guideline quality
Four appraisers (MZ, YH, WL and ZC) independently assessed the selected guidelines using the AGREE-II instrument. The AGREE II is an international, validated and rigorously developed tool to evaluate the quality of clinical practice guidelines and consensus statements. 11 The AGREE II consists of 23 key items organised within six domains (scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability and editorial independence) followed by two global rating items (overall assessment). Each domain points to a unique dimension of guideline quality. 12 Each of the AGREE II items is rated on a 7-point scale (1=strongly disagree to 7=strongly agree). Domain scores are calculated by summing the scores of the individual items in a domain and by scaling the total as a percentage of the maximum possible score for that domain. 12 The score for each domain of each document is calculated as follows: (obtained score−minimal possible score)/(maximal possible score−minimal possible score). 10 All reviewers were trained online using the AGREE training tools. Discrepancies of >3 points were discussed in a consensus meeting.
We extracted descriptive data from the guideline recommendations to identify the consistencies and discrepancies. The recommendations were then summarised according to different items related to the diagnosis and treatment strategies of neonatal hyperbilirubinemia such as the test used for the early prediction and diagnosis, time to start phototherapy and exchange transfusion, recommendation for drug use, criterion for discharge and timing or frequency of follow-up. The intraclass correlation coefficients for the six domains were calculated to assess the reliability of the scores between investigators. The analysis of the reliability study was performed using SPSS V.24.0.
Patient and public involvement
No patient involved.
Search results
Figure 1 illustrates the search and guideline selection process. The systematic search retrieved 725 records, of which 701 were excluded after removing duplicates and articles that did not meet the eligibility criteria. Consequently, after the full-text evaluation of the remaining records, 12 additional clinical practice guidelines were excluded for the following reasons: not written in English or Chinese, not original guidelines and not clinical practice guidelines or consensuses. Ultimately, we included 12 clinical practice guidelines from 12 different national or regional organisations.
- Download figure
- Open in new tab
- Download powerpoint
Study selection diagram. GIN, Guidelines International Network; NHS, NationalHealth Service; NICE, National Institute for Health and Care Excellence; TRIP, TurningResearch Into Practice Database.
General characteristics of the guidelines
Tables 1 and 2 summarise the general characteristics of the included clinical practice guidelines. Twelve clinical practice guideline documents were published by national or regional organisations, including the American Academy of Pediatrics (AAP) Subcommittee on Hyperbilirubinemia, 13 Canadian Pediatric Society (CPS) Fetus and Newborn Committee, 14 Chinese Pediatric Society (ChPS) Chinese medical Association, 15 Israel Neonatal Society (INS), 16 Italian Society of Neonatology (ISN), 17 Malaysia Health Technology Assessment Section (MaHTAS), 18 NICE in the UK, 19 Norwegian Pediatric Association, 20 Queensland Clinical Guidelines (QCG) in Australia, 21 Spanish Association of Pediatrics (SAP), 22 Swiss Society of Neonatology (SSN) 23 and Turkish Pediatric Association (TPA). 24 Five of these guidelines are new and the others have been updated or reaffirmed. Four guidelines from the USA, 13 Canada, 14 Italy 17 and Switzerland 23 were targeted towards neonates born at >35 weeks of gestation, while the other guidelines covered all preterm and term babies. Six organisations (QCG, 21 CPS, 14 SAP, 22 NICE, 19 INS 16 and MaHTAS 18 reported performing a systematic review and appraisal of the evidence and were explicit about the level of evidence that underpinned their recommendations. Three groups were funded by governmental institutions (QCG, 21 NICE 19 and MaHTAS), 18 one declared no financial support (TPA), 24 and the remainder did not disclose a funding source.
- View inline
General characteristics
Appraisal of guidelines
Table 3 shows the scores for each guideline for the six domains of the AGREE II instrument. The overall quality of the guideline development process varied widely both among guidance documents and within guidance documents among different domains. The average score was 36.3%–89.3%. Most guidelines achieved average scores of <50% in four of the six domains, and only two received an average score of >50%. The highest scores were achieved in the domains of clarity of presentation and the lowest scores were achieved for rigour of development.
Domain scores of the nine guidelines assessed by using the AGREE-II instrument (%)
Domain 1: the mean score for scope and purpose was 88.8%±6.5% and the MaHTAS 18 guideline achieved the highest score at 98.6%. Domain 2: the mean stakeholder involvement score was 47.6%±22.4% and ChPS 15 received the lowest score at 9.7%. Domain 3: the mean score for rigour of development was 31.9%±22.6%. NICE 19 scored the highest for this domain at 85.9% with the most extensive development process, while TPA 24 received the lowest at only 9.9%. Domain 4: the mean score for clarity of presentation was 91.7%±5.7%. For this domain, most of the guidelines obtained a score of >90%. Domain 5: the mean score for applicability was 43.0%±18.9%, with five guidelines scoring <30%. Domain 6: the mean score for editorial independence was 36.8%±36.1%, and four guidelines obtained scores of 0% for this domain. In terms of overall quality, 50% of the guidelines received an average score of >50%. The NICE 19 guidelines received the highest score at 89.3%±5.7%.
Table 4 shows the intraclass correlation coefficients, 95% CIs, and p values for each domain between the four evaluators. The intraclass correlation coefficients ranged from 0.818 to 0.995.
Inter rater reliability study results
Clinical guideline recommendations
Nine guidelines covered risk factors for severe neonatal hyperbilirubinemia, including maternal and neonatal risk factors. All guidance documents provided recommendations for diagnosis. Tables 5 and 6 show the main risk factors and some example diagnostic strategies for neonatal hyperbilirubinemia. The guidelines differed somewhat in their report of risk factors. Nearly all guidelines reported prematurity, exclusive breastfeeding and glucose-6-phosphate dehydrogenase (G6PD) deficiency as neonatal risk factors. Cephalohematoma or bruises and male sex were also defined as neonatal risk factors in some guidelines, while NICE 25 stated that the evidence was inconclusive and that the results of most studies revealed no significant association between these factors and hyperbilirubinemia.
Summary of risk factors of severe neonatal jaundice
Summary of recommendations for approaches to diagnosis of neonatal hyperbilirubinemia
Visual assessment was recommended as a first step in diagnosis by most organisations, and the guideline of Malaysia 18 specifically mentioned that Kramer’s rule could be widely practiced. All guidelines advocated TSB measurement as the gold standard for detecting and determining the level of hyperbilirubinemia. Non-invasive methods such as a transcutaneous bilirubinometer are accepted by all guidelines. Other methods of detection such icterometers were not recommended by NICE 19 and MaHTAS 18 because there was no good quality evidence to indicate their reliability. In addition, nearly all guidelines recommended additional laboratory tests for babies with prolonged hyperbilirubinemia that could be of value to evaluate and identify the underlying disease. These tests included complete blood counts, blood group compatibility, a direct antiglobulin test, septic workup, urinalysis, urine culture, thyroid functions, G6PD, reticulocyte count and conjugated component of bilirubin.
Table 7 shows the recommendations for the management of neonatal hyperbilirubinemia. The key areas included the initiating threshold and details of different types of therapies and care for babies during therapy. The guidelines distinguished treatment scenarios based on the level of hyperbilirubinemia, including phototherapy, exchange transfusion and pharmacotherapy.
Summary of recommendations for approaches to treatment of neonatal hyperbilirubinemia
All guidelines discussed the threshold of phototherapy and exchange transfusion, and most of the organisations divided patients into groups according to gestational age and risk factors. As an example, we reported the detailed initiation TSB levels for full-term neonates according to the presence and absence of risk factors in table 7 , finding that there were few differences among the guidelines regarding the initiation of TSB levels. The majority of the guidelines proposed a number of general care strategies during phototherapy, such as temperature measurement, eye protection and continued breastfeeding. Among other forms of phototherapy, home phototherapy was recommended by AAP 13 and MaHTAS, 18 while sunlight exposure was not supported by four organisations (AAP, 13 NICE, 19 QCG, 21 SAP). 22 Moreover, seven guidelines mentioned the complications of phototherapy.
The threshold for initiating exchange transfusion was higher than that for phototherapy in all risk groups. Potential signs of acute bilirubin encephalopathy were highlighted as important in all guidelines. Most guidelines reported the details of performing exchange transfusion such as the blood product and blood volume. Double-volume exchange transfusion was advocated by the majority of guidelines. Furthermore, observations during exchange transfusion including heart rate, blood pressure, respiratory rate, oxygen saturation and skin temperature were only proposed by three organisations (MaHTAS, 18 ChPS 15 and ISN). 17 After the exchange transfusion, seven guidelines recommended maintaining intensive phototherapy and six suggested monitoring the TSB at varied time points. Pharmacotherapy was also mentioned by 10 guidelines. However, the recommendation of medication varied greatly.
Most of the guidelines discussed follow-up after discharge, and some provided different follow-up time recommendations according to the time of discharge and risk factors. In addition, some guidelines focused on the follow-up of children with severe hyperbilirubinemia. The CPS 14 guidelines recommend that the hearing screen of patients with severe hyperbilirubinemia should include brainstem auditory evoked potentials. The MaHTAS 18 guideline reported that term and late preterm babies with TSB of >20 mg/dL or exchange transfusions should have auditory brainstem response (ABR) testing performed within the first 3 months of life. If the ABR is abnormal, neurodevelopmental follow-up should be continued. The ABR test was also recommended by the Turkish guidelines for babies with hyperbilirubinemia requiring treatment. Moreover, two of the guidelines (SSN 23 and ISN) 17 mentioned the national institute for monitoring the incidence of kernicterus and severe hyperbilirubinemia.
This systematic review appraised 12 clinical practice guidelines for the diagnosis and management of neonatal hyperbilirubinemia. The quality of the guidelines was highly variable. The included guidelines received acceptable AGREE II scores in the domains of clarity of presentation and scope and purpose, but the mean scores were moderate or low in the stakeholder involvement, rigour of development, applicability and editorial independence domains. This finding was similar to that of the 2010 review by Alonso-Coello et al . 26 In recent years, although the number of guidelines has increased, the quality of guidelines still needs to be improved.
As evaluated by the AGREE II instrument, most guidelines had good clarity regarding their objective, clinical questions and scope. Further, as the AGREE II revealed in the stakeholder involvement domain, many guideline development groups represented a variety of relevant professional areas. 12 It is valuable to explore the views of the target population, that is, healthcare providers or the parents of neonates with hyperbilirubinemia. However, although some guidelines targeted healthcare providers and parents, almost all development groups ignored the preferences of parents of the hyperbilirubinemia neonates.
The mean score of the rigour of development domain, which was considered the indicator of quality in all domains, 27 varied significantly among different guidelines. Guidelines typically received low scores in this domain because of poor reporting of systematic methods for searching for evidence and formulating recommendations, lack of external review and updating mechanisms. Some guidelines, such as NICE, 19 provided detailed search strategies, evidence tables and reasons for excluded studies to confirm their systematic methods, while some guidelines did not provide complete information regarding methods of searching and selecting evidence. Muka et al 28 provided a 24-step guide on how to perform a systematic review and meta-analysis in 2020. The guide described the most important 24 steps, such as defining the search strategy, designing the data collection form, checking reporting bias and so on. We suggest that these methodologically sound tools should be used to help future guideline designers conduct or appraise systematic reviews. Guidelines need to reflect current research, but most of the guidelines did not provide a statement about the procedure for updating. Alonso-Coello et al 29 conducted an international survey of the updating practices of guidelines in 2011 and concluded that there was an urgent need to develop rigorous international standards for the updating process.
The clarity of presentation of the recommendations was specific and unambiguous in most guidelines. The scores of the applicability domain were highly reflective of the implementation of guidelines. Additional materials, including summary documents and educational tools, could be beneficial in this respect. However, >50% of the included guidelines did not discuss facilitators and barriers to their application or tools for practicing; thus, the guidelines might have a limited effect. 30 Therefore, future guideline developers should afford greater consideration to the potential resource implications and facilitators of application, particularly for guidelines published in developing regions. Regarding the editorial independence domain, the views of the funding body and interests of the developers should be reported as part of the standard practice of guideline development.
In this study, we also summarised and compared the specific recommendations for the diagnosis and treatment of neonatal hyperbilirubinemia. All guidelines covered the threshold of phototherapy and exchange transfusion, while most of the guidelines stated that the threshold graph was reproduced and adapted with permission from the AAP. 13 However, the AAP noted that the suggested levels represented a consensus of committee but were based on limited evidence, and the levels shown were approximations. 13 Therefore, more qualified studies of different populations are needed to standardise treatment methods. In terms of pharmacotherapy, variations also existed among different guidelines. The discrepancies were mainly due to varying evidence quality, limitations in generalisability and lack of approval by a national administration.
The burden of hyperbilirubinemia is highest in South Asia and sub-Saharan Africa. 2 Hyperbilirubinemia is the 7th leading cause of neonatal mortality in South Asia, 8th in sub-Saharan Africa, 9th in western Europe and 13th in North America. 2 In our review, we appraised five guidelines from Europe with a mean score of 55.9%, four guidelines from Asian countries with mean scores of 55.2% and two guidelines from North America with mean scores of 50.6%. In 2015, Olusanya et al 31 provided a practical framework for the management of late-preterm and term infants (≥35 weeks of gestation) with clinically significant hyperbilirubinemia in low-income and middle-income countries lacking local practice guidelines. They provided recommendations for comprehensive management, including primary prevention, early detection, diagnosis, monitoring, treatment and follow-up. 31
To our knowledge, our study is the first systematic critical appraisal of guidelines with diagnostic and treatment recommendations targeted to neonates with hyperbilirubinemia. The strengths of our review include the integration of comprehensive search strategies, use of the validated AGREE II instrument and use of four independent reviewers to minimise subjective bias. Further, in addition to guidelines written in English, a Chinese-language guideline by the Chinese Pediatric Society was appraised in our study. As a representative of developing countries, the inclusion of Chinese-language guidelines may minimise the overestimation of the quality of guidelines to some degree.
However, there were several possible limitations to our study. First, guidelines written entirely in languages other than English and Chinese might have been overlooked. Second, the AGREE-II was used to evaluate guidelines with less attention on detailed recommendations. Although it is thought that a global appraisal of a guideline’s developing process may reflect the strength of recommendations, 9 the quality of specific recommendations has a direct influence on practice. Finally, we only assessed guidelines through the reported literature without the use of additional methods such as contacting guideline developers to obtain further clarification. This may have underestimated the systematic methods of guideline development by organisations.
Our study evaluated the quality of methodologies and rigorous strategies in the guideline development process and summarised the recommendations on the diagnosis and treatment of neonatal hyperbilirubinemia. The results revealed that current guidelines varied in the quality of the development process and were inconsistent in their recommendations, despite some similarities. Therefore, future guidelines should afford greater attention to the quality of methodologies in the guideline development process, and more qualified evidence is needed to standardise the initiating threshold of treatment for neonatal hyperbilirubinemia.
Ethics statements
Patient consent for publication.
Not required.
- Bhutani VK ,
- Lazzeroni LC , et al
- Olusanya BO ,
- Kassebaum NJ
- Shaneyfelt TM ,
- Mayo-Smith MF ,
- Rothwangl J
- Jolliffe L ,
- Lannin NA ,
- Cadilhac DA , et al
- Johnston A ,
- Hsieh S-C , et al
- Nagler EV ,
- Vanmassenhove J ,
- van der Veer SN , et al
- Kwong JS-W , et al
- Huang T-W ,
- Wu M-Y , et al
- Brouwers MC ,
- Browman GP , et al
- Chapman JR ,
- Wong G , et al
- ↵ Agree II training tools . Available: http://wwwagreetrustorg/resource-centre/agree-ii-training-tooles/
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia
- ↵ Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks' gestation) - Summary . Paediatr Child Health 2007 ; 12 : 401 – 7 . doi:10.1093/pch/12.5.401 pmid: http://www.ncbi.nlm.nih.gov/pubmed/19030400 OpenUrl PubMed Web of Science
- Editorial Board of Chinese Journal of Pediatrics
- Subspecialty Group of Neonatology, The Society of Pediatrics, Chinese Medical Association
- Romagnoli C ,
- Pratesi S , et al
- Bratlid D ,
- Nakstad B ,
- Guidelines QC
- Sánchez-Redondo Sánchez-Gabriel MD ,
- Leante Castellanos JL ,
- Benavente Fernández I , et al
- ↵ et al Arlettaz AB R , Buetti, H L , Fahnenstich D . Assessment and treatment of jaundiced newborn infants 35 0/ 7 or more weeks of gestation . Available: https://wwwneonetch/recommendations/authored-ssn 2007
- Türkmen MK ,
- NICE, NIfHaCE
- Alonso-Coello P ,
- Solà I , et al
- Hu Y , et al
- Milic J , et al
- Martínez García L ,
- Carrasco JM , et al
- Sabharwal S ,
- Gauher S , et al
- Ogunlesi TA ,
- Kumar P , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Correction notice This article has been corrected since it first published. The provenance and peer review statement has been included.
Contributors MZ conceptualised and designed the study, screened the titles and abstracts of searched citations, extracted general characteristics and descriptive data from guideline recommendations, assessed the selected guidelines using the AGREE-II instrument and drafted the initial manuscript. JT conceptualised and designed the study, coordinated and supervised guideline assessment, and critically reviewed the manuscript for important intellectual content. YH screened the titles and abstracts of searched citations, extracted general characteristics and descriptive data from guideline recommendations, assessed the selected guidelines using the AGREE-II instrument and revised the manuscript. WL and ZC assessed the selected guidelines using the AGREE-II instrument, reviewed and revised the manuscript. TX, YQ, YL and DM coordinated and supervised guideline assessment, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding This work was supported by the National Science Foundation of China (Numbers 81630038, 81971433), the grant from Ministry of Education of China (IRT0935), the grant of clinical discipline program (Neonatology) from the Ministry of Health of China (1311200003303) and the grants from the Science and Technology Bureau of Sichuan Province (2020YJ0236, 2020YFS0041).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Topic collections
- BMJ Journals
You are here
- Volume 1, Issue 1
- Burden of severe neonatal jaundice: a systematic review and meta-analysis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Tina M Slusher 1 , 2 ,
- Tara G Zamora 1 ,
- Duke Appiah 3 ,
- Judith U Stanke 4 ,
- Mark A Strand 5 ,
- Burton W Lee 6 ,
- Shane B Richardson 7 ,
- Elizabeth M Keating 8 ,
- Ashajoythi M Siddappa 1 , 2 ,
- http://orcid.org/0000-0002-3826-0583 Bolajoko O Olusanya 9
- 1 Department of Pediatrics , University of Minnesota , Minneapolis , Minnesota , USA
- 2 Hennepin County Medical Center , Minneapolis , Minnesota , USA
- 3 Texas Tech University Health Science Center , Abilene , Texas , USA
- 4 Biomedical Library , University of Minnesota , Minneapolis , Minnesota , USA
- 5 Department of Pharmacy , North Dakota State University , Fargo , North Dakota , USA
- 6 Department of Medicine , University of Pittsburgh School of Medicine , Pittsburgh , Pennsylvania , USA
- 7 Department of Family Medicine , University of Arizona , Tucson , Arizona , USA
- 8 Department of Pediatrics , Baylor College of Medicine , Houston , Texas , USA
- 9 Center for Healthy Start Initiative , Lagos , Nigeria
- Correspondence to Dr Tina M Slusher; tslusher{at}umn.edu
Context To assess the global burden of late and/or poor management of severe neonatal jaundice (SNJ), a common problem worldwide, which may result in death or irreversible brain damage with disabilities in survivors. Population-based data establishing the global burden of SNJ has not been previously reported.
Objective Determine the burden of SNJ in all WHO regions, as defined by clinical jaundice associated with clinical outcomes including acute bilirubin encephalopathy/kernicterus and/or exchange transfusion (ET) and/or jaundice-related death.
Data sources PubMed, Scopus and other health databases were searched, without language restrictions, from 1990 to 2017 for studies reporting the incidence of SNJ.
Study selection/data extraction Stratification was performed for WHO regions and results were pooled using random effects model and meta-regression.
Results Of 416 articles including at least one marker of SNJ, only 21 reported estimates from population-based studies, with 76% (16/21) of them conducted in high-income countries. The African region has the highest incidence of SNJ per 10 000 live births at 667.8 (95% CI 603.4 to 738.5), followed by Southeast Asian, Eastern Mediterranean, Western Pacific, Americas and European regions at 251.3 (132.0 to 473.2), 165.7 (114.6 to 238.9), 9.4 (0.1 to 755.9), 4.4 (1.8 to 10.5) and 3.7 (1.7 to 8.0), respectively. The incidence of ET per 10 000 live births was significantly higher for Africa and Southeast Asian regions at 186.5 (153.2 to 226.8) and 107.1 (102.0 to 112.5) and lower in Eastern Mediterranean (17.8 (5.7 to 54.9)), Americas (0.38 (0.21 to 0.67)), European (0.35 (0.20 to 0.60)) and Western Pacific regions (0.19 (0.12 to 0.31). Only 2 studies provided estimates of clear jaundice-related deaths in infants with significant jaundice [UK (2.8%) and India (30.8%).
Conclusions Limited but compelling evidence demonstrates that SNJ is associated with a significant health burden especially in low-income and middle-income countries.
- neonatology
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
https://doi.org/10.1136/bmjpo-2017-000105
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is already known on this topic?
Acute bilirubin encephalopathy (ABE), exchange transfusions and death are frequent and costly outcomes of severe neonatal jaundice (SNJ) especially in low-income and middle-income countries.
Long-term disabilities including cerebral palsy and deafness can occur following ABE.
The actual burden of SNJ is not well documented.
What this study hopes to add?
A review of population-based literature to assess the global impact of severe neonatal jaundice (SNJ) highlighting the importance of this disease as defined by its clinical presentations.
Objective evidence that the burden of SNJ is not evenly distributed and that a heavier burden of disease is born by low-income and middle-income countries.
The limited amount of population-based data currently available and the need to capture this information globally.
