
- Allergy & Immunology
- Anesthesiology
- Critical Care
- Dermatology
- Diabetes & Endocrinology
- Emergency Medicine
- Family Medicine
- Gastroenterology
- General Surgery
- Hematology - Oncology
- Hospital Medicine
- Infectious Diseases
- Internal Medicine
- Multispecialty
- Ob/Gyn & Women's Health
- Ophthalmology
- Orthopedics
- Pathology & Lab Medicine
- Plastic Surgery
- Public Health
- Pulmonary Medicine
- Rheumatology
- Transplantation
- Today on Medscape
- Business of Medicine
- Medical Lifestyle
- Science & Technology
- Medical Students
- Pharmacists

FDA Clears the Omnipod 5 System for Type 2 Diabetes
Miriam E. Tucker
August 26, 2024
The US Food and Drug Administration (FDA) has cleared the Omnipod 5 for use by people aged 18 and older with type 2 diabetes , a first for any automated insulin delivery (AID) system.
The Omnipod 5 system (Insulet) comprises a tubeless plastic "pod" worn on the body that automatically delivers insulin based on data communicated to it wirelessly from a compatible continuous glucose monitor, also worn on the body. Users still need to interact with the system for mealtime insulin dosing and in some cases to adjust for exercise. Previously, the Omnipod 5 was approved only for people with type 1 diabetes aged 2 years and up. Other AIDs are still only approved for people with type 1 diabetes.
The new indication is based on data from the real-world multicenter SECURE-T2D trial of a racially diverse group of 305 adults with type 2 diabetes who were taking insulin. About half were also taking a glucagon -like peptide-1 (GLP-1) receptor agonist. Use of the Omnipod 5 resulted in a significant A1c reduction from 8.2% at baseline to 7.4% at 13 weeks ( P < .001), with no differences in outcome by GLP-1 receptor agonist use.
Study investigator Viral Shah, MD, told Medscape Medical News that use of AIDs in people with type 2 diabetes who require insulin treatment makes sense, especially for those who require premeal as well as basal insulin. "Type 2 diabetes is a progressive disease, and many with longstanding disease don't have enough beta cells...So I don't see a difference between people with type 1 diabetes and those with type 2 diabetes who are on multiple daily injections because they both do not have enough insulin production."
Indeed, Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women's Hospital, Boston, Massachusetts, noted that "there are patients with advanced type 2 diabetes who don't tolerate GLP-1 receptor agonists. That's not an uncommon scenario, and insulin does really become the drug of choice for many of them."
McDonnell, who has no financial ties to Insulet, also pointed out that there are people misdiagnosed with type 2 who actually have autoimmune (type 1) diabetes, as well as other types that often are lumped under the heading of type 2 diabetes, including those with monogenic diabetes, pancreatic disease, or post-transplant diabetes. "It's actually quite complex. This new indication makes a lot of sense just in terms of not having to go through hoops to justify insulin technology that is safer and easier for people with insulin-deficient type 2 diabetes or [those] without type 1 diabetes but with other forms of insulin deficiency."
Both Shah and McDonnell have prescribed the Omnipod 5 off-label to some of their patients with type 2 and other types of diabetes. Private insurance will typically cover it, although prior authorization may be required. Medicare requires certification of C-peptide deficiency for coverage of other insulin pumps under Part B but not for the Omnipod because it's covered under Part D.
Shah, who is professor of medicine in the division of endocrinology and metabolism and director of diabetes clinical research at Indiana University, Indianapolis, said: "We haven't encountered any big issues with the insurance companies approving pumps and AID systems in type 2 diabetes, but at the same time, having that official label helps."
McConnell said that the Omnipod 5 is the most common AID she prescribes for her patients with type 2 diabetes because it is the only one covered under pharmacy rather than as durable medical equipment (as are other pumps with tubing). "Because it goes through as a pharmacy benefit it is an easier transition for most people with type 2 diabetes who have been traditionally less engaged with technology and equipment suppliers," she told Medscape Medical News .
This new indication is likely to increase uptake of the Omnipod 5 into primary care, where the vast majority of people with type 2 diabetes are managed, Shah noted. "This opens up an avenue for primary care to manage more diabetes so that people don't have to wait in line a long time to see an endocrinologist...I'm very optimistic in the way that the diabetes technology field is moving is helping our primary care practitioners to really optimize diabetes care by themselves."
But McConnell worries about the capacity of primary care for both the technical and administrative aspects of the system's use. The diabetes program she runs at Brigham and Women's Hospital has a team that works full-time to deal with the paperwork involved in diabetes device insurance coverage, an advantage that most nonspecialist practices don't have.
And, she notes, use of Omnipod 5 requires proper adjustment of the settings and patient education, particularly around meal dosing. "I've seen patients whose primary care physicians have sent the Omnipod prescription to the pharmacy without any real knowledge of the system or next step plan. It was a problem. You really need guidance, especially patients who have never done premeal insulin dosing. I hope the company will do the right thing by ensuring patients and their providers have sufficient product support."
An Insulet spokesperson told Medscape Medical News : "We are continuing to expand our education, training, and support for [healthcare professionals], including primary care practices. Our team will continue to assist with prior authorizations when needed, as we do today."
Shah has received honoraria from Dexcom, Insulet, Tandem Diabetes Care, Embecta, Sanofi, Novo Nordisk, Genomelink, and LumosFit for consulting, speaking, or advising. McDonnell has conducted research for Dexcom but did not take salary from it.