Introduction
Newborn jaundice occurs in up to 85% of all live births. 1–3 In the absence of haemolysis, sepsis, birth trauma or prematurity, it usually resolves within 3–5 days without significant complications. 1 However, epidemiological evidence suggests that severe neonatal jaundice (SNJ) results in substantial morbidity and mortality. 4 SNJ has been recognised as a significant cause of long-term neurocognitive and other sequelae, cerebral palsy, non-syndromic auditory neuropathy, deafness and learning difficulties. 5 6 The burden is unacceptably high in low-income and middle-income countries (LMICs) and has prompted calls for intense scrutiny and attention. 4 Under the millennium development goals, the potential impact of adverse perinatal conditions such as preterm birth complications and birth asphyxia on thriving and well-being beyond survival rarely received attention. 7 With the current focus on inclusiveness for persons with disability under the sustainable development goals (SDGs), it is essential that we tackle SNJ as one key component of optimising neurodevelopmental outcome. 7 8
A recent report by Bhutani et al 4 noted that at least 481 000 term/near-term neonates are affected by SNJ/hyperbilirubinaemia each year, with 114 000 dying and an additional 63 000 surviving with kernicterus. However, these alarming estimates were based on limited data determined by mathematical modelling as true population-based data are limited and difficult to find. Therefore, the incidence of SNJ and thus its contribution to global neonatal morbidity and mortality presently remain unclear and possibly significantly underestimated.
Jaundice is usually recognised around a total serum bilirubin (TSB) of 5 mg/dL in neonates. 3 SNJ is unlikely to happen before a TSB of at least 20–25 mg/dL in term neonates presenting early. 4 TSB is unfortunately often either not available or delayed in many LMICs. 9 Therefore, for the purposes of this article, severe SNJ is defined as jaundice associated with acute bilirubin encephalopathy (ABE)/kernicterus and/or exchange transfusions (ET) and/or jaundice-related death.
Phototherapy and ET are widely used therapeutic modalities for jaundice. 2 However, due to constrained resources, devices for measuring bilirubin 10 11 and effective phototherapy are often lacking in LMICs. 12 This, together with higher prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency, blood group incompatibilities, late referrals and delayed recognition of excessive bilirubin levels in LMICs, has necessitated excessive use of ETs. 13
We systematically reviewed the available evidence pertaining to the global burden of SNJ to inform child health policy regarding its prevention and management especially in LMICs.
Search criteria
Although most SNJ occurs at TSB at 20 mg/dL (343 µmol/L), there is no standard worldwide definition of SNJ or clinically significant TSB necessitating medical intervention. There is a wide range of definitions of significant jaundice. In studies reviewed in this article, TSB levels considered significant, when results were available, generally ranged from 15 to 30 mg/dL. 14–27 Even though beginning in 2004, the American Academy of Pediatrics recommended ABE be used for acute manifestations of SNJ in the first weeks of life and kernicterus for chronic manifestations of SNJ/ABE, 28 many still use the terms interchangeably. Because of limited availability of TSBs and our attempt to quantify the burden of clinical disease, we defined SNJ clinically using ABE, ET and jaundice-related death.
We systematically reviewed published papers following PRISMA guidelines (online supplementary appendix 1 ). 13 Databases searched included Ovid Medline, PubMed, CINAHL, Global Health, Scopus, Popline, Africa Journal Online and Bioline databases for published articles on SNJ. We used both controlled subject headings and free-text terms for neonatal jaundice (NNJ), jaundice, bilirubin/blood levels, haemolytic anaemia, G6PD deficiency in various forms and in combination with terms for ET, ABE, kernicterus, death, mortality and phototherapy. Other inclusion criteria were jaundice in first month of life; availability of data on incidence of ABE/kernicterus; provision of information on incidence of ETs for SNJ or jaundice-related death which we defined as SNJ. We also reviewed references of selected retrieved articles and review papers, and contacted authors of relevant articles for missing dates. No language restrictions were used. To be included in the meta-analysis, a study must have reported estimates of incidence from a retrospective or prospective population-based study, increasing likelihood that estimates could be generalised to the geographical location where the study was conducted. The search results were limited to publication dates of 1990 to June 2017. See online supplementary appendix 2 for complete Ovid Medline search strategy.
Supplementary Material
Data extraction.
Two authors examined studies using a predetermined checklist (online supplementary appendix 3 ) devised by three authors for selecting articles that met inclusion criteria after one author screened titles and abstracts. Two authors independently confirmed eligibility of all full-text articles. Discrepancies were resolved by discussion and when needed by a third author. The following data were extracted from each article: publication year, study design, country, WHO region, sample size, SNJ definition and outcomes (ET, ABE, mortality). Articles were excluded if neonates were enrolled before 1990; study published after June 2017; sample size <10; ET unrelated to SNJ, results limited to only metabolic or primary liver diseases, studies with defined enrolment period, failure to define neonates as having ABE, ET or jaundice-related death and for the meta-analysis if they included only premature neonates.
Quality assessment
We explored several quality assessment tools reported in the literature for observational studies including the Newcastle-Ottawa Scale, 29 and found none directly applicable for evaluating diagnostic studies on NNJ /hyperbilirubinaemia. We therefore chose to adopt the tool validated by Wong et al 30 with all the critical components for assessing the risk of bias across studies. Two authors examined four important components of quality/risk of bias assessment: selection of subjects (representativeness), case definition for SNJ (exposure ascertainment), diagnostic criteria for jaundice and outcome measurement. Study quality was judged based on number of criteria that were met: all 4 (high), 2–3 (medium) or 1 (low). Finally, two authors determined which studies were population-based. We defined population-based studies as studies that addressed the incidence of SNJ for a defined population with every individual in the population having the same probability of being in the study and the results of the study having the ability to be generalisable to the whole population from which study participants were sampled and not necessarily the individuals included in the study. 31 Disagreements were resolved through consensus after joint reassessment.
Statistical analysis
For the meta-analysis, when multiple reports were obtained from the same population with overlapping study years, the one providing sufficient data (ie, numerator and denominator data) to derive estimates of disease burden was selected. To facilitate meta-analytical techniques, estimates of incidence were logit transformed to enable them to correspond to probabilities under the standard normal and permit use of the normal distribution for significance testing. Pooled estimates were calculated using DerSimonian and Laird’s random effects method, weighting individual study estimates by the inverse of the variance of their transformed proportion as study weight, with their 95% (CI) determined using Clopper-Pearson exact binomial method. 32 For presentation, pooled transformed estimates were back transformed. Statistical heterogeneity among studies was investigated using Cochran’s Q test and I 2 with a conservative p value less than 0.1 chosen as the level of significance. Forest plots were then used to examine the overall effects. Exploration of potential sources of heterogeneity was undertaken using meta-regression. Whether it is an interventional or an observational study, small studies are more likely to show more extreme values given wider CIs compared with larger studies. Since more extreme findings may be more newsworthy and hence more likely to be published, potential for publication bias was assessed by visual inspection of the funnel plot as well as by formal means using Begg’s adjusted rank correlation and Egger’s regression asymmetry tests. 33 All analyses were conducted using R Statistical Software. 34
Search of electronic databases identified 6844 articles ( figure 1 ). Eight hundred and twenty papers were reviewed. After excluding studies not meeting inclusion criteria, 416 studies were selected for further review. Multiple languages (Chinese, English, Farsi, French, German, Hebrew, Italian, Norwegian, Polish, Portuguese, Serbian and Spanish) were represented, with translation of relevant sections, but only 26/416 were non-English, none of which were population based. Of these, 416 papers included at least one marker of SNJ, but only 21 provided population-based data on 4 975 406 neonates ( table 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Flow chart of study selection for the meta‐analysis.
- View inline
Studies that met the inclusion criteria to be included in the meta-analysis
Sixteen (76%) were from high-income countries and 13 (62%) used a prospective study design. High-quality studies tended to report lower incidence compared with low-quality to moderate-quality studies ( figure 2 ). High-quality studies tended to come from high-income countries with less disease while low-quality studies tend to come from LMICs. Overall, incidence estimates of SNJ from high-income countries tended to be lower compared with LMICs ( figure 3 ). Studies which enrolled all neonates regardless of gestational age had a higher incidence of SNJ compared with studies enrolling only term/near-term ( table 2 ).
Pooled incidence (per 10 000) of severe neonatal jaundice among all neonates aged 24 months or less according to study quality.
Pooled incidence (per 10 000) of severe neonatal jaundice among all neonates aged 24 months or less according to income.
Incidence (per 10 000 live births) of severe neonatal jaundice among all neonates aged 24 months or less by gestation and study design
The incidence of SNJ per 10 000 live births was highest in the African region at 667.8, followed by Southeast Asian at 251.3, Eastern Mediterranean with 165.7 and Western Pacific region with 9.4. The Americas and European regions each had substantially lower incidence of 4.4 and 3.2, respectively ( table 3 ).
Incidence of severe neonatal jaundice per 10 000 live births, among all neonates aged 24 months or less
The incidence of ET per 10 000 live births was significantly higher for the African (186.5) and Southeast Asian (107.1) regions and lower in Eastern Mediterranean, Americas, European and Western Pacific regions reporting estimates of 17.8, 0.38, 0.35 and 0.19, respectively ( table 4 ).
Incidence of exchange transfusions, per 10 000 live births, among all neonates aged 24 months or less
Visual inspection of funnel plot in which incidences of SNJ were plotted against their standard errors showed asymmetry. This was confirmed by formal tests of publication bias (Begg-Mazumdar test: p=0.016, Egger: bias, p=0.002). The observed heterogeneity between studies may explain the asymmetric funnel plots. In random effects meta-regression analyses, the overall observed between-study heterogeneity explained by covariates which were selected a priori (study design and duration, income classification of country and gestational age) was 66.23%; p<0.001. However, only income classification of country was statistically significant determinant of the incidence of SNJ ( table 5 ). Only two studies provided information on jaundice-related deaths with estimates of 2.8, 30.8 and 50.0 for UK (European), 22 and India (Southeastern) 35 While one study fromPakistan 3 (Eastern Mediterranean), mentions death in 30% of infants with jaundice but stated they did not feel the deaths could be directly attributed to jaundice.
Meta-regression analysis potential factors* influencing the heterogeneity of incidence of severe neonatal jaundice
Although data are limited despite our extensive literature review, this systematic review and meta-analysis suggests that the incidence of SNJ is high, with regions that include predominantly LMICs bearing the greatest burden of disease. In the systematic review, mentioned earlier by Bhutani et al 4 18% of 134 million live births had SNJ with the greatest burden of disease in LMICs, and therefore supporting this hypothesis. But as previously pointed out, these estimates were generated by mathematical modelling due to lack of accurate incidence data available. Both Bhutani’s data as well as this review, highlight the glaring paucity of studies particularly in LMICs. Although all WHO regions are represented, only 4/136 (2.9%) LMICs countries were represented with most having only one study (India (Southeast) n=2, 25 35 Nigeria (African) n=1 36 and Pakistan (Eastern Mediterranean) n=1, 3 Vietnam (Western Pacific) n=1). 37 In contrast representation among high-income countries, while low was better with 8/79 (10.1%) high-income countries having population-based data (Australia (Western Pacific) n=1, 23 Canada (Americas) n=3, 26 38 Denmark (European) n=3, 15 17 39 Norway (European) n=1, 24 Netherlands (European) n=1, 20 Switzerland (European) n=1, 27 USA (Americas) n=5, 16 18 19 40 UK and Ireland (European) n=1). 22 This general lack of population-based studies worldwide emphasises the need for more accurate data to determine the actual burden of disease.
Jaundice was the primary diagnosis in 17% of neonates ≤1 week in a hospital-based study in Kenya, 41 and several other African-based studies demonstrate that SNJ commonly leads to hospital admissions. 42–44 This pattern is also observed in Asia, including the Middle East. 41 45–49
Although not readily generalisable, all regions do have numerous hospital-based studies among the 416 articles with at least one clinical indicator of SNJ, highlighting the prevalence of SNJ among admissions. For some countries, such as the USA and many European nations where hospital birth is the norm, this data would more accurately reflect true population-based data. However, in LMICs where ‘60 million women give birth outside a facility’ (2012) 50 and recorded data population data spares, hospital data cannot be assumed to reflect true population data. The higher incidence of home births correlates well with the much higher incidence of SNJ noted in the studies from the African, Southeast Asian and Eastern Mediterranean regions compared with substantially lower incidence noted in the regions of the Americas and Europe.
Although only one study each from Africa and Eastern Mediterranean met the definition of population based, these two studies underscore the burden of ETs in LMIC’s with 186.5 and 107.1 ET’s per 10 000 live births in stark contrast to the American and European regions with only 0.38 and 0.35 per 10 000 live births, respectively.
While many paediatricians and even neonatologists in high-income countries never perform an ET, physicians in LMICs continue to perform ETs on a regular basis. 13 Although population-based data were available in only a few LMICs studies, other hospital-based studies support their findings. Of note again is the high prevalence of ETs, reported in studies from many LMIC (22%–86%), particularly Nigeria, 36 51 52 India 53 54 and Bolivia. 55
Access to ET, a proxy indicator of the magnitude of SNJ, is often limited in resource poor countries. 13 56 57 Multiple studies have demonstrated early intervention including phototherapy and appropriate ET can prevent kernicterus. 56 58 59 Despite benefits of ET, there are associated complications 13 making it important to provide effective phototherapy before ET is needed. 60
SNJ is significant due to the associated mortality, but some would argue even more so because of associated long-term morbidity especially in LMICs ill-equipped to handle these disabilities. Farouk et al reported abnormal neurological findings in almost 90% of infants returning for follow-up after ABE in their nursery. 61 Olusanya and Somefun, 62 reported ET as a risk factor for sensorineural hearing loss in their community-based study in Nigeria, as did da Silva et al in Brazil. 63
Contribution of SNJ to neonatal mortality
While only two studies in this review, 22 35 64 provided information on clear jaundice-related deaths, other studies have shown striking numbers of jaundice-related deaths where it reportedly accounted for 34% of neonatal deaths in Port Harcourt Nigeria, 52 15% in Ile-Ife, Nigeria, 65 14% in Kilifi District Kenya, 66 6.7% in Cairo Egypt 67 and 5.5% in Lagos Nigeria. 68
Multiple factors contributing to kernicterus in LMICs and the need for solutions addressing these factors has been spelled out in articles by Olusanya et al 69 and Slusher et al 2 including the need for national guidelines, 9 60 effective phototherapy, rapid reliable diagnostic tools, maternal and healthcare provider education. 70
Contribution of SNJ to long-term disability
Current evidence indicates SNJ continues to contribute significantly to the burden of cerebral palsy, deafness and other auditory processing disorders. 4 In India, Mukhopadhyay et al 71 found an abnormal MRI or brainstem auditory evoked response in 61% and 76%, respectively, of children who underwent ET. In Nigeria, Ayanniyi and Abdulsalam 72 reported NNJ as the leading cause of cerebral palsy (39.9%) trumping birth asphyxia (26.8%), while Ogunlesi et al 73 also from Nigeria, reported cerebral palsy, seizure disorders and deafness as leading sequelae of ABE, occurring in 86.4%, 40.9% and 36.4%, respectively. Oztürk et al from Turkey, 74 observed a history of prolonged jaundice commonly in children affected with cerebral palsy. Summing up available estimates, a recent Lancet article by Lawn et al 75 indicts pathological hyperbilirubinaemia/jaundice in >114 000 deaths and states that there are >63 000 damaged survivors.
The increased global awareness of SNJ has led to improvement in some locations. One notable example of this is Myanmar where a package of services including a photoradiometer, education and intensive phototherapy decreased ET by 69%. 76 Another example is the development, ongoing testing and refinement of filtered sunlight phototherapy in areas without access to continuous electricity or intensive phototherapy. 77 Several studies have shown that maternal and health worker education, screening programmes 14 18 28 38 and national guidelines 78 can and do improve outcomes and decrease the observed clinical sequelae of SNJ. 14 38 78 Many programmes supported by groups such as WHO 79 and Essential Care for Every Baby 80 now strongly support screening for jaundice and highlight it as a danger sign needing urgent care. This increased focus and awareness on SNJ is beginning to lead to decreases of this problem even in LMICs where recent studies though not always population based are beginning to show decreases in severe sequela. 76
Some limitations of this comprehensive review should be noted, besides those inherent in meta-analysis. 81 Only 12/195 sovereign nations 82 are represented in the quantitative data. While highlighting one of the greatest problems in determining the actual burden of disease from SNJ, absence of data from other countries despite searching multiple databases limits generalisability of our findings. Another significant limitation is the marked variability in the actual focus of the articles. The populations studied, availability of a TSB, recommendations and methods of screening, differences in TSBs and many other variables of included articles span an extremely wide range. Finally, the initial search excluding articles by title was done by only one author and the auditory evoked brainstem response, which is rarely available in LMICs, where not included in the criteria for SNJ.
Despite these limitations, this review still fills critical holes in our knowledge about the true burden of disease from this devastating but preventable tragedy. To our knowledge, this is the first attempt to report the global burden of SNJ derived from population-based studies. While providing strong evidence for the burden of disease, it highlights the notable lack of population-based data from most countries, especially LMICs where the disease is more prevalent and most devastating. The burden of SNJ and its acute and chronic ramifications establish a strong case for appropriate health education, routine screening, early diagnosis and effective treatment. The spectrum of disease crosses ethnic and socioeconomic boundaries, impacting children everywhere, and is a commonly encountered hospital diagnosis worldwide. SNJ may represent the most common unrecognised and/or under-reported neonatal cause of preventable brain damage. 83 More research with capacity building especially in LMICs and other areas where data are limited are needed to truly quantify the impact of this disease and to better understand how to integrate screening and therapy to eliminate this disease in the future.
Compelling but limited evidence from the literature demonstrates that SNJ is associated with a significant acute and chronic health burden, especially in LMICs. There is an urgent need to address this preventable disease in these regions, consistent with the inclusiveness advocated for erstwhile disadvantaged populations under the current SDGs dispensation.
Acknowledgments
We thank Dr Vinod Bhutani, Ms. Judith Hall RNC-NIC, and Dr Mark Ralston for their edits to an earlier version of the manuscript. We also thank Dr Philip Fischer, MD, Dr Reza Khodaverdian, Dr Janielle Nordell, Ms Ann Olthoff, RN, Dr Clydette Powell, Dr Hoda Pourhassan, Dr Maryam Sharifi-Sanjani, Ms Olja Šušilović, Dr Deborah Walker, Ms Allia Vaez and Ms Agnieszka Villanti, RN, for their help in the translation of foreign language literature used in this review. We also thank Ms Ayo Bode-Thomas, Dr Katie Durrwachter Erno, Mr Jeffrey Flores, Ms Judith Hall, RNC-NIC, Mr Jonathan Koffel, Ms Toni Okuyemi, Dr Mark Ralston, Mr Del Reed, Mr Paul Reid, Dr Yvonne Vaucher, Ms Mabel Wafula, Ms Katherine Warner, Dr Olga Steffens for their help in editing the article/tables including retrieving articles, verifying numbers, and managing Endnote.
- 1. ↵ National Institutes for Health and Clinical Excellence . Neonatal jaundice. (clinical guidelines 98 ) , 2010 .
- Slusher TM ,
- Zipursky A ,
- Tikmani SS ,
- Warraich HJ ,
- Abbasi F , et al
- Bhutani VK ,
- Blencowe H , et al
- Mwaniki MK ,
- Lawn JE , et al
- Blencowe H ,
- Darmstadt GL , et al
- Njelesani J
- Olusanya BO ,
- Ogunlesi TA ,
- Donaldson KM , et al
- Mabogunje CA ,
- Olaifa SM ,
- Olusanya BO
- Knauer Y , et al
- Bjerre JV ,
- Petersen JR ,
- Christensen RD ,
- Lambert DK ,
- Henry E , et al
- Ebbesen F ,
- Andersson C ,
- Verder H , et al
- Eggert LD ,
- Wiedmeier SE ,
- Wilson J , et al
- Flaherman VJ ,
- Kuzniewicz MW ,
- Escobar GJ , et al
- Gotink MJ ,
- Benders MJ ,
- Lavrijsen SW , et al
- Escobar GJ ,
- Wi S , et al
- Manning D ,
- Maxwell M , et al
- McGillivray A ,
- Polverino J ,
- Badawi N , et al
- Johansen KB
- 25. ↵ National neonatal perinatal database . National neonatal perinatal database: report: 2002-2003 . New Delhi : Nodal Centre , 2005 .
- Campbell D ,
- Berrut S , et al
- 28. ↵ American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation . Pediatrics 2004 ; 114 : 297 – 316 . OpenUrl Abstract / FREE Full Text
- Sanderson S ,
- Cheung CS ,
- DerSimonian R ,
- Davey Smith G ,
- Schneider M , et al
- 34. ↵ R: A language and environment for statistical computing [computer program] . Vienna, Austria : R Foundation for Statistical Computing , 2013 .
- Baitule S , et al
- Akande AA ,
- Emokpae A , et al
- Partridge JC ,
- Tran BH , et al
- Parmar SM ,
- Allegro D , et al
- Vandborg PK
- Wickremasinghe AC ,
- Wu YW , et al
- Iroha E , et al
- Mukhtar-Yola M ,
- 45. ↵ Subspecialty Group of Neonatology . Epidemiologic survey for hospitalized neonates in China . China J Contemp Pediatr 2009 ; 11 : 15 – 20 . OpenUrl
- Taşçilar E , et al
- Partridge J ,
- Chaudhary D ,
- Adhikary V , et al
- Abd El-Latif M ,
- Abd El-Latif D
- 50. ↵ Newborn Health: The Issue . http://www.savethechildren.org/ accessed 13 Dec 2016 , 2016
- Oruamabo RS
- Gathwala G ,
- 54. ↵ National Neonatal Perinatal Database . Morbidity and mortality among outborn neonates at 10 tertiary care institutions in India during the year 2000 . J Trop Pediatr 2004 ; 50 : 170 – 4 . OpenUrl CrossRef PubMed Web of Science
- Ogunlesi TA
- Ogunfowora OB
- Agrawal VK ,
- Misra PK , et al
- Johnson L ,
- Kumar P , et al
- Farouk ZL ,
- Muhammed A ,
- Gambo S , et al
- da Silva LP ,
- Queiros F ,
- Adeolu AA ,
- Arowolo OA ,
- Alatise OI , et al
- Gatakaa HW ,
- Mturi FN , et al
- Iskander I ,
- Gamaleldin R ,
- El Houchi S , et al
- Emokpae AA ,
- Imam ZO , et al
- Zamora TG , et al
- Mukhopadhyay K ,
- Chowdhary G ,
- Singh P , et al
- Ayanniyi O ,
- Abdulsalam KS
- Dedeke IO ,
- Adekanmbi AF , et al
- Demirci F ,
- Yavuz T , et al
- Oza S , et al
- Arnolda G ,
- Trevisanuto D , et al
- Vreman HJ , et al
- Kandasamy S ,
- Shah V , et al
- 79. ↵ World Health Organization . Newborn: reducing mortality , 2014 . http://www.who.int/mediacentre/factsheets/fs333/en/ ( accessed 15 Aug 2017 ).
- 80. ↵ Essential Care for Every Baby - AAP.org . 2017 https://www.aap.org/en-us/advocacy.babies./Essential-Care-Every-Baby.aspx (accessed 2 Sep 2017) .
- Greenland S
- 82. ↵ Independent States of the World . http://www.nationsonline.org/
Contributors TS and BOO conceptualised and designed the study, acquired data, analysed and interpreted data, supervised the study, drafted the manuscript and conducted a critical revision of the manuscript for intellectual content and approved the manuscript as submitted. TGZ conceptualised and designed the study, and assisted with acquiring data and approved the manuscript as submitted. AMS assisted in acquiring data and approved the manuscript as submitted. EMK and JUS were responsible for acquisition of data as well as providing administrative, technical and material support and approved the manuscript as submitted. SBR was responsible for acquisition of data, analysing and interpreting data and provided administrative, technical and material support and approved the manuscript as submitted. DA, MAS and BWL analysed and interpreted the data, conducted the statistical analysis, and conducted a critical revision of the manuscript for important intellectual content and approved the manuscript as submitted. All authors had full access to all data, take responsibility for the accuracy and integrity of the data and approved the manuscript as submitted.
Funding Support for the quantitative analysis was provided in part by The Programme for Global Paediatric ReSouth-East Asianrch, Centre for Global Child Health, The Hospital for Sick Children, Toronto, Canada.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Published: 01 February 2021
Neonatal jaundice and autism spectrum disorder: a systematic review and meta-analysis
- Monica L. Kujabi ORCID: orcid.org/0000-0002-2901-3417 1 ,
- Jesper P. Petersen 2 ,
- Mette V. Pedersen 2 ,
- Erik T. Parner 3 &
- Tine B. Henriksen 2
Pediatric Research volume 90 , pages 934–949 ( 2021 ) Cite this article
2489 Accesses
9 Citations
18 Altmetric
Metrics details
Two meta-analyses concluded that jaundice was associated with an increased risk of autism. We hypothesize that these findings were due to methodological limitations of the studies included. Neonatal jaundice affects many infants and risks of later morbidity may prompt physicians towards more aggressive treatment.
To conduct a systematic literature review and a meta-analysis of the association between neonatal jaundice and autism with particular attention given to low risk of bias studies . Pubmed, Scopus, Embase, Cochrane, and Google Scholar were searched for publications until February 2019. Data was extracted by use of pre-piloted structured sheets. Low risk of bias studies were identified through predefined criteria.
A total of 32 studies met the inclusion criteria. The meta-analysis of six low risk of bias studies showed no association between neonatal jaundice and autism; cohort studies risk ratio 1.09, 95% CI, 0.99–1.20, case-control studies odds ratio 1.29 95% CI 0.95, 1.76. Funnel plot of all studies suggested a high risk of publication bias.
Conclusions
We found a high risk of publication bias, selection bias, and potential confounding in all studies. Based on the low risk of bias studies there was no convincing evidence to support an association between neonatal jaundice and autism.
Meta-analysis of data from six low risk of bias studies indicated no association between neonatal jaundice and autism spectrum disorder.
Previous studies show inconsistent results, which may be explained by unadjusted confounding and selection bias.
Funnel plot suggested high risk of publication bias when including all studies.
There is no evidence to suggest jaundice should be treated more aggressively to prevent autism.
You have full access to this article via your institution.
Similar content being viewed by others
Consensus definition and diagnostic criteria for neonatal encephalopathy—study protocol for a real-time modified delphi study

Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants

Italian neonatologists and SARS-CoV-2: lessons learned to face coming new waves
Introduction.
Autism spectrum disorder (ASD) is a disease defined by symptoms in the following three domains; social interaction, communicative disorders, and stereotyped, repetitive or restricted behavior. 1 This review focuses on ASD, including all subtypes. The prevalence of ASD is 1−2%, and has increased since the 1940s. 2 , 3 , 4 ASD is more than four times as prevalent in boys than in girls. 2 The etiology of ASD is unknown, but studies indicate involvement of both genetic 5 , 6 , 7 and non-inheritable factors. 8 , 9 ASD is a disease with long-term consequences for both the child and the family. 10 Accordingly, there is a need to identify preventable causes of ASD. Neonatal jaundice occurs in some 80% of neonates. 11 Unconjungated bilirubin crosses the blood−brain barrier in the newborn and high levels may cause acute bilirubin-induced encephalopathy and permanent brain damage. 12 The most common neuropathological findings in children with ASD are a decreased number of purkinje cells in the cerebellum, decreased neuronal cell size, and increased cell packing density in the cerebral cortex. 13 , 14 These areas may also be damaged by bilirubin deposition in brain tissue. 12 , 15 , 16 Accordingly, an association between hyperbilirubinemia and ASD seems plausible. 17 Reviews by Amin et al. 16 and Jenabi et al. 18 concluded that neonatal jaundice was associated with an increased risk of ASD. In the review by Amin et al. no structured quality assessment was performed and the conclusion was based on a meta-analysis of all studies regardless of their quality. Jenabi et al. rated 19 out of 21 studies as high quality despite methodological limitations of some studies including no adjustment for confounders. The purpose of this systematic review was to compile and critically review the existing evidence of the association between jaundice and ASD and to base the conclusion only on studies with low risk of bias .
Search strategy
This study is conducted in accordance with the PRISMA guideline (see PRISMA checklist). A systematic literature search was carried out according to the review protocol published in PROSPERO, protocol number: CRD42016025927. Pubmed, Scopus, Embase, Cochrane, and Google Scholar were searched for publications until February 2019. The search terms included autism, autistic disorder, pervasive developmental disorder (PDD), ASD, Asperger, hyperbilirubinemia, jaundice, icterus, bilirubin, newborn/perinatal/neonatal risk factor (s), phototherapy. MESH terms were used whenever available. The full search strategy can be found in Supplementary Text S1 (online). References of included studies and other relevant reviews were screened to identify additional studies.
Inclusion/exclusion criteria
All case−control and cohort studies examining the association between jaundice, hyperbilirubinemia, or phototherapy and ASD, that provided absolute numbers were eligible.
Exposure measures had to be either neonatal hyperbilirubinemia or jaundice based on clinical assessment, parental report, laboratory confirmation by estimating serum bilirubin during the neonatal period (within 28 days after birth), or phototherapy treatment.
The outcome measure was ASD, which include childhood/infantile autism, autistic disorder, pervasive developmental disorder—not otherwise specified, and Asperger’s. In the literature the terms autism, ASD, and PDD are often used interchangeably; thus, all were included.
To be able to tease out the details of each study, only studies in English peer-reviewed journals were included. Conference abstracts and studies without a reference group such as case series or case reports were excluded.
Studies that adjusted for confounding factors, but did not include the adjusted results, were excluded from the meta-analysis. Studies that investigated preterm infants only were included in a sub-analysis of preterm infants.
Study selection and data extraction
Titles and abstracts of all identified records were screened for eligibility according to the inclusion and exclusion criteria. If immediate exclusion based on title and abstract was not possible, the full text was assessed for eligibility. Structured sheets piloted prior to the search were used for data extraction from each study (see Table 1 ).
Low risk of bias studies
Studies passed the threshold for strong methodological quality, if they met the following criteria: ASD diagnosis based on International Classification of Diseases/Diagnostic and Statistical Manual of Mental Disorder (ICD/DSM), jaundice was based on TSB measurement or jaundice diagnosis from medical records, and adjustment for at least sex 2 and either gestational age (e.g. term vs. preterm or gestational week at birth) or birth weight. 19 These quality criteria were defined after the development of the PROSPERO protocol, but prior to data extraction. Studies that met the quality criteria were defined as low risk of bias studies . Only low risk of bias studies were subjected to further quality assessment.
Quality assessment
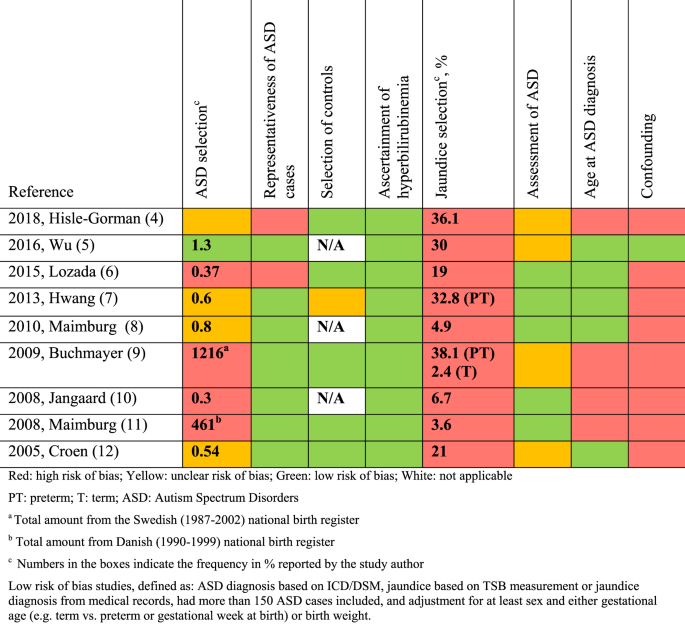
The quality-assessment was guided by the Cochrane Handbook for systematic reviews of interventions, 20 the STROBE checklist 21 (STrengthening the Reporting of OBservational studies in Epidemiology), and the Newcastle-Ottawa Scale. 22 We defined essential confounders as: sex, 2 gestational age 19 or birth weight, 19 birth year, 4 and Apgar score. 19 According to current evidence, these may likely influence the association and should be adjusted for. 23 Other potential confounding factors such as pregnancy complications, parental age, education, and socioeconomic status were also considered, but not deemed essential due to the paucity of studies between these variables and ASD. To further evaluate the quality of the low risk of bias studies , the risk of bias in predefined areas (ASD selection, representativeness of ASD cases, selection of controls, ascertainment of hyperbilirubinemia, jaundice selection, assessment of ASD, age at ASD assessment, confounding) were rated as low, high or unclear risk of bias (Fig. 1 ). This assessment aimed to show the quality of the studies without suggesting how that might influence the effect estimates. The quality-assessment was based on a risk of bias table (Supplementary Table S2 (online)) and assessment of confounders (Supplementary Table S3 (online)) made a priori by the authors.
Qualitative assessment of low risk of bias studies based on predefined criteria (Supplementary Table S2 ).
Literature search, inclusion, data extraction, selection of low risk of bias studies , and quality assessment of low risk of bias studies were conducted independently by two authors (M.L.K. and M.V.P.). In case of discrepancy between the two authors, a third author (T.B.H.) was conferred.
Data analysis
Data were analyzed using the Cochrane Collaboration Review Manager Software (RevMan version 5.3). 24 Adjusted effect measures were used when available. The unadjusted risk ratio (RR) or odds ratio (OR) was calculated from absolute numbers with 95% confidence intervals (CIs) if adjusted estimates were unavailable. Effect measures were entered into RevMan using the “generic inverse variance” outcome. OR and RR were analyzed separately in the meta-analysis because case−control and cohort studies are heterogenic and may have different challenges related to methodology. A random-effects model was used to analyze the included studies as a random sample of a hypothetical population of studies. Between-study heterogeneity was assessed using I 2 , which describes the percentage of variation across studies that is due to heterogeneity rather than chance. 25 , 26 A forest plot and meta-analyses using a logarithmic scale were made for all studies, the low risk of bias studies , and for preterm infants. A funnel plot was used to assess selective reporting.
Literature search
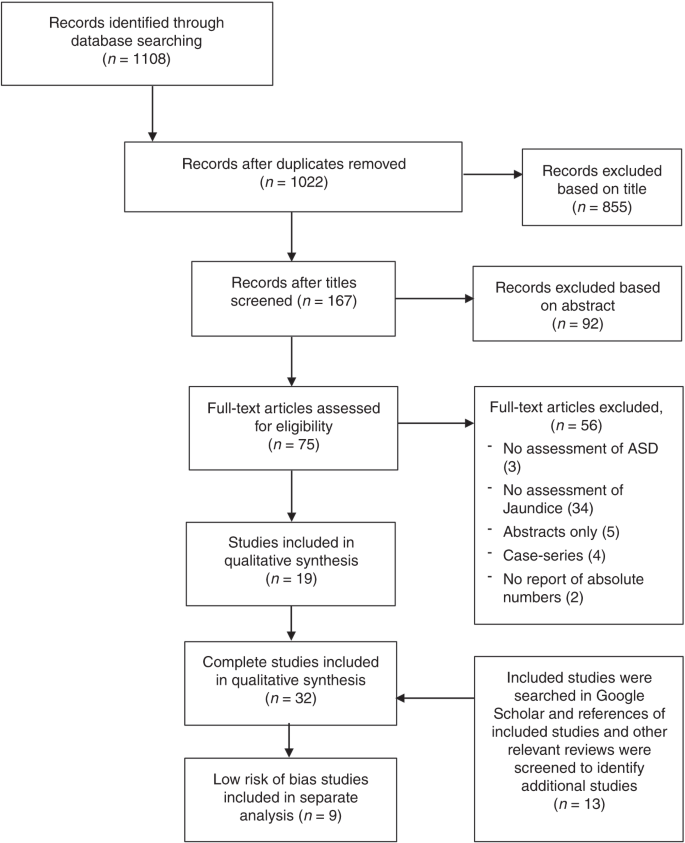
Literature search was conducted in February 2019 (PRISMA flow chart in Fig. 2 ) identifying a total of 32 studies to be included in this review. Two studies by Maimburg et al. 27 , 28 were both included, despite overlapping by 5 years. However, they also represent 10 years without overlap.
PRISMA flow chart for the systematic review detailing the number of abstracts and full-text screened and number of studies excluded.
Study characteristics
Table 1 shows the main characteristics and effect estimates from all 32 included studies. The earliest study dates back to 1979. The total number of children with ASD across all studies was 29,299. Differences in the definition of jaundice (parental assessment by self-administered questionnaires, clinical diagnosis, diagnosis by TSB levels, the need for treatment by phototherapy) and the definition of ASD (diagnosis by ICD-8, 9 or 10 or DSM-III, IV or V) compromised overall comparability.
Nine studies met the low risk of bias criteria. The low risk of bias studies included 24,440 children with ASD. The studies that were not included in the low risk of bias studies failed to adjust for any potential confounding factors or they based the information on jaundice on parental recall. Fig. 1 shows the quality assessment of each of these nine studies and Supplementary Table S3 (online) shows the potential confounders adjusted for. As seen in Fig. 1 even the studies we considered low risk of bias studies had several limitations. Of the nine studies two reported an increased risk of ASD with jaundice, 28 , 29 the seven remaining studies showed no association between jaundice and ASD. 27 , 30 , 31 , 32 , 33 , 34 , 35 These nine studies were thoroughly reviewed and their main characteristics are summarized in the following narrative syntheses ordered according to their weight in the meta-analyses, with cohort studies first.
Narrative description of low risk of bias studies
Wu et al. 31 based their cohort study on 457,855 children born 1995–2011 at 15 Kaiser Permanente Northern California hospitals (KPNC) covering 40% of the insured population. They found no association between jaundice and ASD (RR 1.07, 95% CI, 0.98–1.17). Neonatal jaundice was found in 30% and ASD in 1.3% of the included population. Jaundice was defined as TSB > 10 mg/dL, and 51% of all newborns in the study had TSB measured. ASD was defined according to ICD-9 and retrieved from the KPNC registry. Children were either diagnosed at autism evaluation centres, by a clinical specialist outside the ASD center, or by a general pediatrician. The study adjusted for all our predefined essential confounders. They estimated the effect of phototherapy, and found that use of phototherapy did not change the association between jaundice and ASD.
Maimburg et al. 27 (revised ASD selection 36 ) based their cohort study on all Danish children born 1994–2004. They found no association between jaundice and ASD (RR 1.07, 95% CI, 0.94–1.21). They included 733,826 children, 5% were jaundiced and 0.8% had ASD. Jaundice was defined according to ICD-10 retrieved from the National Patient Registry. Several neurodevelopmental disorders (F80−F84.9 and F88−F88.9), including autism/pervasive developmental disorders, were studied. ASD was defined by ICD-10 from the Danish Psychiatric Central Register (in- and outpatients). Results were adjusted for all the predefined essential confounders except birth year. In children born preterm no association was found (RR 1.05, 95% CI, 0.83–1.33).
Jangaard et al. 32 based their cohort study on the Canadian Nova Scotia Atlee Perinatal Database including 94% of all newborns 1994–2000 in the province. They found an association between jaundice and ASD (RR 1.60, 95% CI, 1.00–2.56). A total of 56,019 children were included, 7% were jaundiced and 0.33% had ASD. The study assessed the association between serum bilirubin levels and four outcomes including autism. Jaundice was defined as TSB level above 13.5 mg/dL. The Medical Service Insurance (physician billings) and the hospital Discharge Abstract Database provided ASD diagnosis by ICD-9. The study adjusted for all predefined essential confounders except birth year and Apgar score.
Lozada et al. 29 found that neonatal jaundice was associated with an increased risk of ASD (OR 1.18, 95% CI, 1.06–1.31). This case−control study was based on data from the United States (US) Military Health System database. It included 2917 cases born 2000–2009 and 8751 controls matched by sex and age. Jaundice and ASD was defined according to ICD-9-CM; only inpatient diagnoses were used for jaundice. Eighteen percent of infants in the control group were jaundiced. ASD was ascertained from a minimum of one outpatient visit to a pediatric specialist, with no description of how children were referred. ASD was found in 0.37% of 783,047 births recorded. Our defined essential confounders apart from Apgar score and birth year were assessed. When studying preterm children only, the association disappeared (OR 1.06, 95% CI, 0.77–1.46).
Buchmayer et al. 33 based their case−control study on the Swedish Medical Birth Register and included 1216 ASD cases born 1987–2002 and 6080 controls matched by sex, birth year and birth hospital. They found no association between jaundice and ASD (OR 1.18, 95% CI, 0.83–1.68). Jaundice was one of many perinatal factors studied. Jaundice and ASD was defined by ICD-9 and ICD-10 from inpatient medical records, 5% of infants in the control group were jaundiced. ASD was verified by a child psychiatrist. The study adjusted for all the predefined essential confounders and 16 other risk factors. When preterm infants were assessed no association was seen (OR 0.70, 95% CI, 0.50–0.98).
Croen et al. 30 based their case−control study on children born at one of the KPNC hospitals in Northern California covering 30% of the insured population. They found no association between jaundice and ASD (OR 0.67, 95% CI, 0.43−1.04). It included 338 ASD cases born 1995–1998 and 1718 controls matched by sex, birth year, and hospital of birth. Jaundice was defined as TSB > 15 mg/dL, 28% of cases and controls had TSB measured and 12% of infants in the control group were jaundiced. ASD was defined by ICD-9-CM and obtained from the outpatient databases. The study adjusted for all our predefined essential confounders except Apgar score.
Maimburg et al. 28 found that TSB > 17.5 mg/dL (300 µmol/L) was associated with increased odds of ASD (OR 3.70, 95% CI, 1.30–10.53). Maimburg et al. based their case−control study on all children born in Denmark 1990–1999. The study included 461 cases and 461 controls from the national civil registration system matched by sex, birth year and county of birth. The study assessed the association between seven neonatal risk factors and ASD. TSB values were retrieved from medical records; 18% of cases and 13% of controls had a TSB measured, jaundice frequency was 3.6%. ASD was defined by psychiatrists’ ICD-8 and ICD-10 codes. ASD cases were ascertained from the Danish Psychiatric Central Register including all inpatients in Denmark 1990–1995 and in- and outpatients 1995–1999. Apgar score was the only essential confounder not adjusted for. When preterm infants were considered the association disappeared (OR 1.00, 95% CI, 0.06–16.67).
Hilse-Gorman et al. 35 based their case−control study on the US Military Health System. They included 8760 ASD cases born 2000–2013. They claimed to find no association in the adjusted analyses. However, the adjusted results were not presented. Each case was matched by three controls by age, sex, and enrollment time frame. Jaundice was one of 28 different risk factors studied. Information on jaundice and ASD was based on ICD-9 from inpatient and outpatient data. Thirty-six percent of infants in the control group were jaundiced (highest rate in any study in this review). All essential confounders were adjusted for. Adjusted results were not shown, and therefore not included in our meta-analysis.
Hwang et al. 34 based their case−control study on Taiwan National Health Insurance Research Database covering 99% of Taiwanese population. They found no association between jaundice in preterm neonates and ASD (OR 0.99, 95% CI, 0.81–1.21). The aim was to identify neonatal risk factors for autism in preterm children. The study included 411 ASD cases and 29,614 controls born 1998–2001. Jaundice was defined by ICD-9-CM from in- and outpatient databases, ASD was only from outpatient databases, 33% of infants in the control group were jaundiced. All predefined essential confounders except for Apgar score were adjusted for.
Meta-analysis and funnel plot
When restricting the analysis to the low risk of bias studies , there was no significant association between neonatal jaundice and ASD. Three case−control studies were excluded from the meta-analysis, one only studied preterm infants 34 and one did not show the adjusted OR. 35 The third study had an overlapping population with that of Wu et al. 31 Croen et al. 30 included one KNPC hospital while Wu et al. 31 included 15 KNPC hospitals of Northern California. The meta-analysis of the three low risk of bias cohort studies revealed an RR of 1.09 (95% CI, 0.99–1.20), and of the three low risk of bias case−control studies an OR of 1.29 (95% CI, 0.95–1.76) (Fig. 3 ). If the study by Croen et al. 30 was included in the meta-analysis of the case−control studies, the OR was 1.14 (95% CI, 0.80–1.61). In addition, we found no statistically significant association from the meta-analysis of all four cohort studies (RR 1.14, 95% CI, 0.99–1.30), while the meta-analysis of all 29 case−control studies showed an association OR 1.74 (95% CI, 1.42–2.12) (Fig. 4 ). The meta-analysis based on preterm infants only showed no significant association (OR 0.93, 95% CI, 0.77–1.12) (Fig. 5 ). The meta-analysis of all studies found a high degree of heterogeneity ( I 2 of 51% (cohort studies) and 83% (case−control studies)). Furthermore, funnel plots (Fig. 6 ) indicated selective reporting of studies that found an association.
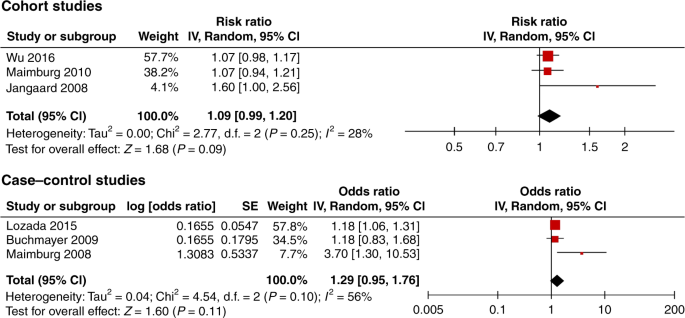
The squares show the average effect size of each study. The diamonds show the combined average effect size.
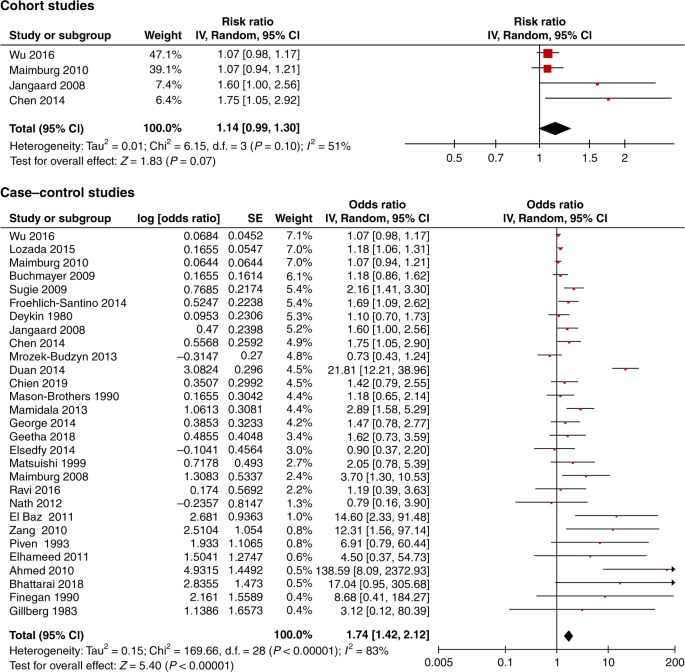
The squares show the average effect size of each study. The diamonds show the combined average effect size.
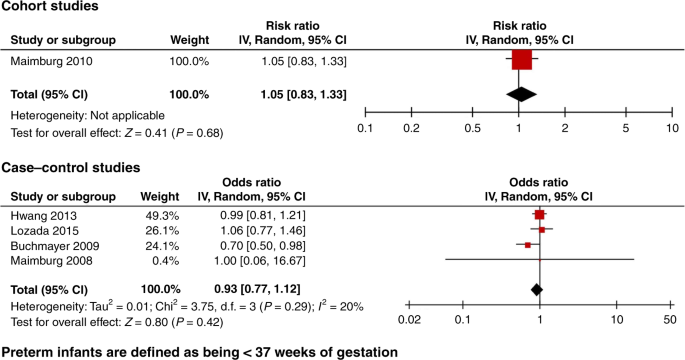
Funnel plot of studies investigating the association between neonatal jaundice and ASD.
We identified 32 studies that qualified for this review of the association between neonatal jaundice and ASD. In the meta-analysis of all studies we found an association between neonatal jaundice and ASD. A funnel plot demonstrated a high risk of publication bias. Due to the large variation in the quality of the studies, a meta-analysis of all studies should be interpreted with caution. The low risk of bias studies were based on ICD/DSM and not on parental recall, and most of them had a predefined primary aim to study jaundice and ASD, making publication bias and type 1 errors less likely. Although not significant, our meta-analysis restricted to the three low risk of bias cohort studies showed an increased risk of ASD of 9% (RR 1.09, 95% CI, 0.99–1.20). If not due to random variation, this could be explained by methodological limitations such as residual confounding and selection bias even in the low risk of bias studies (Fig. 1 ).
A challenge in all studies was a reliable jaundice diagnosis. No studies defined the criteria for diagnosing jaundice or measuring TSB level, e.g., referral criteria. In most settings bilirubin testing is not used as a screening procedure for all newborns, and since jaundice often develops some days after birth, discharged newborns may be less likely to be diagnosed. Accordingly, the neonate who has been discharged may rarely have a diagnosis of hyperbilirubinemia from the hospital system 37 , 38 , 39 ; at nine KPNC hospitals the number of infants with TSB 15–19.9 mg/dL increased by 56% after implementation of universal bilirubin screening. 40 This suggests that the jaundice diagnosis is an indicator of being hospitalized rather than having a bilirubin level different from non-hospitalized newborns, in particular when jaundice is defined by the lower cut-off levels of bilirubin. In our low risk of bias studies we included jaundice based on medical records and even among studies using serum values 28 , 30 , 31 , 32 , 41 , 42 highly variable definitions of jaundice were seen resulting in frequencies differing between 1 and 36%. In conclusion, availability and criteria of TSB testing and TSB cut-off values may influence the frequency of jaundice, the risk of selection bias, and the interpretation of the exposure in the studies. All studies qualified as low quality on jaundice selection, because they did not explain which infants had TSB measured or controlled for hospitalization or in other ways reflected on the frequency of TSB measurement/hyperbilirubinemia.
If hospitalized children are more likely to be categorized as exposed, interpretation of results may be difficult. Compared to the background population, hospitalized newborns may differ in several ways: they are more likely to be the first child, to have had a complicated delivery, to be of low birth weight, or to be preterm. These are all factors associated with ASD. Comparing children hospitalized in the newborn period who may much more often be diagnosed with jaundice to non-hospitalized children with a much lower risk of being diagnosed with jaundice might lead to bias towards an association between jaundice and ASD. We have illustrated this by a directed acyclic graph (DAG) 43 ; if hospitalization is a cause of jaundice diagnosis it opens numerous potential biasing pathways (Supplementary Fig. 5 ). According to the DAG, studies of a causal relationship should either adjust for all covariates causing both neonatal hospitalization and ASD, should be based on exposures obtained from universal bilirubin screening, or should adjust for hospitalization for reasons other than suspected hyperbilirubinemia. Using a conservative cut-off level may decrease but not eliminate this bias.
Accordingly, studies in preterm newborns that are all hospitalized after birth may illustrate the points made on jaundice and hospitalization; in six of the included studies, preterm neonates were analyzed independently. Five of these studies were low risk of bias and all showed no association between jaundice and ASD in preterm newborns (Fig. 5 ).
Confounding factors may influence the relationship between bilirubin levels and ASD. Potential confounders could be newborn infections, asphyxia, parental age, and complicated delivery; however, other factors such as genetic and socioeconomic factors may also be involved. Whether it is possible to fully adjust for all potential confounders is questionable.
Several studies used parental recall of neonatal jaundice as the exposure, which may result in recall bias. None of these studies were considered low risk of bias studies in this review.
The Autism and Development Disabilities Monitoring Network suggested an increase in estimated prevalence of ASD by roughly 123% since 2002, which is supported by several other sources. 4 , 44 , 45 , 46 This is thought to be explained by other factors than a true increase, i.e., diagnostic criteria, service availability, increased funding, and population awareness. 3 , 46 , 47 , 48 , 49 Furthermore, new guidelines on the diagnosis of hyperbilirubinemia (one particularly from 1994 37 ) have emerged, and contributed to an increase in admissions for neonatal jaundice. 32 , 50 , 51 The majority of studies collected data over time periods of some 15 years. Therefore, if time is not adjusted for, changes in diagnostic practices, could bias results related to the association between jaundice and ASD.
The majority of included studies offered no description on how infants with ASD were referred for diagnostic evaluation. Reported frequencies of ASD were as low as 0.3% 29 , 32 and as high as 1.3% 31 (the latter being close to the expected prevalence 2 .) The low number of ASD cases seen in some studies could be due to the use of hospital-based databases. 28 , 30 , 33 , 34 , 35 , 52 In somatic hospital databases only children with somatic diseases will be admitted to the hospital and an additional ASD diagnosis may depend on availability of patient history from other contacts e.g., general practice or history taken from parents. While studies with small numbers of children with autism argue that they have more severe cases, the cases might also differ in other aspects. Thus, studies with a low frequency that did not provide valid arguments for the occurrence were rated as low quality on ASD selection.
Maimburg et al. published two studies based on information from Danish health registries with overlapping study periods. They differed substantially in the number of identified cases; a case−control study including 461 cases born 1990−1999 28 and a cohort study including 6171 cases born between 1994 and 2004. 27 The case−control study showed a threefold increased risk of ASD with jaundice, while the cohort study found no association. Thus, selection bias might contribute significantly to the associations seen.
The study by Wu et al. 31 investigated the effect of phototherapy and found no indication of a protective effect. So, even if there would be an association between jaundice and ASD, it does not seem to be affected by the use of phototherapy.
Strengths and limitations
Our inclusion criteria were broad to allow for a high number of studies. Consequently, we made no restrictions to studies with particular methodological strengths. Many studies examined a variety of newborn complications with no a priori hypotheses related to jaundice. 41 , 42 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 A number of studies had other methodological weaknesses such as the use of parents’ information to diagnose neonatal jaundice 55 , 56 , 57 , 58 , 59 , 61 , 62 , 63 , 67 , 68 , 69 , 70 , 71 and no adjustment for confounding factors. 41 , 42 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 61 , 63 , 66 , 67 , 69 , 70 , 72 , 73 However, we were able to restrict our main analysis to include only low risk of bias studies . The low risk of bias studies were identified based on a priori defined quality criteria. Thus, providing a reliable final conclusion based on low risk of bias studies . Our criteria could have been stricter, since the low risk of bias studies also had limitations.
We identified a high risk of publication bias in all studies on jaundice and ASD. We also pointed out selection and information bias and lack of adjustment for potential confounding factors in a number of studies, which may explain previous findings. When restricting the meta-analysis to low risk of bias studies , we found no convincing evidence of an association between neonatal jaundice and ASD. Furthermore, one study investigated the effect of phototherapy and found no indication of a protective effect. However, further high-quality studies are warranted to provide more firm conclusions. A more aggressive use of phototherapy to lower any potential risk of ASD in jaundiced infants should not be encouraged based on current evidence.
WHO. International Statistical Classification of Diseases and Related Health Problems (WHO, 2018).
Baio, J. et al. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67 , 1–23 (2018).
PubMed PubMed Central Google Scholar
Hansen, S. N., Schendel, D. E. & Parner, E. T. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 169 , 56–62 (2015).
PubMed Google Scholar
Nevison, C., Blaxill, M. & Zahorodny, W. California autism prevalence trends from 1931 to 2014 and comparison to National ASD Data from IDEA and ADDM. J. Autism Dev. Disord. 48 , 4103–4117 (2018).
Bill, B. R. & Geschwind, D. H. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr. Opin. Genet. Dev. 19 , 271–278 (2009).
CAS PubMed PubMed Central Google Scholar
Chaste, P. & Leboyer, M. Autism risk factors: genes, environment, and gene−environment interactions. Dialogues Clin. Neurosci. 14 , 281–292 (2012).
Bailey, A. et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol. Med. 25 , 63–77 (1995).
CAS PubMed Google Scholar
Guinchat, V. et al. Pre-, peri- and neonatal risk factors for autism. Acta Obs. Gynecol. Scand. 91 , 287–300 (2012).
Google Scholar
Gardener, H., Spiegelman, D. & Buka, S. L. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128 , 344–355 (2011).
Karst, J. S. & Van Hecke, A. V. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev. 15 , 247–277 (2012).
Bhutani, V. K. et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 162 , 477–482.e1 (2013).
Shapiro, S. M. Bilirubin toxicity in the developing nervous system. Pediatr. Neurol. 29 , 410–421 (2003).
Bauman, M. L. & Kemper, T. L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 23 , 183–187 (2005).
Palmen, S. J., van Engeland, H., Hof, P. R. & Schmitz, C. Neuropathological findings in autism. Brain 127 , 2572–2583 (2004).
Hansen, T. W. & Bratlid, D. Bilirubin and brain toxicity. Acta Paediatr. Scand. 75 , 513–522 (1986).
Amin, S. B., Smith, T. & Wang, H. Is neonatal jaundice associated with autism spectrum disorders: a systematic review. J. Autism Dev. Disord. 41 , 1455–1463 (2011).
Verhoeven, J. S., De Cock, P., Lagae, L. & Sunaert, S. Neuroimaging of autism. Neuroradiology 52 , 3–14 (2010).
Jenabi, E., Bashirian, S. & Khazaei, S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clin. Exp. Pediatr. 63 , 8–13 (2020).
Schieve, L. A., Clayton, H. B., Durkin, M. S., Wingate, M. S. & Drews-Botsch, C. Comparison of perinatal risk factors associated with autism spectrum disorder (ASD), intellectual disability (ID), and co-occurring ASD and ID. J. Autism Dev. Disord. https://doi.org/10.1007/s10803-015-2402-0 (2015).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/ (2011).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61 , 344–349 (2008).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2009).
Gardener, H., Spiegelman, D. & Buka, S. L. Prenatal risk factors for autism: comprehensive meta-analysis. Br. J. Psychiatry 195 , 7–14 (2009).
Review Manager 5 (RevMan 5) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (2014).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 , 1539–1558 (2002).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj 327 , 557–560 (2003).
Maimburg, R. D., Bech, B. H., Væth, M., Møller-Madsen, B. & Olsen, J. Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics 126 , 872–878 (2010).
Maimburg, R. D. et al. Neonatal jaundice: a risk factor for infantile autism? Paediatr. Perinat. Epidemiol. 22 , 562–568 (2008).
Lozada, L. E. et al. Association of autism spectrum disorders with neonatal hyperbilirubinemia. Glob. Pediatr. Heal 2 , 2333794x15596518 (2015).
Croen, L. A., Yoshida, C. K., Odouli, R. & Newman, T. B. Neonatal hyperbilirubinemia and risk of autism spectrum disorders. Pediatrics 115 , e135–e138 (2005).
Wu, Y. W. et al. Risk of autism associated with hyperbilirubinemia and phototherapy. Pediatrics 138. https://doi.org/10.1542/peds.2016-1813 (2016).
Jangaard, K. A., Fell, D. B., Dodds, L. & Allen, A. C. Outcomes in a population of healthy term and near-term infants with serum bilirubin levels of ≥325 μmol/L (≥19 mg/dL) who were born in Nova Scotia, Canada, between 1994 and 2000. Pediatrics 122 , 119–124 (2008).
Buchmayer, S. et al. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 124 , e817–e825 (2009).
Hwang, Y. S., Weng, S. F., Cho, C. Y. & Tsai, W. H. Higher prevalence of autism in Taiwanese children born prematurely: a nationwide population-based study. Res. Dev. Disabil. 34 , 2462–2468 (2013).
Hisle-Gorman, E. et al. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr. Res. https://doi.org/10.1038/pr.2018.23 (2018).
Maimburg, R. D., Bech, B. H., Væth, M., Møller-Madsen, B. & Olsen, J. [personal communication]. Response from authors. Comment to Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics. 126 , 872–878 (2010).
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114 , 297−316 (2004).
Bjerre, J. V. & Petersen, J. R. & Ebbesen, F. Surveillance of extreme hyperbilirubinaemia in Denmark. A method to identify the newborn infants. Acta Pediatr. https://doi.org/10.1111/j.1651-2227.2008.00879.x (2008).
Lain, S. J., Roberts, C. L., Bowen, J. R. & Nassar, N. Early discharge of infants and risk of readmission for jaundice. Pediatrics https://doi.org/10.1542/peds.2014-2388 (2015).
Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124 , 1031–1039 (2009).
Gillberg, C. & Gillberg, I. C. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J. Autism Dev. Disord. 13 , 153–166 (1983).
Finegan, J. A. & Quarrington, B. Pre-, peri-, and neonatal factors and infantile autism. J. Child Psychol. Psychiatry 20 , 119–128 (1979).
Williams, T. C., Bach, C. C., Matthiesen, N. B., Henriksen, T. B. & Gagliardi, L. Directed acyclic graphs: a tool for causal studies in paediatrics. Pediatr. Res. 84 , 487–493 (2018).
Idring, S. et al. Changes in prevalence of autism spectrum disorders in 2001–2011: findings from the Stockholm Youth Cohort. J. Autism Dev. Disord. 45 , 1766–1773 (2014).
Rutter, M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 94 , 2–15 (2005).
Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5 , 160–179 (2012).
Nassar, N. et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int. J. Epidemiol. 38 , 1245–1254 (2009).
Williams, J. G., Higgins, J. P. & Brayne, C. E. Systematic review of prevalence studies of autism spectrum disorders. Arch. Dis. Child 91 , 8–15 (2006).
Lundström, S., Reichenberg, A., Anckarsäter, H., Lichtenstein, P. & Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 350 , h1961 (2015).
Burgos, A. E., Schmitt, S. K., Stevenson, D. K. & Phibbs, C. S. Readmission for neonatal jaundice in California, 1991−2000: trends and implications. Pediatrics 121 , e864–e869 (2008).
Burke, B. L. et al. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988−2005. Pediatrics 123 , 524–532 (2009).
Rosti, L., Lambertini, L., Stucchi, I. & Condo, V. Neonatal jaundice: should we go crazy? Pediatrics 127 , e859–e860 (2011). author reply e860−1.
Mrozek-Budzyn, D., Majewska, R. & Kieltyka, A. Prenatal, perinatal and neonatal risk factors for autism. Cent. Eur. J. Med. 8 , 424–430 (2013).
Nath, S., Roy, R. & Mukherjee, S. Perinatal complications associated with autism—a case control study in a neurodevelopment and early intervention clinic. J. Indian Med. Assoc. 110 , 526–529 (2012).
Zhang, X. et al. Prenatal and perinatal risk factors for autism in China. J. Autism Dev. Disord. 40 , 1311–1321 (2010).
Piven, J. et al. The etiology of autism: pre-, peri- and neonatal factors. J. Am. Acad. Child Adolesc. Psychiatry 32 , 1256–1263 (1993).
Ravi, S., Chandrasekaran, V., Kattimani, S. & Subramanian, M. Maternal and birth risk factors for children screening positive for autism spectrum disorders on M-CHAT-R. Asian J. Psychiatry https://doi.org/10.1016/j.ajp.2016.04.001 (2016).
Geetha, B., Sukumar, C., Dhivyadeepa E., Reddy, J. K. & Balachandar, V. Autism in India: a case–control study to understand the association between socio-economic and environmental risk factors. Acta Neurol. Belg. https://doi.org/10.1007/s13760-018-01057-4 (2018).
Deykin, E. Y. & MacMahon, B. Pregnancy, delivery, and neonatal complications among autistic children. Am. J. Dis. Child. 134 , 860–864 (1980).
El-Baz, F., Ismael, N. A. & El-Din, S. M. N. Risk factors for autism: an Egyptian study. Egypt J. Med. Hum. Genet. 12 , 31–38 (2011).
Elhameed, M. A. A., Elbaky, A. & Kamel, E. A. A. A controlled study of the risk factors and clinical picture of children with autism in an Egyptian sample. Egypt J. Neurol. Neurosurg. Psychiatry 48 , 271–276 (2011).
Froehlich-Santino, W. et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J. Psychiatr. Res. 54 , 100–108 (2014).
George, B., Padmam, M. S. R., Nair, M. K. C., Leena, M. L. & Russell, P. S. S. CDC Kerala 13: Antenatal, natal and postnatal factors among children (2null6 y) with autism null: a case control study. Indian J. Pediatr. 81 , 133–137 (2014).
Juul-Dam, N., Townsend, J. & Courchesne, E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder—not otherwise specified, and the general population. Pediatrics 107 , E63 (2001).
Lord, C., Mulloy, C., Wendelboe, M. & Schopler, E. Pre- and perinatal factors in high-functioning females and males with autism. J. Autism Dev. Disord. 21 , 197–209 (1991).
Matsuishi, T. et al. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J. Autism Dev. Disord. 29 , 161–166 (1999).
Bhattarai, A. et al. Prenatal and perinatal risk factors for autism at National Children’s Hospital. J. Gandaki Med. Coll. 11 , 67–73 (2018).
Mamidala, M. P. et al. Prenatal, perinatal and neonatal risk factors of Autism Spectrum Disorder: a comprehensive epidemiological assessment from India. Res. Dev. Disabil. 34 , 3004–3013 (2013).
Mason-Brothers, A. et al. The UCLA-University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics 86 , 514–519 (1990).
Elsedfy, G. O. & Abdelraheem, T. High autism risk in children. Middle East Curr. Psychiatry 21 , 106–112 (2014).
Chien Chien, Y. L., Gau, S. S. F. & Wu, Y. Y. Prenatal and perinatal risk factors in autism spectrum disorders and their association on autistic symptoms severity. Eur. Child Adolesc. Psychiatry 22 , S262 (2013).
Ahmed, E. S., Helaly, M. & Gemeay E. M. Effect of neonatal hyperbilirubinemia on the occurrence of Autism in children. AAMJ 8 (2010).
Sugie, Y. & Sugie, H. Perinatal and neonatal risk factors for autism spectrum disorders. Seishin Shinkeigaku Zasshi 111 , 1397–1403 (2009).
Duan, G., Yao, M., Ma, Y. & Zhang, W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res 220 , 410–417 (2014).
Chen, M. H. et al. Is neonatal jaundice associated with autism spectrum disorder, attention deficit hyperactivity disorder, and other psychological development? A nationwide prospective study. Res. Autism Spectr. Disord. 8 , 625–632 (2014).
Download references
Author information
Authors and affiliations.
Perinatal Epidemiology Research Unit, Department of Pediatrics, Aarhus University Hospital, Aarhus, Denmark
Monica L. Kujabi
Department of Pediatrics, Aarhus University Hospital, Aarhus, Denmark
Jesper P. Petersen, Mette V. Pedersen & Tine B. Henriksen
Department of Public Health, Section for Biostatistics, Aarhus University, Aarhus, Denmark
Erik T. Parner
You can also search for this author in PubMed Google Scholar
Contributions
Each author has met the Pediatric Research authorship requirements listed below: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. M.L.K., J.P.P., and T.B.H. contributed substantially to conception and design, M.L.K. and M.V.P. contributed substantially to the acquisition of data. All authors contributed substantially to the analysis and interpretation of data; M.L.K. drafted the article; the remaining authors contributed in revising it critically for important intellectual content. All authors have approved the final version to be published.
Corresponding author
Correspondence to Monica L. Kujabi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary information
Supplemental material, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Kujabi, M.L., Petersen, J.P., Pedersen, M.V. et al. Neonatal jaundice and autism spectrum disorder: a systematic review and meta-analysis. Pediatr Res 90 , 934–949 (2021). https://doi.org/10.1038/s41390-020-01272-x
Download citation
Received : 13 May 2020
Revised : 07 October 2020
Accepted : 13 October 2020
Published : 01 February 2021
Issue Date : November 2021
DOI : https://doi.org/10.1038/s41390-020-01272-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Neonatal phototherapy and risk of epilepsy—a danish population based study.
- Yuelian Sun
- Julie Werenberg Dreier
- Rikke Damkjær Maimburg
European Journal of Pediatrics (2024)
The association between post-term births and autism spectrum disorders: an updated systematic review and meta-analysis
- Ensiyeh Jenabi
- Sajjad Farashi
- Hamideh Parsapoor
European Journal of Medical Research (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The burden and management of neonatal jaundice in Nigeria: A scoping review of the literature
Affiliation.
- 1 Centre for Healthy Start Initiative, 286A Corporation Drive, Dolphin Estate, Ikoyi, Lagos, Nigeria.
- PMID: 26755212
- DOI: 10.4103/1119-3077.173703
Neonatal jaundice is a leading cause of hospitalization in the first week of life worldwide. If inappropriately managed, it may result in significant bilirubin-induced mortality and disability. We set out to describe the epidemiology of neonatal hyperbilirubinemia as well as the practices and challenges in the care of infants with significant neonatal hyperbilirubinemia (SNH) in Nigeria, as basis for policy intervention and research priorities. We systematically searched PubMed, Scopus, EMBASE, Cumulative Index to Nursing and Allied Health Literature, WHO Library Database, African Index Medicus, African Journals Online, and local journals for studies published between January 1960 and December 2014. We included studies, without restriction on methodological design that provided evidence on the incidence/prevalence, etiological /risk factors and adverse outcomes of hyperbilirubinemia, care-seeking practices, diagnosis and treatment, as well as follow-up evaluation of infants with SNH in Nigeria. A total of 558 studies were identified from all sources out of which 198 (35.5%) were finally selected. SNH accounted for about one in five neonatal admissions and has been associated consistently with substantial case fatality and neuro-developmental sequelae such as cerebral palsy and auditory impairments, especially among out-born babies. Glucose-6-phosphate dehydrogenase (G6PD) deficiency, prematurity/low birth weight, infection, and ABO incompatibility were most frequently, and Rhesus disease rarely, associated with SNH. Late presentation at appropriate health facilities was common and resulted in high rates of acute bilirubin encephalopathy (ABE), kernicterus and avoidable exchange transfusions. Uniform practice guidelines, including developmental assessment and surveillance of infants with SNH, were rare at all levels of healthcare delivery. In summary, since 1960, SHN persists as a major contributor to neonatal mortality and developmental disabilities in Nigeria. The underpinning maternal, perinatal and neonatal factors as well as systems-based constraints are not insurmountable. Systematic and sustained interventions are warranted to curtail the disproportionate and perennial burden of this condition in this population.
PubMed Disclaimer
Similar articles
- Infants at risk of significant hyperbilirubinemia in poorly-resourced countries: evidence from a scoping review. Olusanya BO, Slusher TM. Olusanya BO, et al. World J Pediatr. 2015 Nov;11(4):293-9. doi: 10.1007/s12519-015-0037-z. Epub 2015 Oct 11. World J Pediatr. 2015. PMID: 26454433 Review.
- Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Johnson L, et al. J Perinatol. 2009 Feb;29 Suppl 1:S25-45. doi: 10.1038/jp.2008.211. J Perinatol. 2009. PMID: 19177057
- Heliotherapy for Neonatal Hyperbilirubinemia in Southwest, Nigeria: A Baseline Pre-Intervention Study. Emokpae AA, Mabogunje CA, Imam ZO, Olusanya BO. Emokpae AA, et al. PLoS One. 2016 Mar 22;11(3):e0151375. doi: 10.1371/journal.pone.0151375. eCollection 2016. PLoS One. 2016. PMID: 27003893 Free PMC article.
- Risk factors for severe neonatal hyperbilirubinemia in low and middle-income countries: a systematic review and meta-analysis. Olusanya BO, Osibanjo FB, Slusher TM. Olusanya BO, et al. PLoS One. 2015 Feb 12;10(2):e0117229. doi: 10.1371/journal.pone.0117229. eCollection 2015. PLoS One. 2015. PMID: 25675342 Free PMC article. Review.
- Jaundice and kernicterus in the moderately preterm infant. Wallenstein MB, Bhutani VK. Wallenstein MB, et al. Clin Perinatol. 2013 Dec;40(4):679-88. doi: 10.1016/j.clp.2013.07.007. Epub 2013 Sep 20. Clin Perinatol. 2013. PMID: 24182955 Review.
- Delivery of a post-natal neonatal jaundice education intervention improves knowledge among mothers at Jinja Regional Referral Hospital in Uganda. Alinaitwe B, Francis N, Ngabirano TD, Kato C, Nakamya P, Uwimbabazi R, Kaplan A, McCoy M, Ayebare E, Winter J. Alinaitwe B, et al. PLoS One. 2024 Apr 4;19(4):e0301512. doi: 10.1371/journal.pone.0301512. eCollection 2024. PLoS One. 2024. PMID: 38574088 Free PMC article.
- An assessment of Nigeria's health systems response to COVID-19. Okeke C, Uzochukwu B, Onyedinma C, Onwujekwe O. Okeke C, et al. Ghana Med J. 2022 Sep;56(3 Suppl):74-84. doi: 10.4314/gmj.v56i3s.9. Ghana Med J. 2022. PMID: 38322737 Free PMC article. Review.
- Bilirubin impairs neuritogenesis and synaptogenesis in NSPCs by downregulating NMDAR-CREB-BDNF signaling. Zhang Y, Li S, Li L, Huang H, Fu Z, Hua Z. Zhang Y, et al. In Vitro Cell Dev Biol Anim. 2024 Mar;60(2):161-171. doi: 10.1007/s11626-023-00844-5. Epub 2024 Jan 12. In Vitro Cell Dev Biol Anim. 2024. PMID: 38216855
- Demystifying non-invasive approaches for screening jaundice in low resource settings: a review. Abiha U, Banerjee DS, Mandal S. Abiha U, et al. Front Pediatr. 2023 Nov 20;11:1292678. doi: 10.3389/fped.2023.1292678. eCollection 2023. Front Pediatr. 2023. PMID: 38054187 Free PMC article. Review.
- Trends in Neonatal Mortality at Princess Marie Louise Children's Hospital, Accra, and the Newborn Strategic Plan: Implications for Reducing Mortality in Hospital and the Community. Tette EMA, Nartey ET, Nyarko MY, Aduful AK, Neizer ML. Tette EMA, et al. Children (Basel). 2023 Oct 29;10(11):1755. doi: 10.3390/children10111755. Children (Basel). 2023. PMID: 38002846 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Knowledge, attitudes and practices regarding neonatal jaundice among caregivers in a tertiary health facility in Ghana
Solomon mohammed salia.
1 Department of Nursing, School of Nursing and Midwifery, University of Health and Allied Sciences, Ho, Ghana
Agani Afaya
2 College of Nursing, Yonsei University, Seoul, South Korea
Abubakari Wuni
3 Nurses’ and Midwives’ Training College, Tamale, Ghana
Martin Amogre Ayanore
4 Department of Health Policy Planning and Management, School of Public Health, University of Health and Allied Sciences, Ho, Ghana
Emmanuel Salia
5 Central Laboratory, Korle-Bu Teaching Hospital, Accra, Ghana
Doreen Dzidzor Kporvi
Peter adatara, vida nyagre yakong.
6 Department of Midwifery, School of Nursing and Midwifery, University for Development Studies, Tamale, Ghana
Sean Augustine Eduah-Quansah
Shine seyram quarshie, eric kwame dey, dominic amoah akolga, robert kaba alhassan.
7 Centre for Health Policy and Implementation Research, Institute of Health Research, University of Health and Allied Sciences, Ho, Ghana
Associated Data
All relevant data are within the paper and its Supporting Information files.
Neonatal jaundice is a major reason babies are frequently re-admitted after hospital discharge following delivery. One means of improving neonatal care and reducing potential mortality associated with neonatal jaundice in resource-limited settings is to create awareness among caregivers. Caregivers who tend to have higher knowledge and awareness, also have positive attitudes, and are not guided by outmoded socio-cultural beliefs and practices are more likely to seek early care and treatment for neonatal jaundice.
This study investigated caregivers’ knowledge, attitude and practices regarding neonatal jaundice in a tertiary health facility in the Volta region of Ghana.
This was a descriptive cross-sectional study that employed a quantitative approach for data collection. A total of 202 caregivers from the Ho Teaching Hospital in the Volta region of Ghana were sampled using a systematic random sampling strategy where quantitative data was collected using a questionnaire and analyzed with STATA version 14.0. Ordered logistic regression was used to determine the factors that were associated with caregivers’ knowledge regarding neonatal jaundice and attitude after controlling for relevant covariates.
Less than half of the caregivers demonstrated good knowledge (45.5%) and attitude (47.5%) but 58.9% had good practices regarding neonatal jaundice. Caregivers who had prior awareness and education on neonatal jaundice were three times more likely to have good knowledge about jaundice than those without previous education [AOR = 3.02, (95%CI: 1.59–5.74), p = 0.001]. A caregiver employed in the public sector was two times more likely to have a good attitude about jaundice than those employed in the private sector [AOR = 2.08, (95%CI: 1.03–4.21), p = 0.042].
Less than two thirds of the caregivers demonstrated good practice with limited knowledge and poor attitude. Efforts to promote well informed and improved caregivers’ attitude will advance positive maternal health-seeking behavior and reduce disabilities and death through early detection and intervention of infants with neonatal jaundice. Public awareness and education about neonatal jaundice especially among caregivers in the private sector should also be intensified.
Introduction
Empirical evidence indicates that neonatal jaundice is a major reason babies are frequently re-admitted after hospital discharge, and it affects about 60% of term infants and 80% of preterm within the first week of life [ 1 ]. The term neonatal jaundice is used to described the yellowish coloration of the skin and other membranes of the newborn, which signifies elevated levels of unconjugated bilirubin in their blood [ 1 , 2 ]. Blackburn [ 3 ] asserts that jaundice results from an imbalance between the rate of bilirubin production and bilirubin excretion leading to increased levels of bilirubin in the blood of the newborn. The presence of jaundice on clinical examination indicates hyperbilirubinemia, which is defined as a total amount of serum bilirubin more than 1.5 mg/dL [ 4 ].
Physiological jaundice which is a form of jaundice is the elevation of unconjugated bilirubin in the blood of the newborn occurring during the third to fourth day of life, as a result of an inability of the newborn’s liver due to immaturity to convert the unconjugated bilirubin for excretion [ 5 ]. It may be benign and self-limiting [ 6 ] and may resolve by the end of the first week of life [ 7 ]. Factors responsible for the development of this jaundice includes shortened lifespan of red blood cells (70 to 90 days), high number of circulating erythrocytes, lower plasma binding capacity, and delayed passage of meconium [ 6 ]. Furthermore, pathological jaundice is the manifestation of jaundice in the newborn within the first 24 hours of life when the serum bilirubin levels rise to more than 5mg/dL and may be due to factors such as ABO and Rh incompatibilities, polycythemia, and septicemia [ 6 ].
Although neonatal jaundice affects several babies, many of them recover. In some babies, high levels of unconjugated bilirubin may result in acute and chronic bilirubin encephalopathy or kernicterus leading to irreversible brain damage [ 8 ] which may cause death [ 9 , 10 ]. If jaundice is not detected and treated early in life, it may cause major disabilities in babies such as cerebral palsy, mental retardation, and deafness [ 11 ].
Evidence from the Ghana Health Service (GHS) shows that the incidence of neonatal jaundice among newborns has been on the rise in recent years. For instance, from 2015 to 2019, Ghana recorded 3,031, 4,251, 5,338, 7,175 and 9,273 cases of neonatal jaundice respectively [ 12 ]. Also, a study by Adoba et al [ 13 ] in Ghana that focused on the knowledge and determinants of neonatal jaundice reported that the prevalence of neonatal jaundice was 66.7%. Nevertheless, neonatal jaundice remains one of the most important contributors to neonatal mortality [ 14 , 15 ] and as a result, all necessary efforts to reduce its prevalence are imperative.
Ghana’s attempt to meet the global targets of reducing neonatal mortality saw the country endorse the health components of the Sustainable Development Goals (SDGs) and the World Health Organization (WHO) targets of reducing neonatal mortality rate to 12 and 7 per 1000 live births by 2030 and 2035 respectively [ 16 – 18 ]. In resource-limited settings, one important measure of reducing neonatal mortality is to create awareness in a bid to improve the knowledge and attitudes of caregivers. This will help dispel some myths and socio-cultural beliefs and misconceptions regarding neonatal jaundice among caregivers who are essential in meeting a continuum of care for neonates after birth.
There is a plethora of data regarding caregivers’ perspectives of neonatal jaundice globally [ 8 , 19 – 25 ], however, there is a paucity of information regarding caregivers’ perspectives of neonatal jaundice in the Ghanaian setting especially, the Volta region. Several studies have reported gaps about caregivers’ knowledge and attitude of neonatal jaundice [ 5 , 8 , 19 , 20 , 25 – 27 ] in low and middle-income countries including Ghana. In the studies of [ 22 , 25 , 27 ], though some of the mothers of neonates knew the definition and how to recognize jaundice in their babies when it develops, the majority demonstrated knowledge gaps in the causes and treatment. For instance, a majority of the mothers indicated that they did not know the causes and treatment of neonatal jaundice and some mothers however prefer to use herbal treatment and exposure to sunlight as a means of treatment. Furthermore, on the aspect of attitude, the studies of [ 19 , 20 , 28 ] have all reported poor attitude with regards to treatment because mothers delay in seeking medical care when their babies develop jaundice. They will further expose babies to sunlight as means of treatment. It is worth noting that inadequate knowledge and poor practices passed on to parents from previous generations as well as perceptions and attitudes of parents toward neonatal jaundice may explain reasons for delay in seeking medical advice immediately [ 29 ] and adherence to inappropriate treatment methods.
To address the above knowledge gaps concerning neonatal jaundice, this study assessed a broad range of caregivers’ knowledge, attitudes and practices regarding neonatal jaundice in a tertiary referral facility in the Volta region of Ghana. Understanding the knowledge level, attitude and identifying the practices of caregivers are key in improving newborn survival and reducing neonatal mortality rates. The study findings will also add to the body of knowledge of existing data on neonatal jaundice in Ghana and also serve as useful information for educating expectant parents on appropriate health-seeking behaviors in a bid to reduce neonatal mortality rates in Ghana.
Materials and methods
Ethics approval and consent to participate
Ethical approval was obtained from the University of Health and Allied Sciences Research Ethics Committee (UHAS-REC) with protocol number (UHAS-REC/A.7[ 11 ]18–19). Administrative approval was also obtained from the management of Ho Teaching Hospital and the nurse in-charge of the postnatal unit. A voluntary written informed consent was obtained from the caregivers which included a well explained description and objectives of the study. Caregivers were informed that they had the right to voluntarily withdraw from the study without any penalty. They were also informed and assured of confidentiality and anonymity during the study by protecting their information and identity. Caregivers were informed that they will not receive direct material benefits for participation, however, the findings of this study will be published for wider reading and will also help to improve the caring practices of newborns among caregivers and healthcare providers regarding the prevention of neonatal jaundice. They were however informed that their participation in this study will have no risk or discomfort. The caregivers were informed that data collection will be done on an individual basis and that privacy will be provided at all times during the process of data collection.
The study was conducted in the Ho Teaching Hospital, Volta region of Ghana. The Ho Teaching Hospital is located in the Volta regional capital (Ho), and the sole tertiary and referral facility in the region. The referral facility is a 313-bed capacity. The hospital has a neonatal intensive care unit (NICU) that provides specialized care to sick babies. The NICU has an admission capacity of 30 beds, six (6) functional incubators and two (2) phototherapy machines. In 2019, the total number of deliveries for the hospital stood at spontaneous vagina deliveries (1006) and caesarian section (803). The NICU recorded 411 admissions in 2019 with a neonatal mortality rate of 11.7% (42 deaths). It also has two pediatricians, three medical officers, 20 professional and four nonprofessional nurses. The postnatal unit operates under the Obstetrics and Gynecology department from Monday to Friday each week. The unit has one doctor and two midwives. Care provided in the unit include general head to toe examination of postnatal clients, neonates, removal of caesarian section stitches and wound care. Additionally, the midwives also educate women on exclusive breastfeeding and perineal hygiene as well as cord care. The hospital provides both general and specialized medical and surgical services including obstetric and gynecological, Ear Nose and Throat, Eyecare among others. Data collection took place at the postnatal unit of the hospital which offers services to parents and their babies after delivery.
Study design
This was a hospital-based descriptive cross-sectional study that employed a quantitative approach to investigate caregiver’s knowledge, attitude and practices regarding neonatal jaundice. This design was adopted because it is good at collecting information that describes a phenomenon as it exists.
Sample size and sampling determination
The sample size was determined using Yamane [ 30 ] formula for sample calculation based on a known population and a total sample of 202 caregivers with babies was determined. The study employed a systematic random sampling strategy for data collection. This method was chosen to ensure that all the caregivers who formed part of the sampling frame had equal chances of being selected to be part of the study. Nurses and midwives gave the caregivers numbers based on the time of arrival at the postnatal unit. The first person was given the number 1 in that order until the last person. Therefore, based on these numbers, the caregivers were arranged to sit in rows. Their names were then documented into a book which served as the register of attendance. In this study, the sampling interval for systematic sampling was determined by dividing the sample size (202) by the total target population of interest (410). The sampling interval was 2. Therefore, every second caregiver in the row was chosen to participate in the study. Balloting was done to select the first two caregivers to determine the starting point.
Study population
The study population were caregivers (father or mother) of the babies who sought care at the postnatal unit of the referral hospital.
Inclusion/Exclusion criteria
Caregivers aged 18 years and above who sought postnatal services at Ho Teaching Hospital with their babies and consented to participate were included in the study. Those who were present during the period of the study but did not offer voluntary consent were excluded from the study. The study also excluded other caregivers who were not the parents of the babies but brought the babies for postnatal care. Caregivers were recruited for the study for a period of two months from March to April 2019.
Study variables
The main variables in this study were the Independent and Dependent variables. The independent variables of interest were classified as follows; Age (18–25, 26–35, 36–45 and >45); Sex (Male and Female); Religion (Christian, Muslim and Traditionalist); Marital status (Single, Married, Divorced and Widowed); Ethnicity (Ewe, Akan, Ga and Others); Educational level (None, Basic, Secondary and Tertiary); Residence (Rural, Urban and Estate); Number of previous children (None, One, Two and more than two); Employment status (Self/Private, Public Servant, Unemployed/Student and Unknown); Previous education on neonatal jaundice (Yes or No); Child previously diagnosed with neonatal jaundice (Yes or No); Number of Antenatal Care (ANC) (less than 4 visits and more than 4 visits). Although the WHO has changed the minimum recommended antenatal visits from 4 to 8 [ 31 ], in this study, we studied an attendance of 4 visits [ 32 ].
The dependent variables of interest for the ordered logistic regression were the composite variables for the overall level of knowledge, attitude and practice. The overall level of knowledge was computed using 28 items grouped to (definition, causes, complications, danger signs, sites for checking, treatment and prevention of neonatal jaundice) that measured knowledge, while nine items each were used to compute for the attitude and practices. These are captured in more detail in the results section.
Data collection instrument
A structured questionnaire was used to collect information regarding caregivers’ knowledge, attitude and practice on neonatal jaundice. The questionnaire designed was informed by the objectives of the study and after a careful review of literature relevant to the study objectives. The instrument was organized into four parts; part one collected information on caregivers’ sociodemographic characteristics such age, marital status, educational background, ethnicity, number of children, number of Antenatal Care (ANC) attendance etc. The second part consisted of 28 items that asked knowledge questions in areas of definition, causes, clinical manifestations, danger signs, treatment, complications and prevention. In part three, nine items gathered information on attitude while part four comprised of questions relating to beliefs and practices of neonatal jaundice with 8 items. The questionnaire was ranked on a five-point Likert scale with appropriate descriptions as: 1 = “Strongly disagree”, 2 = “Disagree”, 3 = “Undecided”, 4 = “Agree” and 5 = “Strongly disagree”. Questionnaires were serially numbered to allow for easy identification and accuracy of input into data entry sheet for easy analysis.
Data collection procedure
Data was collected from 1 st March, 2019 to 30 th April, 2019 at the postnatal unit of the Ho Teaching Hospital. The objectives of the study were explained to the caregivers and voluntarily informed consent was obtained. Items in the questionnaire were explained to the understanding of the caregivers before they were administered. Caregivers were approached at the postnatal unit on each day of data collection and data collection was done on an individual basis in a private restroom in the unit to ensure privacy. Respondents who were proficient in speaking and writing in the English language answered the questionnaires by themselves. Those who could neither read nor write in the English language were guided to complete the questionnaire. Four of the authors were native speakers of the “Ewe” language and three authors who could speak “Ewe”, “Twi” and other languages were used to orally translate the questionnaire into these local languages for the understanding of the caregivers.
Clarity was however given to those who wanted further explanation about the questions. Each day after data gathering, the filled questionnaires were kept in a sealed A4 brown envelope and kept in a safe place from the reach of others to maintain anonymity. All questionnaires were crossed checked for completeness before leaving the data collection site each day.
Validity and reliability
The questionnaire was peer-reviewed by all authors. Also, two midwives specialized in neonatal nursing and three pediatricians reviewed the questionnaire for content validity and appropriateness of questions. Further validation was done through piloting as well as pretesting which took place in a government hospital in the Ho municipality. The instrument was tested for scale reliability which yielded the following scale reliability coefficients; knowledge (0.78), attitude (0.51) and beliefs and practices (0.84). The reliability coefficient (r) for knowledge (0.78) was measured as adequate, attitude (0.51) was below the required coefficient value of 0.7, and beliefs and practices (0.84) showed a good coefficient value. The closer the reliability coefficient is to 1.0, the greater the internal consistency of the items in the scale. The items in the study instrument showed a good internal consistency except for attitude which showed poor internal consistency. George and Mallery [ 33 ] provide a rule of thumb that when the reliability coefficient is below 0.5 it is unacceptable. However, the attitude subscale was not below 0.5 so it was not excluded from the instrument.
Data analysis
For data quality checks, the field data were first entered into Microsoft Excel and cleaned before exporting to STATA version 14.0 for analysis. Descriptive statistics was used for the demographic characteristics and categorical variables using simple frequencies and percentages.
Overall level of knowledge, attitude and practice were measured by scoring responses that measured caregivers’ knowledge, attitude and practices. These were classified as good, moderate and poor. A score of ‘1’ was assigned if respondents had a correct response in knowledge, attitude and practice and ‘0’ if respondents had a wrong response in knowledge, attitude and practice. Total scores were computed and a cumulative score of 80% and above was considered to be good, 60–80% was considered as moderate and below 60% was considered to be poor. The level of knowledge, attitude and practice were coded 0 for poor, 1 for moderate and 2 for good.
Ordered logistic regression analysis was used to determine the association between dependent (knowledge, attitude and practice) and independent variables (socio-demographic characteristics) and statistical significance was considered based on the p-value <0.05 at a confidence level of 95%. All explanatory variables of interest were tested for multicollinearity and none had a Variance Inflation Factor (VIF) above 10 necessary for exclusion from the regression model.
Socio-demographic characteristics of caregivers
Of the 202 caregivers who participated in the study, a majority of them were aged 26–35 years (56.9%) and 64.9% were married, 31% had two children. Most of them (45.0%) had tertiary education and 35.7% were engaged in self or private employment. A majority, 71.8% attended ANC visit at least four times. Socio-demographic characteristics are presented in Table 1 .
| Variable | Frequency (f) | Percent (%) |
|---|---|---|
| 18–25 | 45 | 22.3 |
| 26–35 | 115 | 56.9 |
| 36–45 | 34 | 16.8 |
| >45 | 8 | 4.0 |
| Female | 187 | 92.6 |
| Male | 15 | 7.4 |
| Christian | 173 | 85.6 |
| Muslim | 22 | 10.9 |
| Traditional | 7 | 3.5 |
| Single | 53 | 26.2 |
| Married | 131 | 64.9 |
| Divorced | 13 | 6.4 |
| Widowed | 5 | 2.5 |
| None | 5 | 2.5 |
| Basic | 37 | 18.3 |
| Secondary | 69 | 34.2 |
| Tertiary | 91 | 45.0 |
| Self/Private | 72 | 35.7 |
| Public Servant | 56 | 27.7 |
| Unemployed/Student | 14 | 6.9 |
| Unknown | 60 | 29.7 |
| Ewe | 134 | 66.3 |
| Akan | 41 | 20.3 |
| Ga | 14 | 6.9 |
| Others | 13 | 6.5 |
| None | 36 | 17.8 |
| One | 60 | 29.7 |
| Two | 63 | 31.2 |
| More than two | 43 | 21.3 |
| <4 visits | 57 | 28.2 |
| 4+ visits | 145 | 71.8 |
| Urban | 115 | 56.9 |
| Rural | 72 | 35.7 |
| Estate | 15 | 7.4 |
| No | 116 | 58 |
| Yes | 84 | 42 |
| No | 173 | 86.5 |
| Yes | 27 | 13.5 |
Summary of knowledge, attitude and practice scores of caregivers on neonatal jaundice
A summary of knowledge, attitude and practice scores of caregivers is presented in Fig 1 . The results revealed that a little above 50% of the caregivers (54.5%) and (52.0%) had poor knowledge and attitude regarding neonatal jaundice. However, a majority (58.9%) recorded good practice scores towards neonatal jaundice.

Caregivers’ knowledge on neonatal jaundice
The majority, (89.6%) correctly defined jaundice as the yellowish discoloration of the skin and eyes. About 53% identified prematurity as a cause of neonatal jaundice while 77.7% identified the death of a baby as a complication of jaundice. The majority (91.6%) correctly identified that skin and eyes are the commonest sites for checking jaundice. On the aspect of treatment, less than 50% correctly identified traditional methods as an inappropriate choice of treatment for jaundice (32.7%). Table 2 shows caregivers’ knowledge in neonatal jaundice.
| Variable | Correct responses | |
|---|---|---|
| Frequency (f) | Percent (%) | |
| Jaundice is the yellowish discoloration of the skin and eyes | 181 | 89.6 |
| Disparity between blood groups can cause jaundice | 70 | 34.7 |
| Prematurity of the baby can cause jaundice | 107 | 53.0 |
| Infection is a cause of jaundice | 103 | 51.0 |
| Feeding the baby with breastmilk can cause jaundice | 39 | 19.3 |
| Jaundice can be caused by giving your baby cold water | 94 | 46.5 |
| Delay passage of meconium can cause jaundice | 59 | 29.2 |
| Jaundice can bring about brain damage in the baby | 83 | 41.1 |
| Jaundice can render a child physically handicapped | 92 | 45.5 |
| A baby with jaundice can develop convulsions | 112 | 55.5 |
| A baby diagnosed with jaundice can die | 157 | 77.7 |
| A jaundiced baby feeds very poorly | 131 | 64.9 |
| Arching of the back is a danger sign of jaundice | 64 | 31.7 |
| Convulsion is a danger sign in a baby with jaundice | 101 | 50.0 |
| Refusal to eat is also a danger sign in a baby with jaundice | 99 | 49.0 |
| High pitch cry is a danger sign of jaundice | 65 | 32.2 |
| Down turning of the eye is a danger sign of jaundice in a baby | 80 | 39.6 |
| The skin and eyes are sites for checking jaundice | 185 | 91.6 |
| The palms are also sites used to check jaundice | 168 | 83.2 |
| The urine of the baby is used to check for jaundice in a baby | 20 | 9.9 |
| Feaces of the child can be used to determine if the baby has jaundice or not | 38 | 18.8 |
| Phototherapy is one method used to treat neonatal jaundice | 92 | 45.5 |
| Exchange blood transfusion is also a method of treating neonatal jaundice | 78 | 38.6 |
| Going to church with frequent fasting and prayers are ways of treating neonatal jaundice | 111 | 55.0 |
| Traditional methods are also used to treat neonatal jaundice | 66 | 32.7 |
| 32 | 15.8 | |
| Neonatal Jaundice can be prevented | 171 | 84.7 |
| Early initiation of breast milk can prevent neonatal jaundice | 125 | 61.9 |
Multiple response system was allowed
Attitude of caregivers towards neonatal jaundice
The majority of the caregivers 86.1% indicated that jaundice is treated in the hospital in early life, 81.2% correctly indicated that frequent ANC attendance will prevent jaundice in newborns. Furthermore, only 21.8% and 30.2% of the caregivers correctly identified the use of herbal medications and exposing a baby to sunlight as inappropriate modes of treatment of neonatal jaundice. Caregivers attitude towards neonatal jaundice is presented in Table 3 .
| Variable | Correct responses | |
|---|---|---|
| Frequency (f) | Percent (%) | |
| If I attend ANC frequently, I will receive education on the prevention and recognition of jaundice to help me prevent the condition | 164 | 81.2 |
| Jaundice in early life can be treated in the hospital | 174 | 86.1 |
| Herbal medications are used to treat jaundice | 44 | 21.8 |
| Breastfeeding is a means of treating my baby’s jaundice | 32 | 15.8 |
| Expose of baby to early morning sunlight is a way of treating jaundice. | 61 | 30.2 |
| Jaundice in early life is caused by evil spirits | 144 | 71.3 |
| Poor personal and environmental hygiene causes jaundice | 75 | 37.1 |
| Exclusive breastfeeding prevents jaundice | 119 | 58.9 |
| Going to church frequently and engage in prayer and fasting are means of preventing jaundice | 120 | 59.4 |
Caregivers’ beliefs and practices of neonatal jaundice
A majority 84.7%, believe that jaundice is not a curse from the gods while 83.2% engaged in good practices by not putting their jaundiced babies in a dark room for at least 7 days. Also, 89.6% believe the yellowish color of their babies does not signify that their babies are growing well. Caregivers’ beliefs and practices are shown in Table 4 .
| Variable | Correct response | |
|---|---|---|
| Frequency (f) | Percent (%) | |
| I believe jaundice is a curse from the gods | 171 | 84.7 |
| I will drop breast milk on my baby’s eyes as a means of managing jaundice | 140 | 69.3 |
| I will not feed my baby with first breast milk as means to prevents jaundice | 158 | 78.2 |
| I drop seawater on my baby’s eyes to help cure jaundice | 163 | 80.7 |
| I keep my baby away from light to help prevent jaundice | 143 | 70.8 |
| I cut the areas between my baby’s eyebrow to help prevent jaundice | 147 | 72.8 |
| I put my jaundiced baby in the darkroom for at least 7 days | 168 | 83.2 |
| I believe jaundice makes a baby’s skin look more beautiful | 176 | 87.1 |
| I believe the yellowish discoloration of the baby’s skin is a good sign that the baby is growing healthy and beautiful | 181 | 89.6 |
Factors associated with knowledge of neonatal jaundice among caregivers
A multivariate ordered logistic regression analysis revealed that caregivers’ who had prior awareness and education on neonatal jaundice were three times likely to exhibit a good knowledge on neonatal jaundice compared to caregivers without prior awareness and education on neonatal jaundice at a p = 0.001 [AOR = 3.02, (95%CI: 1.59–5.74), p = 0.001]. The factors associated with caregivers’ knowledge is shown in Table 5 .
| Variable | COR | 95%CI | p-value | AOR | 95%CI | p-value |
|---|---|---|---|---|---|---|
| 18–25 | ||||||
| 26–35 | 1.04 | 0.52–2.06 | 0.907 | |||
| 36–45 | 1.10 | 0.45–2.68 | 0.835 | |||
| >45 | 1.04 | 0.25–4.30 | 0.955 | |||
| Female | ||||||
| Male | 3.29 | 1.21–8.91 | 2.64 | 0.90–7.66 | 0.074 | |
| Christian | ||||||
| Muslim | 0.37 | 0.14–0.98 | 0.45 | 0.56–1.31 | 0.143 | |
| Traditional | 0.39 | 0.07–2.03 | 0.267 | 0.28 | 0.05–1.68 | 0.164 |
| Single | ||||||
| Married | 098 | 0.53–1.85 | 0.966 | |||
| Divorced | 0.31 | 0.08–1.24 | 0.097 | |||
| Widowed | 0.26 | 0.03–2.45 | 0.239 | |||
| None | ||||||
| Basic | 0.27 | 0.05–1.54 | 0.139 | |||
| Secondary | 0.50 | 0.09–2.62 | 0.411 | |||
| Tertiary | 1.40 | 0.27–7.18 | 0.688 | |||
| Self/Private | ||||||
| Public Servant | 2.46 | 1.23–4.92 | 0.011 | 1.83 | 0.88–4.07 | 0.102 |
| Unemployed/Student | 4.82 | 1.51–15.32 | 0.008 | 3.19 | 0.92–11.02 | 0.067 |
| Ewe | ||||||
| Akan | 1.65 | 0.85–3.21 | 0.137 | |||
| Ga | 1.20 | 0.42–3.45 | 0.729 | |||
| Others | 0.37 | 0.10–1.37 | 0.136 | |||
| None | ||||||
| One | 0.40 | 0.17–0.89 | 0.025 | 0.56 | 0.23–1.35 | 0.197 |
| Two | 0.54 | 0.24–1.21 | 0.134 | 0.87 | 0.35–2.09 | 0.751 |
| More than two | 0.45 | 0.19–1.07 | 0.070 | 0.96 | 0.34–2.66 | 0.935 |
| <4 visits | ||||||
| 4+ visits | 0.60 | 0.33–1.09 | 0.092 | |||
| Urban | ||||||
| Rural | 0.47 | 0.26–0.85 | 0.79 | 0.40–1.54 | 0.486 | |
| Estate | 1.04 | 0.39–2.77 | 0.935 | 0.62 | 0.22–1.83 | 0.396 |
| No | ||||||
| Yes | 2.93 | 1.67–5.13 | 3.02 | 1.59–5.74 | ||
| No | ||||||
| Yes | 1.61 | 0.76–0.55 | 0.211 |
Ordered logistic regression significant, p < 0.05
Odds ratios (OR) were all adjusted for possible confounding covariates
Factors associated with caregivers’ attitude towards neonatal jaundice
In a multivariate logistic analysis, the odds of a having a good attitude regarding neonatal jaundice was two times more likely to occur among public service employers compared to those who were self/privately employed [AOR = 2.08, (95%CI: 1.03–4.21), p = 0.042]. Furthermore, caregivers who had one child were 58% less likely to demonstrate positive attitudes regarding neonatal jaundice [AOR = 0.42, (95%CI: 0.18–0.99), p = 0.049]. Factors associated with caregivers’ attitude is shown in Table 6 .
| Variable | COR | 95%CI | p-value | AOR | 95%CI | p-value |
|---|---|---|---|---|---|---|
| 18–25 | ||||||
| 26–35 | 1.37 | 0.70–2.70 | 0.356 | |||
| 36–45 | 1.51 | 0.63–3.62 | 0.553 | |||
| >45 | 1.17 | 0.28–4.79 | 0.830 | |||
| Female | ||||||
| Male | 0.70 | 0.24–1.98 | 0.497 | |||
| Christian | ||||||
| Muslim | 0.58 | 0.23–1.44 | 0.241 | |||
| Traditional | 0.16 | 0.02–1.33 | 0.090 | |||
| Single | ||||||
| Married | 0.85 | 0.46–1.56 | 0.615 | |||
| Divorced | 0.38 | 0.11–1.33 | 0.130 | |||
| Widowed | 0.54 | 0.09–2.67 | 0.504 | |||
| None | ||||||
| Basic | 1.04 | 0.16–6.60 | 0.964 | |||
| Secondary | 1.65 | 0.27–9.97 | 0.581 | |||
| Tertiary | 1.77 | 0.30–10.38 | 0.528 | |||
| Self/Private | ||||||
| Public Servant | 2.17 | 1.09–4.31 | 2.08 | 1.03–4.21 | ||
| Unemployed/Student | 2.98 | 1.05–8.42 | 2.37 | 0.76–7.35 | 0.535 | |
| Ewe | ||||||
| Akan | 0.71 | 0.36–1.41 | 0.329 | |||
| Ga | 0.68 | 0.23–2.02 | 0.491 | |||
| Others | 0.38 | 0.11–1.28 | 0.119 | |||
| None | ||||||
| One | 0.34 | 0.15–0.76 | 0.42 | 0.18–0.99 | ||
| Two | 0.60 | 0.28–1.32 | 0.206 | 0.77 | 0. 33–1.77 | 0.535 |
| More than two | 0.51 | 0.22–1.18 | 0.116 | 0.67 | 0.27–1.64 | 0.383 |
| <4 visits | ||||||
| 4+ visits | 0.90 | 0.50–1.62 | 0.728 | |||
| Urban | ||||||
| Rural | 0.90 | 0.51–1.59 | 0.712 | |||
| Estate | 1.13 | 0.38–3.31 | 0.829 | |||
| No | 1.28 | 0.74–2.18 | 0.386 | |||
| Yes | ||||||
| No | ||||||
| Yes | 0.82 | 0.38–1.82 | 0.635 |
Factors associated with caregivers’ beliefs and practices of neonatal jaundice
Multivariate logistic regression analysis revealed that rural resident caregivers were 48% less likely to have good practices compared to urban residents [COR = 0.52 (95%CI: 0.29–0.93), p = 0.026]. Factors associated with caregivers’ beliefs and practices are presented in Table 7 .
| Variable | COR | 95%CI | p-value |
|---|---|---|---|
| 18–25 | |||
| 26–35 | 1.40 | 0.70–2.79 | 0.341 |
| 36–45 | 1.19 | 0.51–2.80 | 0.692 |
| >45 | 1.34 | 0.29–6.20 | 0.705 |
| Female | |||
| Male | 2.19 | 0.69–6.96 | 0.184 |
| Christian | |||
| Muslim | 1.30 | 0.54–3.16 | 0.561 |
| Traditional | 0.30 | 0.06–1.43 | 0.133 |
| Single | |||
| Married | 1.39 | 0.75–2.59 | 0.298 |
| Divorced | 0.78 | 0.23–2.59 | 0.682 |
| Widowed | 0.90 | 0.13–6.06 | 0.917 |
| None | |||
| Basic | 0.29 | 0.03–2.81 | 0.283 |
| Secondary | 0.31 | 0.03–2.99 | 0.314 |
| Tertiary | 0.58 | 0.06–5.50 | 0.636 |
| Self/Private | |||
| Public Servant | 1.56 | 0.79–3.11 | 0.201 |
| Unemployed/Student | 1.57 | 0.49–5.03 | 0.445 |
| Ewe | |||
| Akan | 1.08 | 0.53–2.20 | 0.836 |
| Ga | 1.04 | 0.33–3.25 | 0.956 |
| Others | 0.62 | 0.24–1.60 | 0.325 |
| None | |||
| One | 0.62 | 0.27–1.40 | 0.251 |
| Two | 1.04 | 0.45–2.39 | 0.926 |
| More than two | 0.76 | 0.54–1.84 | 0.536 |
| <4 visits | |||
| 4+ visits | 0.85 | 0.46–1.58 | 0.608 |
| Urban | |||
| Rural | 0.52 | 0.29–0.93 | |
| Estate | 0.45 | 0.15–1.34 | 0.151 |
| No | |||
| Yes | 1.30 | 0.74–2.26 | 0.356 |
| No | |||
| Yes | 1.42 | 0.61–3.28 | 0.414 |
The current study was undertaken to investigate knowledge, attitude and practice among caregivers’ regarding neonatal jaundice. As a matter of importance, the reduction in neonatal mortality through the achievement of the health component of Sustainable Development Goals (SDGs) is imperative and timely, and one means of achieving this is through the creation of awareness, improving the knowledge, attitude and practices among caregivers. Improvement in maternal knowledge and early care-seeking behavior serve as fundamental components of effective management of neonatal jaundice and an implication for reducing neonatal mortality [ 11 ].
The current study reported that most caregivers have a poor knowledge regarding neonatal jaundice. Only 8.9% of the caregivers had good knowledge about neonatal jaundice. Comparing our findings to other results in some LMICs, it was realized that our 8.9% good knowledge score was lower [ 15 , 24 , 27 , 34 ]. However, despite an overall poor knowledge regarding neonatal jaundice in the current study, the majority of the caregivers demonstrated good knowledge regarding the definition and sites for checking for jaundice. These results largely corroborate similar findings in Ghana and other LMICs in which the majority of the caregivers correctly defined and stated the sites to identify neonatal jaundice [ 20 , 25 , 27 , 35 , 36 ]. The implication of good knowledge in the identification of jaundice is that caregivers will detect jaundice immediately after birth and take appropriate steps towards seeking prompt treatment in the hospital. Regarding the cause of neonatal jaundice, most caregivers in our study identified correctly prematurity and infection as the main causes which reflects a good knowledge. Contrary to this, several studies [ 8 , 24 , 25 , 27 ] have reported significant knowledge gaps where 73%, 63%, 57.1% and 73.1% of mothers respectively indicated they did not know the cause of neonatal jaundice. The study of Amegan-Aho et al [ 25 ] in Ghana further reported that about 24.1% and 3.8% of mothers wrongly identified “consumption of too much oil” and mosquito bites as causes of neonatal jaundice despite more than 90% of the mother receiving ANC services. Mothers’ inability to identify the right causes of jaundice could be attributed to the low level of tertiary education as observed in their study. Nonetheless, the majority (76.0%) of mothers in their study demonstrated overall good awareness of neonatal jaundice than the current study. Importantly, a good knowledge in identifying the cause of jaundice is an indication that mothers will likely put in measures to prevent it and seek prompt treatment. Knowledge on the treatment of jaundice was low in our study. Despite the high literacy level of caregivers in this study, most of the caregivers reported going to church and engaging in fasting and prayers as means of treating neonatal jaundice. However, caregivers also correctly identified phototherapy and exchange blood transfusion as the main treatment modalities of jaundice. Though in the minority, the identification of phototherapy and exchange blood transfusion signified good knowledge, and this corroborates several other studies in LMICs [ 21 , 22 , 24 , 37 ]. Having poor knowledge of identifying the cause of neonatal jaundice is a recipe for poor decision-making on the choice of treatment and a risk of not identifying avoidable factors that may lead to jaundice, and this was largely demonstrated in our study. Health education should emphasize phototherapy as the main treatment plan and the need to seek early care at the hospital when neonatal jaundice is detected. Furthermore, on the aspect of caregivers’ knowledge regarding the complications of jaundice, the majority (77.7%) mentioned the death of the baby. The current figure is higher than the 57.8%, 55.1% and 71% reported in Nigeria and Malaysia respectively [ 26 , 28 , 38 ].
In a multivariate analysis, caregivers with prior awareness and education on neonatal jaundice were three times more likely to have good knowledge of jaundice than those who have never received education on jaundice. The study of Huq et al corroborates the current finding [ 28 ]. Though our study did not find an association between knowledge and caregivers’ educational status, however, the majority had secondary and tertiary education which suggested that their knowledge on neonatal jaundice will be high. Shockingly, most of the caregivers demonstrated an overall poor knowledge regarding neonatal jaundice. This may be due to inadequate information given regarding jaundice which may be a recipe for the poor knowledge observed or it may as well be as a result of poor health-seeking behavior on the part of the caregivers. The poor knowledge could also be due to low health literacy even in the midst of a high level of education. Collaborated effort is needed from healthcare professionals to impart knowledge on neonatal jaundice among caregivers during ANC visits using recommended guidelines as provided by the Ministry of Health GHS.
Findings from the current study revealed that most of the caregivers demonstrated a poor attitude towards neonatal jaundice. Despite the poor attitude, some of the caregivers correctly responded that they will not expose their babies to sunlight and will not use herbal medications to treat jaundice. Though these were positive responses, the majority will indulge in negative practices which signifies poor attitude. The practice of exposing jaundiced babies to sunlight is however common in Africa and other developing countries which explains the reason for its practice in our study [ 19 , 20 , 26 , 28 , 38 , 39 ]. The majority of caregivers in our study will visit ANC frequently to prevent jaundice and most will send a jaundice baby to the hospital for treatment which is an indication of a good attitude. Other studies [ 20 , 34 , 40 , 41 ] corroborates this finding in other resource-limited settings. A positive attitude implies that it will lead to early detection and correct diagnosis as well as prompt treatment with phototherapy or exchange blood transfusion with the ultimate aim of reducing neonatal mortality. The negative attitudes of exposing babies to the sunlight and the use of herbal preparation need to be discouraged through appropriate education during ANC as these are inimical to the survival of the babies and may lead to delays in early care-seeking at the hospital which may result in complications and even death.
Beliefs and practices regarding neonatal jaundice among the caregivers were good in this study. On the aspect of the belief questions, the majority of the caregivers answered positively. However, despite the positive responses regarding their practices, caregivers also responded negatively. For instance, about 30.3% of caregivers indicated that they will put breast milk in the baby’s eyes as a means of managing jaundice, 29.2% will keep babies away from light to help prevent jaundice while 27.2% will cut the areas between the baby’s eyebrow to help prevent jaundice.
Multivariate logistic regression analysis revealed that those who resided in rural areas were less likely to have good practices of neonatal jaundice compared to urban residents. This could probably be possible due to the availability of adequate health facilities and health care professionals in the urban areas who offer frequent education to caregivers at health facilities thereby improving their attitudes. Also, the majority of the caregivers resided in urban areas.
Limitation of the study
First of all, the study was conducted in a single health facility making the findings not generalizable. Secondly, the study focused only on real parents of the babies and did not include significant others such as grandmothers, sisters or aunts etc. Also, the study excluded adolescent mothers/fathers who could have provided more revealing information about neonatal jaundice. Despite the above limitations, the study provided an insight into the caregivers’ perspectives regarding jaundice and adds to the body of knowledge that will help reform newborn care practices in Ghana.
More than 50% of the caregivers demonstrated overall poor knowledge and attitude regarding neonatal jaundice while about 58.9% had good practices. That notwithstanding, some caregivers demonstrated good knowledge regarding neonatal jaundice including but not limited to defining jaundice as the yellowish coloration of eyes and skin, identifying prematurity as a cause of jaundice, and indicating that jaundice in the newborn can be prevented. Contrary to the good knowledge, some indicated that going to church and engaging in fasting and prayers were means of treating jaundice which depict poor knowledge. Despite the overall good belief and practice score seen this study, caregivers will put breast milk in the baby’s eyes to treat jaundice while others will cut the areas between the baby’s eyebrow to help prevent jaundice and a few of them indicating correctly that traditional methods are not used to treat neonatal jaundice.
Caregivers with prior awareness and education of neonatal jaundice were more likely to demonstrate good knowledge of neonatal jaundice. Also, caregivers employed in the public sector were more likely to have a good attitude towards neonatal jaundice while those who resided in rural were less likely to have good practices.
In the light of the nation’s dire need to reduce neonatal mortality rates which is in line with the health aspects of the United Nations SDGs, creating awareness, improving knowledge and attitudes and dispelling misconceptions about neonatal jaundice is timely. Therefore, concerted effort is required from frontline healthcare professionals to collaboratively intensify the education on jaundice in the areas of early identification of cases, causes, treatment, danger signs and prevention.
Supporting information
Acknowledgments.
The authors express their profound gratitude to the caregivers for allowing time to take part in this study. Further gratitude goes to the management of the Ho Teaching Hospital for permitting us to conduct the study in the hospital.
Abbreviation
| ANC | Antenatal Care |
| REC | Research Ethics Committee |
| SDGs | Sustainable Development Goals |
| UHAS | University of Health Allied Science |
| VIF | Variance Inflation Factor |
| LMICs | Low- and Middle-Income Countries |
| GHS | Ghana Health Service |
Funding Statement
The author(s) received no specific funding for this work.
Data Availability
- PLoS One. 2021; 16(6): e0251846.
Decision Letter 0
27 Nov 2020
PONE-D-20-19725
Determinants of Neonatal Jaundice Knowledge, Attitudes and Practice Among Caregivers in a Tertiary Health Facility in Ghana: Implications for Neonatal Care policy Reforms and Reducing Neonatal Mortality in a Low Resource Setting.
Dear Dr. Salia,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
I would like to sincerely apologise for the delay you have incurred with your submission. It has been exceptionally difficult to secure reviewers to evaluate your study. We have now received two completed reviews; their comments are available below.
Please revise the manuscript to address all the reviewer's comments in a point-by-point response in order to ensure it is meeting the journal's publication criteria. Please note that the revised manuscript will need to undergo further review, we thus cannot at this point anticipate the outcome of the evaluation process.
Please submit your revised manuscript by Jan 11 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
- A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
- A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
- An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols
We look forward to receiving your revised manuscript.
Kind regards,
Miquel Vall-llosera Camps
Senior Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. Please include a separate caption for each figure in your manuscript.
[Note: HTML markup is below. Please do not edit.]
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Partly
Reviewer #2: Yes
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: Yes
Reviewer #2: I Don't Know
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: No
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: Determinants of Neonatal Jaundice Knowledge, Attitudes and Practice among Caregivers in Ghana
Introduction and Literature Review:
Neonatal Jaundice is an important topic in the West African sub-region, the significance of jaundice being emphasized by the complications that attend uncontrolled rise in unconjugated hyperbilirubinaemia . Jaundice may progress to acute bilirubin encephalopathy with hearing loss and cerebral palsy or death besides lingering kernicterus spectrum disorder. The author needs to elaborate this outcome of jaundice because it is ruinous to families that become unfortunate to experience the disorder.
Jaundice occurs in physiological or pathological forms. While the former is harmless the latter is deleterious. What is concerning is the pathological jaundice which may lead to neonatal mortality. Literature review however scanty it may be needs to cover the entire understanding of the subject matter as the KAP of parents may be influenced by this knowledge.
Study Population:
The study focused on the real fathers/mothers of the neonates excluding other significant patient relatives. In the study location of West Africa, a good number of caregivers are neither the real father/mother of the neonate. Very often the grandmother, aunt, sister or other significant relative play an important, sometimes dominant role in neonatal care. Thus excluding them from a study of this nature could mean a major information loss and interventions targeted at real mothers/fathers may fall short of success without them. In the author’s area of practice the grandmothers often overrule their daughters in child care practices irrespective of the daughter’s knowledge or educational background.
Inclusion/Exclusion Criteria:
The author excluded adolescent mothers/fathers from the study. In West Africa, teenage pregnancy and delivery is common. These mothers are inexperienced and therefore more likely to be ignorant of good child care practices. Studies of under-five mortality show that infants born to these teenagers succumb by far more than the experience mothers of older age group. This study would have been more revealing and perhaps more beneficial if adolescent mothers (12-18 years) were included in the study.
Study Design:
The study design was entirely quantitative but the issues were more dominantly qualitative. A study which set out to evaluate the KAP of respondents should have given them the opportunity to express themselves freely in order to optimize that information that the researcher may not have thought about. This is a major concern in this study. How can we be sure that the investigators captured the reflections of the respondents’ true inclination?
Data Collection procedure:
The sampling of study participants was by convenient method. How could this methodology have navigated around bias? The questionnaire was administered to two groups differently, to one group by self-administration while to the second group it was by interviewer administration. A number of the respondents had no formal education requiring interpretation of the questionnaire. How exactly was this bottle neck sorted out? Was translation into local languages carried out? Please specify the details.
Assessment of Knowledge of mothers/caregivers on NJ:
It is eminently observable that death as an outcome of untreated NJ was not evaluated in this study, neither as a complication nor as danger sign. Could knowledge of this adverse outcome influence respondents’ attitude and change their health seeking behavior? If this was evaluated, it would be important to analyze the data to enrich the present study.
Treatment of NJ:
Several treatment modalities were indicated by the authors in evaluating the KAP of the respondents. All modes of treatment were investigated individually. Were there respondents who practiced a combination of more than one mode of treatment of NJ in the study? Fifty-five respondents volunteered that they sought help by visiting “church” or by “fasting and praying”. How many of these respondents also visited the health facility for treatment of the NJ? It is possible to criminalize a respondent’s practice without fully exploring all the actions taken towards treatment of their neonates’ NJ. In West Africa usually a combination of approaches are resorted to in order to overcome health challenges.
Attitude of Caregivers towards NJ:
An overwhelming number of the respondents demonstrated the attitude of attending ANC and a large number of respondents showed awareness that NJ early in life can be treated in the hospital. However, the investigators did not evaluate how many of the respondents actually practiced taking their infants to the hospital for treatment of NJ. In West Africa most health care seekers would apply traditional methods of treatment of ailments and would also visit the hospital for treatment. Was it a deliberate action not to include this point or an omission?
Confounding variables:
KAP may be influenced by other considerations that may complicate the ones studied herein. Such other factors include health infrastructure and availability of equipment, economic realities, and distance to the health facility, availability of transportation and insecurity. How were these confounding variable eliminated so we can trust the validity of this study findings?
Reviewer #2: Reviewer:
Title: Determinants of Neonatal Jaundice Knowledge, Attitudes and Practice Among Caregivers in a Tertiary Health Facility in Ghana: Implications for Neonatal Care policy Reforms and Reducing Neonatal Mortality in a Low Resource Setting.
Date: 26/11/2020
General comment:
Thank you for allowing me to review this article by Salia, et al. The manuscript is about neonatal jaundice a common condition affecting newborns which can lead to disability and sometimes mortality. Generally, the manuscript reads well and is of relevance to those with an interest in the public health aspect of newborn care. However there are several abbreviations such as Neonatal Jaundice, neonatal mortality and postnatal which are simple words or phrases, that can be obtrusive to reading and a few other items that need to be corrected prior to publication. I have made some suggestions below:
Major corrections
• Page 1: Title: Suggest revision of title to: Knowledge, Attitudes and Practices Regarding Neonatal Jaundice Among Caregivers in a Tertiary Health Facility in Ghana.
• This was what was done. The study is a small study and finding are not so different from what is already known or so novel to warrant a reform in policy.
• Page 6 Sample size and sampling determination (change determination to methods) - it is not clear what sampling method was actually applied. While is says in this section that systematic sampling was done, on Page 8 line 10 it says ...caregivers were conveniently selected – this is in contrast with the assertion on page 6 that systematic sampling was used. Please clarify.
• Page 6 Line 12 and 13: was selection of the first skip interval done by balloting or other random sampling method? How were the patients arranged ina row? Were they arranged according to time of arrival as one would imagine would be the same as the attendance records or in another way? This needs to be specified.
• Page 2 abstract line 12 under methods: It does not say where the study was done and what data was collected.
Minor essential corrections
• Abstract
• Page 2 line 23: suggest rephrase to “good attitude about neonatal jaundice” rather than “...attitude in jaundice”.
• Page 2 line 29: suggest rephrase to “,,, will help reduce disability and deaths from neonatal jaundice.”
• Page 3 line 14: it will be useful to also capture global targets (SDG)
• Page 3 lines 9-11. Although neonatal jaundice affects a number of babies, many of them recover. A few may die from kernicterus or bilirubin encephalopathy however underlying causes such as neonatal sepsis are more often the cause of neonatal death in babies with jaundice. Nonetheless neonatal jaundice is a major cause of disability i.e. cerebral palsy and emphasis of this role is lacking. Thus need to rephrase to capture this.
• Page 4 line 9: should read “...as a means of treatment”
• Page 4 line 10 change “generations back” to “previous generations”
• Page 4 line18 suggest rephrasing to read...serve as useful information
• Page 5 ethical approval: were the benefits and risks also explained to them?
• Study area: Information on the delivery rate, presence of a newborn unit, its capacity, admission and neonatal mortality rates will be useful.
• Page 5 line 17: ...the health facility serves as a major facility for neighbouring Togo residents – needs to be put in the right perspective as unintentional.
• Page 5 line 17 bed capacity” remove approximately- be specific
• Page 6 study design – reads better without the abbreviations. i.e. if they are written in full -. KAP and NJ
• Page 6 line 20 - 21 Inclusion criteria- need to include the recruitment period
• Page 7 line 1: rephrase to “...not the parents of the baby” rather than “...not the real father or mother”
• Page 7 line 7 - no mention of northern tribes – they form a significant proportion of the Ghanaian population, were they few?
• Page 7 line 12: Adequacy of antenatal visits is now pegged at 8 or more visits but 4 or more can still be used as information on this is more readily available for comparison. Need to mention
• Page 7 line 15 -17. Add - these are captured in more details in the results section .
• Page 8 line 17: “...further explanation” seems a more appropriate to understanding
• Page 9 line 4 comment of significance of these coefficients.
• Page 10. Table 1 title: choose caregivers or participants
• Page 13: frequent ANC attendance alone will not prevent jaundice unless it provides education on prevention and recognition. Sentence should reflect this.
• Page 13 Lines 4 and 5 only a few identified traditional practices as being wrong should be reflected in the discussion and conclusion
• Page 10. Table 1 an unusually large number of caregivers with tertiary education needs an explanation and additional information on catchment area for hospital or was there a selection before the sampling method was applied
• Page 13 Attitude questions seem like knowledge questions.
• Page 13 last but one sentence spelling of colour –color
• Page 17 last line & Table 7 suggests - factors associated rather than determinants Having one child was associated p-0.009 (with) ones practices, but not explained or discussed.
• Conclusion poor knowledge regarding traditional treatments not mentioned
• Discussion
• Page 20 line 22: Is it possible that the reason for the disparity between your finding and Amegan-Aho et al‘s is because there were several caregivers with tertiary education in this study?
• Page 21 line 6 suggest “...though in the minority...”
• Page 21 line 8 suggest “... poor knowledge of (omit “a”)”
• Page 21 line 14 suggest “... knowledge of... rather than “in”.
• Page 21 last line: what is meant by “positive” knowledge?
• Page 22 last but 3 line suggest removal of “will” to read who “offer”
Discretionary corrections
• Page 4 line 7 suggest revising “where” to read “because”.
• Page 8 line 11: questions asked in the questionnaire –suggest rephrase
• Page 20 Line 12: suggest revising “...where majority to “in which majority”
• Page 21 line 17 suggest removal of “pre” before suggested
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #2: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif . Please note that Supporting Information files do not need this step.
Submitted filename: Reviewer plosone Nov 2020.docx
Author response to Decision Letter 0
Dear editor,
We appreciate your efforts in helping us to shape our manuscript to the required standard for publication in your reputable journal. We would like to thank the reviewer for the insightful comments on our manuscript. We are particularly grateful for the opportunity to resubmit the manuscript for another round of review. We find the comments very useful and have responded to them to the best of our knowledge. We acknowledge that the comments have no doubt helped improve the quality of our manuscript.
We have therefore provided further details by showing point-by-point feedback on how each of the comments by the reviewer were addressed. For easy identification of our responses, the reviewers’ comments have been repeated while the Authors’ responses appear in BOLD text in the main manuscript.
REVIEWER #1
Neonatal Jaundice is an important topic in the West African sub-region, the significance of jaundice being emphasized by the complications that attend uncontrolled rise in unconjugated hyperbilirubinaemia. Jaundice may progress to acute bilirubin encephalopathy with hearing loss and cerebral palsy or death besides lingering kernicterus spectrum disorder. The author needs to elaborate this outcome of jaundice because it is ruinous to families that become unfortunate to experience the disorder.
Authors response
Page 3 line 76-91: The authors agreed that the above comment is relevant in the area of neonatal jaundice as it will reveal the deleterious effects of jaundice to the reader. Given this, the introduction section of the manuscript has been revised to address this comment.
The authors appreciate the in-depth review carried out on the manuscript and the study subject by the reviewer in relation to the geographic area of the study. We acknowledged that the addition of these issues by the reviewer could by far improved the quality of the manuscript. However, these issues escaped us when we were designing the study and we have therefore included this comment as part of the limitations of the study. In subsequent related studies, we will take cognizance of these comments.
We greatly agree with the reviewer on this comment that the inclusion of these groups of mothers could have brought out information/findings that would have contributed to policy reforms in the area of newborn care in Ghana. Despite the omission, we believe the study has revealed some key findings that will add to the body of knowledge and worthy of influencing newborn care positively in Ghana. We have therefore revised the manuscript to include this comment as part its limitation on page 25.
The authors reviewed a wide range of relevant literature regarding this subject area which were equally done as quantitative studies and had included a wide range of relevant questions from literature and questions arising from discussions with some mothers. We however agree that allowing the mothers to express themselves freely on such an important topic would have revealed more information. We therefore believe that the questions asked in our study are by far the reflections of the caregivers’ true inclination. Subsequently, a mixed-method could be considered in related studies.
The method of sampling in this study was a systematic random sampling as found in the abstract and on page 2 line 52 of the manuscript. The manuscript has been revised to remove the convenient method from the manuscript as this was purely a mistake. The use of the systematic random sampling method eliminated any possibility of bias as a sampling interval was obtained to be (2). Participants were arranged to sit in rows and the first respondent who formed the starting point was selected by balloting. This is found on page 7 line 180-184.
Respondents who were proficient in speaking and writing in the English language answered the questionnaires by themselves. For those who could neither read nor write in the English language were guided to complete the questionnaire. Four of the authors were native speakers of the “Ewe” language and three authors who could speak “Ewe”, “Twi” and other languages were used to orally translate the questionnaire into these local languages for the understanding of the caregivers. This has subsequently been included in the data collection procedure section of the manuscript on page 10 line 237-242.
The authors find the comment very useful. Indeed, knowing that poor health seeking behaviour could lead to the death of a child could definitely influence caregivers attitude and their health-seeking behaviours. However, on page 15 (table 2: caregivers knowledge of neonatal jaundice) under the complication section, death has been captured where the majority (77.7%) of caregivers indicated that death could occur as a result of jaundice. We have further included this in the discussion section of the manuscript on page 24 line 422-424.
We appreciate this comment. The authors allowed multiple responses from the caregivers. We therefore believed that the caregivers responded multiple times by choosing responses that applied to them.
The authors agreed with the reviewer on this comment that most healthcare seekers would apply traditional methods of treatment of ailments and would also visit the hospital for treatment. However, it was purely an oversight on the part of the authors not to have included whether “caregivers actually took their infants to the hospital for treatment of neonatal jaundice” in our study. We appreciate that the addition of this to our study would have revealed more attitudes from the caregivers.
KAP may be influenced by other considerations that may complicate the ones studied herein. Such other factors include health infrastructure and availability of equipment, economic realities, and distance to the health facility, availability of transportation and insecurity. How were these confounding variables eliminated so we can trust the validity of this study findings?
This study investigated knowledge, attitude and practice of neonatal jaundice among caregivers. The authors agreed that newborn care practices may be influenced by the factors stated in the reviewer’s comment. However, our questionnaire was designed based on the main aim of the study which was to evaluate caregivers knowledge, attitude and practice of neonatal jaundice. The questionnaire went through a thorough validity process where it was peer-reviewed by all the authors, nursing and medical specialists in related field to the study topic as well as piloting and pretesting. This made it possible that all questions included in the study were relevant and specific in achieving the intended purpose of the study.
Also, the authors carried out multicollinearity diagnostics and found the Variance Inflation Factors (VIFs) were all below the 5-10 rule of thumb range, suggesting there is no collinearity among the independent variables to be fitted in the regression model! Indeed.
REVIEWER #2
MAJOR CORRECTIONS
Reviewer’s comment
Page 1: Title: Suggest revision of title to: Knowledge, Attitudes and Practices Regarding Neonatal Jaundice Among Caregivers in a Tertiary Health Facility in Ghana.
Page 1 line 2-3: We agree with the reviewer that the main outcome variables of this study were knowledge, attitude and practice and that the study size was small to influence policy. We have therefore revised the title of the manuscript to reflect the reviewer’s comment.
Page 6 Sample size and sampling determination (change determination to methods) - it is not clear what sampling method was actually applied. While it says in this section that systematic sampling was done, on Page 8 line 10 it says ...caregivers were conveniently selected – this is in contrast with the assertion on page 6 that systematic sampling was used. Please clarify.
Page 7 line 175-178: The manuscript was formatted according to the journal specifications, however, because of the comment, “sampling determination” has been revised to “sampling method”. The sampling method for this study was systematic random sampling. Therefore, the manuscript was revised to remove “caregivers were conveniently selected” to reflect the correct sampling method as stated under sample size and sampling method.
Page 6 Line 12 and 13: was selection of the first skip interval done by balloting or other random sampling method? How were the patients arranged in a row? Were they arranged according to time of arrival as one would imagine would be the same as the attendance records or in another way? This needs to be specified.
Page 7 line 170-184: The authors have revised this section of the manuscript to address these concerns.
Page 2 abstract line 12 under methods: It does not say where the study was done and what data was collected.
Page 2 line 51-53: The method section of the abstract was revised to include where the study was done and the data collected.
MINOR ESSENTIAL CORRECTIONS
Page 2 line 23: suggest rephrase to “good attitude about neonatal jaundice” rather than “...attitude in jaundice”.
Page 2 line 61-62: The authors revised the abstract to include the reviewer’s suggestion.
Page 2 line 29: suggest rephrase to “,,,will help reduce disability and deaths from neonatal jaundice.”
Page 2 line 67-68: The abstract aspect of the manuscript has been revised to include the reviewer’s suggestion.
INTRODUCTION
Page 3 line 14: it will be useful to also capture global targets (SDG)
Page 5 line 104-107: The introduction section of the manuscript has been revised to include the global targets of reducing neonatal deaths
Page 3 lines 9-11. Although neonatal jaundice affects a number of babies, many of them recover. A few may die from kernicterus or bilirubin encephalopathy however underlying causes such as neonatal sepsis are more often the cause of neonatal death in babies with jaundice. Nonetheless neonatal jaundice is a major cause of disability i.e. cerebral palsy and emphasis of this role is lacking. Thus need to rephrase to capture this.
Page3 line 92-96: The authors agreed that the above suggestion is relevant in the area of neonatal jaundice as it will reveal the deleterious effects of jaundice to the reader. Given this, the introduction section of the manuscript has been revised to capture this information.
Page 4 line 9: should read “...as a means of treatment”
Page 5line 121-122: The manuscript has been revised to include this suggestion.
Page 4 line 10 change “generations back” to “previous generations”
Page 5 line 126: The authors changed “generations back” to “previous generations”.
Page 4 line18 suggest rephrasing to read...serve as useful information
Page 5 line 134: The manuscript was revised and the phrase “serve as a useful information” was changed to “serve as useful information”.
Page 5 ethical approval: were the benefits and risks also explained to them?
Page 6 line 149-154: The authors at the point of data collection explained the ethical issues including benefits and risks to the caregivers. However, in the final write up of the manuscript, the benefits and risks aspects were not included in the ethical consideration as this was an oversight. We acknowledge the importance of benefits and risks to the participants. Because of the reviewer’s comments, the manuscript has been revised to include the benefits and risks.
Study area: Information on the delivery rate, presence of a newborn unit, its capacity, admission and neonatal mortality rates will be useful.
Page 6-7 line 160-166: The manuscript was revised to include the above comment.
Page 5 line 17: ...the health facility serves as a major facility for neighbouring Togo residents – needs to be put in the right perspective as unintentional.
Page 6: The authors revised the manuscript and have decided to remove this portion “the health facility serves as a major facility for neighbouring Togo residents” from the study area.
Page 6 line 17 bed capacity” remove approximately- be specific
Page 6 line 160: The authors removed “approximately” from the study area section of the manuscript.
Page 6 study design – reads better without the abbreviations. i.e. if they are written in full -. KAP and NJ
Page 7 line 172: The authors have revised the manuscript and KAP and NJ have been written in full as “knowledge, attitude and practice” and “neonatal jaundice”.
Page 6 line 20 - 21 Inclusion criteria- need to include the recruitment period
Page 8 line 197: The manuscript was revised in the inclusion and exclusion section to include the recruitment period of the caregivers.
Page 7 line 1: rephrase to “...not the parents of the baby” rather than “...not the real father or mother”
Page 8 line 196: In the inclusion and exclusion section, the phrase “not the real father or mother” was revised to “not the parents of the babies”.
Page 7 line 7 - no mention of northern tribes – they form a significant proportion of the Ghanaian population, were they few?
From the 2010 national housing and population census, Ewes constituted the majority (73.8%) of the ethnic groups in Volta region, followed by Gurma (11.3%) and the Guan (8.1%). Specifically, the largest ethnic group in the Ho municipality where the study was conducted was Ewes (91.1%) followed by Akan (2.0) and the northern tribes (0.5%). However, the authors did not have any empirical data regarding the ethic stratification in the volta region and especially Ho at the time of designing the data collection instrument. Therefore, based on the statistics above, the authors decided to put all other tribes aside those mentioned in the study as “others ethnicity” which includes the northern tribes as contained on page 8 (study variables) and 12 (demographic characteristics).
Ghana Statistical Service (2013). 2010 Population and Housing Census. Regional Analytical Report. GSS, Volta Region
Page 7 line 12: Adequacy of antenatal visits is now pegged at 8 or more visits but 4 or more can still be used as information on this is more readily available for comparison. Need to mention
Page 9 line 208-210: The authors are grateful for this revelation on the adequacy of Antenatal Visit being pegged at 8 or more visits. We have therefore mentioned this in our manuscript but have maintained the 4 visits or more since the data was taken based on this.
Page 7 line 15 -17. Add - these are captured in more details in the results section.
Page 9 line 215-216: The study variables section of the manuscript was revised to include the phrase “these are captured in more details in the results section” of the manuscript.
Page 8 line 17: “...further explanation” seems a more appropriate to understanding
Page 10 line 243: The data collection section was revised where “further understanding” was replaced with “further explanation”.
Page 9 line 4 comment of significance of these coefficients.
Page 11 line 253-260: The manuscript was revised to include the significance of the coefficient values.
Page 10. Table 1 title: choose caregivers or participants
The manuscript was revised replacing “participants” with “caregivers”.
Page 13: frequent ANC attendance alone will not prevent jaundice unless it provides education on prevention and recognition. Sentence should reflect this.
Page 16: The authors largely agreed to the above comment and have therefore revised this item under the caregivers' attitude regarding neonatal jaundice.
Page 13 Lines 4 and 5 only a few identified traditional practices as being wrong should be reflected in the discussion and conclusion
Page 26 line 454-459: The manuscript was revised in the discussion and conclusion sections to include the reviewer’s suggestion on.
Page 10. Table 1 an unusually large number of caregivers with tertiary education needs an explanation and additional information on catchment area for hospital or was there a selection before the sampling method was applied
The Ho Teaching Hospital is located in the Ho municipality. The municipality is predominantly an urban area. Data from the Ghana Statistical Service (2013) showed that about 53.9% and 46.1% of women and men in the municipality are literate. Also, 30.8% of the women and 34.0% of men in the municipality can speak English and other languages.
The increased number of caregivers with tertiary education in the current study could have been a coincidence since data collection was mainly through systematic random sampling. As a teaching hospital, many elite families who are highly educated would prefer to seek care there to receive the best care and this could have increased the number of caregivers with tertiary education. The authors employed the sampling strategy in selecting the caregivers, therefore, there was no selection of participants before applying the sampling strategy.
Page 13 Attitude questions seem like knowledge questions.
Page 17: The attitude questions were revised in the main manuscript.
Page 13 last but one sentence spelling of colour –color
The word “color” was revised to “colour” in the main manuscript.
Page 17 last line & Table 7 suggests - factors associated rather than determinants. Having one child was associated p-0.009 (with) ones practices, but not explained or discussed
The authors have revised the results section of the manuscript to change “determinants” to “factors associated with” from page 17-22. Also, in the description section of the multivariate analysis of caregivers’ attitudes regarding neonatal jaundice, caregivers who had one child were 58% less likely to demonstrate a positive attitude of neonatal jaundice. This is captured on page 19 line 352-354 of the revised manuscript.
Conclusion poor knowledge regarding traditional treatments not mentioned
Page 27 line 477-480: The conclusion section of the has been revised to include this comment.
DISCUSSIONS
Page 20 line 22: Is it possible that the reason for the disparity between your finding and Amegan-Aho et al‘s is because there were several caregivers with tertiary education in this study?
In our study, the majority of caregivers had tertiary education (45.0%) than Amegan-Aho et al (2019) but demonstrated poor knowledge (8.9%) compared to 76% good knowledge in Amegan-Aho et al with only 5.1% tertiary education.
Ideally, one would have thought that a high level of tertiary education would be associated with a good knowledge in health-related issues as revealed by Greenaway et al (2012) and low level of education will lead to poor health-seeking behaviours including neonatal jaundice (Ogunlesi & Abdul, 2015). As revealed in our study on page 23 line 404-407, there was no association between caregivers’ educational status with knowledge on neonatal jaundice.
Greenaway, E. S., Leon, J., & Baker, D. P. (2012). Understanding the association between maternal education and use of health services in Ghana: exploring the role of health knowledge. Journal of biosocial science, 44(6), 733.
Ogunlesi, T. A., & Abdul, A. R. (2015). Maternal knowledge and care. Seeking behaviors for newborn jaundice in Sagamu, Southwest Nigeria. Nigerian journal of clinical practice, 18(1), 33-40.
Page 21 line 6 suggest “...though in the minority...”
Page 24 line 415: The discussion section was revised to capture “though in the minority”.
Page 21 line 8 suggest “... poor knowledge of (omit “a”)”
Page 24 line 417: The manuscript was revised in the discussion section to remove “a” and include “of”
Page 21 line 14 suggest “... knowledge of... rather than “in”.
Page 24 line 417: The manuscript was revised in the discussion section to replace “in” with “of”.
Page 21 last line: what is meant by “positive” knowledge?
Page 24 line 426: The intention was to indicate good knowledge and not positive knowledge. This has been revised as “good knowledge” and not “positive knowledge”.
Page 22 last but 3 line suggest removal of “will” to read who “offer”
Page 26 line 463: The word “will” was removed from the sentence.
DISCRETIONARY CORRECTIONS
Page 4 line 7 suggest revising “where” to read “because”.
Page 5 line 123: Revision was made in which “where” was changed to “because”.
Page 8 line 11: questions asked in the questionnaire –suggest rephrase
Questions in the questionnaire has been revised from 15-17.
Page 20 Line 12: suggest revising “...where majority to “in which majority”
Page 23 line 396-397: Revision was made in the discussion section in which “where majority” was changed to “in which majority”.
Page 21 line 17 suggest removal of “pre” before suggested
Page 25 line 429: Revision was made and “pre” was removed.
Submitted filename: Response to Reviewers.docx
Decision Letter 1
12 Mar 2021
PONE-D-20-19725R1
Manuscript title: Knowledge, Attitudes and Practices Regarding Neonatal Jaundice Among Caregivers in a Tertiary Health Facility in Ghana.
Please submit your revised manuscript by Apr 26 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Bolajoko O. Olusanya, MBBS, FMCPaed, FRCPCH, PhD
Academic Editor
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Reviewers' comments:
Reviewer's Responses to Questions
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: (No Response)
2. Is the manuscript technically sound, and do the data support the conclusions?
3. Has the statistical analysis been performed appropriately and rigorously?
4. Have the authors made all data underlying the findings in their manuscript fully available?
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #1: The reviewer is satisfied by the responses given to questions raised towards the original MS contents. I believe the article is now in a better shape to be accepted for publication and so recommend same.
Reviewer #2: General Comment.
The manuscripts reads much better and most of my previous comments have been addressed. However, there are still some minor essential corrections and discretionary corrections that need to be addressed. Suggest further English language editing.
Minor Essential Corrections
Page 2 line 47: suggest changing “...of neonatal jaundice...” to “...regarding neonatal jaundice...”
Page 2 line 52-53: suggest revision: “...sampling strategy. Quantitative data was collected using a questionnaire and analysed with STATA....
Page 2 line 54: the phrase “...neonatal jaundice knowledge...” does not seem right, kindly revise.
Page 2 line 57 sentence beginning with “More than 50% (54.5%) and (52.5%) of caregivers...” is unclear, kindly revise
Page 2 line 59: suggest revising “....knowledge in...” to “knowledge about...”
Conclusion – needs to be more specific and focused on findings.
Page 2 Line 65: suggest changing “...of jaundice...” to “...about jaundice...”
Page 3 line 76-77: “Jaundice ...manifestation of a disease.” kindly add a reference.
Page 3 line 99: reference 12: Reference from Graphic should be changed to a more appropriate source of data on prevalence of neonatal jaundice in Ghana.
Page 3 line 101 suggest changing revealed to reported
Page 6 line146: the word reinforced is unclear in this context.
Page 6 line 153-154: invasive procedures are not the only sources of risk or discomfort so suggest you remove or revise the reference. The inconvenience of additional waiting to complete the questionnaire, though minimal may be seen as a source of discomfort to some.
Page 6 line 157: study area you have described the neonatal unit however it is still not clear how neonatal services are delivered at the hospital as you have not described the postnatal ward in adequate detail.
Page 7 line 172: remove capitalisation
Page 7 line 175: Change methods to determination to meet journal specification
Page 7 line 184: sentence “Balloting was done...”. needs to be re-sequenced as the skip interval is usually determine before the starting point.
Page 9 line 208 to 2010: suggest revision- Although WHO has changed the minimum recommended antenatal visits from 4 to 8 visits, in this study we studied an attendance of 4 visits.[reference] No need to mention availability of information for comparison..
Page 9 line 218-219 remove capitalisation
Page 10 line 238 remove” For”
Format tables to fit with journals recommendations and also provide information on Total respondents
Table1: Title - change caregiver to caregivers; Characteristics - Education received on neonatal jaundice and Child with a diagnosis of neonatal jaundice reads better (page 14)
Table 2: title- suggest - Caregivers knowledge about neonatal jaundice; Prevention of neonatal jaundice: Neonatal jaundice is a common problem on newborns...so there is no need to prevent it reads better
Table 5 and 6; more information needed on the logistic regression model. What aspects of the knowledge, attitudes, beliefs and practices and were examined?
Page 19 line 354. There is no decimal place in the p-value.
Conclusion: Does not provide a succinct summary of some of the good practices and gaps in knowledge.
Lines 477-480 no need to bring in percentages.
Page 2 line 42: suggest removing word “trends”
Page 8 line 198; suggest removing “thus”
References - Need to examine references again. Inconsistency in formatting noted,; date of accessing online publication omitted in some instances
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Reviewer #1: Yes: Stephen Oguche
Author response to Decision Letter 1
20 Apr 2021
Please see response to reviewers letter at the end of the PDF for review
Decision Letter 2
29 Apr 2021
PONE-D-20-19725R2
Thank you for submitting your revised manuscript to PLOS ONE. This current version is much improved but the key messages are still hazy and imprecise. At this stage I invite you to revise your abstract by addressing the following edits carefully to improve the clarity of your message to our audience. You will then need to amend your results and conclusions in line with the suggested changes in the abstract.
1. Abstract - Results
DELETE: ‘ Most of the caregivers demonstrated poor knowledge (54.5%) and attitude (52.5%) while 58.9%
had good practices regarding neonatal jaundice’.
REPLACE WITH: ‘Less than half of the caregivers demonstrated good knowledge (45.5%) and attitude (47.5%) but 58.9% had good practices regarding neonatal jaundice’.
2. Abstract Conclusion:
DELETE: ‘Most of the caregivers demonstrated poor knowledge and attitude about jaundice while the majority demonstrated good practice. Healthcare professionals need to intensify education regarding jaundice among caregivers in low resource settings throughout antenatal and postnatal periods. Good knowledge may possibly lead to positive attitudes towards jaundice. Overall, improved knowledge and attitude improve maternal health-seeking behaviours which may help to reduce disabilities and deaths from neonatal jaundice’.
REPLACE WITH: 'Less than two thirds of the caregivers demonstrated good practice with limited knowledge and poor attitude. Efforts to promote well informed and improved caregivers’ attitude will advance positive maternal health-seeking behaviour and reduce disabilities and death through early detection and intervention of infants with neonatal jaundice. Public awareness and education about neonatal jaundice especially among caregivers in the private sector should also be intensified'.
3. Reference 12 is incomplete without a URL.
Please submit your revised manuscript by Jun 13 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
Author response to Decision Letter 2
Manuscript title: Knowledge, Attitudes and Practices Regarding Neonatal Jaundice Among Caregivers in a Tertiary Health Facility in Ghana
*Solomon Mohammed Salia1, Agani Afaya1,2, Abubakari Wuni3, Martin Ayanoore4, Emmanuel Salia5, Doreen Dzidzor Kporvi1, Peter Adatara1, Robert Kaba Alhassan6, Vida Nyagre Yakong7, Sean Augustine Eduah-Quansah1, Shine Seyram Quarshie1, Eric Kwame Dey1, Dominic Amoah Akolga1
We are very happy that you are giving us another chance to shape our manuscript to the required journal’s standard for publication. We would like to thank the reviewers for the thorough review and insightful comments on our manuscript. The comments though minor are very useful and we have responded to them to the best of our knowledge. We acknowledge that the comments have no doubt helped improve the quality of our manuscript.
We have therefore provided further details by showing point-by-point feedback on how each of the comments was addressed. For easy identification of our responses, the comments have been repeated while the Authors’ responses appear in BOLD text in the main manuscript.
Editor’s Comments
Abstract - Results
DELETE: ‘Most of the caregivers demonstrated poor knowledge (54.5%) and attitude (52.5%) while 58.9% had good practices regarding neonatal jaundice’.
REPLACE WITH: ‘Less than half of the caregivers demonstrated good knowledge (45.5%) and attitude (47.5%) but 58.9% had good practices regarding neonatal jaundice’.
Authors Response
Page 2 line 60-61: The results section of the abstract was deleted and replaced with the suggested statement by the editor which adds more meaning to the results.
Editor’s Comment
2. Abstract Conclusion:
DELETE: ‘Most of the caregivers demonstrated poor knowledge and attitude about jaundice while the majority demonstrated good practice. Healthcare professionals need to intensify education regarding jaundice among caregivers in low resource settings throughout antenatal and postnatal periods. Good knowledge may possibly lead to positive attitudes towards jaundice. Overall, improved knowledge and attitude improve maternal health-seeking behaviours which may help to reduce disabilities and deaths from neonatal jaundice’.
REPLACE WITH: 'Less than two thirds of the caregivers demonstrated good practice with limited knowledge and poor attitude. Efforts to promote well informed and improved caregivers’ attitude will advance positive maternal health-seeking behaviour and reduce disabilities and death through early detection and intervention of infants with neonatal jaundice. Public awareness and education about neonatal jaundice especially among caregivers in the private sector should also be intensified'.
Page 2 line 68-73: The authors have deleted the conclusion section of the abstract and have replaced it with the editor’s suggested phrase. This has made the understanding of the conclusion clearer.
3. Reference 12 is incomplete without a URL.
Reference to the second revision, the reviewer requested that we change the graphic Online source to a more appropriate reference. However, the authors have searched and have realized that the information used by graphic online was from the Ghana Health Service (GHS), but this information from the GHS was not published as at the time the authors used the information, and therefore, did not have a URL.
Page 31 line 610-612: The authors have revised the reference and have added the URL
Decision Letter 3
PONE-D-20-19725R3
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
Additional Editor Comments (optional):
Acceptance letter
26 May 2021
Knowledge, Attitudes and Practices Regarding Neonatal Jaundice Among Caregivers in a Tertiary Health Facility in Ghana.
Dear Dr. Salia:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno .
If we can help with anything else, please email us at gro.solp@enosolp .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. Bolajoko O. Olusanya

COMMENTS
Neonatal jaundice is a clinical manifestation of elevated total serum bilirubin (TSB), termed neonatal hyperbilirubinemia, which results from bilirubin that is deposited into an infant's skin. ... Karagol BS, Kundak A, Dursun A, Hakan N, Okumus N. Literature review and outcome of classic galactosemia diagnosed in the neonatal period. Clin Lab ...
Introduction. Neonatal jaundice is a common pediatric condition that refers to the yellowish coloration of the skin, sclera of the eye, and other organs of the body caused by the accumulation of bilirubin (American Academy of Pediatrics et al., 2004; Huang et al., 2022).It affects nearly 50% to 60% of full-term babies and 80% of premature babies develop jaundice within the first week ...
Introduction. Jaundice, which is yellowish discolouration of the sclera and the skin, is common in term and preterm neonates. Severe unconjugated hyperbilirubinemia, if not managed urgently, is associated with acute and chronic bilirubin encephalopathy or kernicterus and even death. 1 Neonatal jaundice (NNJ) is a preventable cause of neonatal morbidity and mortality. 2 The common causes of ...
Abstract. "Common" neonatal jaundice can lead to dangerous levels of hyperbilirubinemia, causing neurological damage and even death. This article outlines evidence-based assessment techniques, management guidelines, and treatments for neonatal hyperbilirubinemia, addressing complexities that have arisen with new technologies and research results.
Neonatal jaundice is a common clinical sign that indicates hyperbilirubinemia. Clinicians should become familiar with the differential diagnoses of hyperbilirubinemia in newborns and young infants and the importance of early referral of all patients with cholestatic jaundice to a pediatric gastroenterologist or hepatologist.After completing this article, readers should be able to:The term ...
Neonatal jaundice (NNJ) is a common pediatric condition, affecting 60% and 80% of term and preterm neonates, respectively, globally (Khan et al., 2015; Salia et al., 2021).Clinically, NNJ is defined by the yellowish coloration of the skin and sclera of the eye, suggesting raised levels of unconjugated bilirubin in the blood circulation (Khan et al., 2015).
Jaundice caused by hyperbilirubinaemia is a common phenomenon during the neonatal period. Population-based studies evaluating assessment, management, and incidence of jaundice and need for ...
Abstract. Neonatal jaundice is common, and usually harmless, because of physiological jaundice or breast-feeding. In some neonates unconjugated bilirubin concentration, coupled with other risk factors, is sufficient to allow free bilirubin to cross the blood-brain barrier and cause kernicterus. Another subgroup of infants is jaundiced because ...
Neonatal jaundice is common, and usually harmless, because of physiological jaundice or breast-feeding. ... Neonatal jaundice: a critical review of the role and practice of bilirubin analysis. Jean M Kirk [email protected] ... The few references in the peer-reviewed literature to this interference 60 may be quoted by manufacturers, but may not ...
Neonatal Hyperbilirubinemia. Icterus neonatorum, or neonatal jaundice, has long been recognized. 1 The term "kernicterus" was introduced in the early 1900s to refer to the yellow staining of ...
Physiological jaundice accounts for 75% of neonatal hyperbilirubinemia and results from physiological alterations in neonatal bilirubin metabolism. Healthy adults have normal total
Neonatal jaundice can be classified as physiological. and pathological and can have several causes such as breast milk feeding, blood group incompatibi lity, hemolysis, or. genetic defects of ...
The underlying causes of neonatal jaundice are not well understood. Here, the authors identify genetic variants associated with neonatal jaundice, including a variant in the gene UGT1A, finding a ...
The burden of hyperbilirubinemia is highest in South Asia and sub-Saharan Africa.2 Hyperbilirubinemia is the 7th leading cause of neonatal mortality in South Asia, 8th in sub-Saharan Africa, 9th in western Europe and 13th in North America.2 In our review, we appraised five guidelines from Europe with a mean score of 55.9%, four guidelines from ...
The mechanism of neonatal jaundice is the imbalance between bilirubin production and conjugation, which results in increased bilirubin levels. 4 This imbalance is mainly because of the immature liver of the neonate and the rapid breakdown of red blood cells, which may be multifactorial. 3,4,5,6 At bilirubin levels of between 85 µmol/L and 120 ...
This review gives a brief introduction to hyperbilirubinemia and jaundice and the recent advancement in the treatment of neonatal hyperbilirubinemia. It reports modifications in the previously used methods and findings of some newly developed ones. At present, ample literature is available discussing the issues regarding hyperbilirubinemia and ...
Neonatal jaundice identification has always posed a challenge, mainly in LMICs. 18,19 Over the last years, with the advancement of technology, different solutions have emerged for SNH screening.
Neonatal jaundice was measured using the Kj-8000 device on day 3-6 after birth by measuring skin bilirubin level and also the need for phototherapy (severe jaundice) was determined based on AAP ...
1. Introduction. Severe neonatal jaundice (SNJ) in a neonate may manifest as acute bilirubin encephalopathy (ABE) [] with a range of symptoms including difficulty feeding, tone abnormalities, abnormal cry and the kernicteric facies [] scored using the bilirubin-induced neurological dysfunction (BIND) score or modified BIND [3,4].Persistent abnormalities which are now known as the Kernicterus ...
A review of population-based literature to assess the global impact of severe neonatal jaundice (SNJ) highlighting the importance of this disease as defined by its clinical presentations. Objective evidence that the burden of SNJ is not evenly distributed and that a heavier burden of disease is born by low-income and middle-income countries.
A review of population-based literature to assess the global impact of severe neonatal jaundice (SNJ) highlighting the importance of this disease as defined by its clinical presentations. Objective evidence that the burden of SNJ is not evenly distributed and that a heavier burden of disease is born by low-income and middle-income countries.
To conduct a systematic literature review and a meta-analysis of the association between neonatal jaundice and autism with particular attention given to low risk of bias studies. Pubmed, Scopus ...
mothers revealed that most of the mothers engaged in bad practices (60%) towards neonatal jaundice. The majority (66.8%) of the respo. dents indicated that a neonate diagnosed with neonatal jaundice is given herbal drinks and glucose. Whereas a proportion of (54%) respondents was induced by cultural belief.
Neonatal jaundice is a leading cause of hospitalization in the first week of life worldwide. If inappropriately managed, it may result in significant bilirubin-induced mortality and disability. ... The burden and management of neonatal jaundice in Nigeria: A scoping review of the literature Niger J Clin Pract. 2016 Jan-Feb;19(1):1-17. doi: 10. ...
Aluminum phosphide (ALP) poisoning poses a significant public health concern worldwide, with a high mortality rate and no established definitive treatment. This case report highlights a 30-year-old male with G6PD deficiency who ingested ALP tablets, presenting with jaundice and anemia. Despite the severity of ALP poisoning, the concurrent G6PD deficiency appeared to confer a protective effect ...
Introduction and Literature Review: Neonatal Jaundice is an important topic in the West African sub-region, the significance of jaundice being emphasized by the complications that attend uncontrolled rise in unconjugated hyperbilirubinaemia. Jaundice may progress to acute bilirubin encephalopathy with hearing loss and cerebral palsy or death ...