Miriam E. Tucker is a freelance journalist based in the Washington DC area. She is a regular contributor to Medscape, with other work appearing in the Washington Post, NPR's Shots blog, and Diatribe. She is on X (formerly Twitter) @MiriamETucker.
Send comments and news tips to [email protected] .
TOP PICKS FOR YOU
- Perspective
- Drugs & Diseases
- Global Coverage
- Additional Resources
- Biosimilars May Finally Stop the Rocketing Cost of Insulin
- Reducing Albumin Improves Kidney and Heart Function in People With Type 2 Diabetes
- Atherosclerosis and Diabetes Synergistic, Deadlier in South Asians
- Diseases & Conditions Type 2 Diabetes Mellitus
- Diseases & Conditions Pediatric Type 2 Diabetes Mellitus
- Diseases & Conditions Type 2 Diabetes Mellitus and TCF7L2
- Drugs exenatide injectable suspension
- Type 2 Diabetes Mellitus
- Pediatric Type 2 Diabetes Mellitus
- Type 2 Diabetes Mellitus and TCF7L2
- Pediatric Type 1 Diabetes Mellitus
- Fast Five Quiz: Atrial Fibrillation and Diabetes
- Fast Five Quiz: How Much Do You Know About Diabetic Neuropathy?
- Bedridden Breakdown: Classifying Pressure Injuries
- Diabetes Mellitus Type 2 News & Perspectives
- What's the Goal in Treating Type 2 Diabetes?
- Denosumab for Osteoporosis May Prevent Type 2 Diabetes
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Management of microcomplications of diabetes mellitus: challenges, current trends, and future perspectives in treatment.

1. Introduction
2. role of metabolic pathway in microvascular complications, 3. new molecular pathogenesis, 3.1. epigenetics mechanisms, 3.2. role of gut microbiota, 3.3. diabetic retinopathy, 3.4. treatment and current advances in diabetic retinopathy, diabetic neuropathy, 3.5. treatment and current advances in diabetic neuropathy, diabetic nephropathy, 3.6. treatment and current advances in diabetic nephropathy, 4. impact of glucose-lowering drugs on microvascular complications, author contributions, conflicts of interest.
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020 , 21 , 6275. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chan, J.C.N.; Lim, L.L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2021 , 396 , 2019–2082. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004 , 27 , 1047–1053. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Martinez-Ortega, A.J.; Munoz-Gomez, C.; Gros-Herguido, N.; Remon-Ruiz, P.J.; Acosta-Delgado, D.; Losada-Vinau, F.; Pumar-Lopez, A.; Mangas-Cruz, M.A.; Gonzalez-Navarro, I.; Lopez-Gallardo, G.; et al. Description of a Cohort of Type 1 Diabetes Patients: Analysis of Comorbidities, Prevalence of Complications and Risk of Hypoglycemia. J. Clin. Med. 2022 , 11 , 1039. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021 , 44 , 2438–2444. [ Google Scholar ] [ CrossRef ]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022 , 400 , 1803–1820. [ Google Scholar ] [ CrossRef ]
- Dabelea, D. The accelerating epidemic of childhood diabetes. Lancet 2009 , 373 , 1999–2000. [ Google Scholar ] [ CrossRef ]
- Li, L.; Holscher, C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review. Brain Res. Rev. 2007 , 56 , 384–402. [ Google Scholar ] [ CrossRef ]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020 , 21 , 3165. [ Google Scholar ] [ CrossRef ]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care 2023 , 46 , S158–S190. [ Google Scholar ] [ CrossRef ]
- Soyoye, D.O.; Abiodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes 2021 , 12 , 827–838. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Vithian, K.; Hurel, S. Microvascular complications: Pathophysiology and management. Clin. Med. 2010 , 10 , 505–509. [ Google Scholar ] [ CrossRef ]
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus 2023 , 15 , e45835. [ Google Scholar ] [ CrossRef ]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016 , 11 , 95–104. [ Google Scholar ] [ CrossRef ]
- Marcovecchio, M.L.; Lucantoni, M.; Chiarelli, F. Role of chronic and acute hyperglycemia in the development of diabetes complications. Diabetes Technol. Ther. 2011 , 13 , 389–394. [ Google Scholar ] [ CrossRef ]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005 , 54 , 1615–1625. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001 , 414 , 813–820. [ Google Scholar ] [ CrossRef ]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Osko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczynska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021 , 22 , 13384. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012 , 3 , 87. [ Google Scholar ] [ CrossRef ]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Physiological and Pathological Roles of Aldose Reductase. Metabolites 2021 , 11 , 655. [ Google Scholar ] [ CrossRef ]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res. 2007 , 2007 , 61038. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022 , 12 , 542. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yamagishi, S.; Nakamura, K.; Imaizumi, T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr. Diabetes Rev. 2005 , 1 , 93–106. [ Google Scholar ] [ CrossRef ]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front. Endocrinol. 2012 , 3 , 170. [ Google Scholar ] [ CrossRef ]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008 , 27 , 331–371. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kumar Pasupulati, A.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016 , 7 , 293–309. [ Google Scholar ] [ CrossRef ]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010 , 106 , 1319–1331. [ Google Scholar ] [ CrossRef ]
- Pan, D.; Xu, L.; Guo, M. The role of protein kinase C in diabetic microvascular complications. Front. Endocrinol. 2022 , 13 , 973058. [ Google Scholar ] [ CrossRef ]
- Hart, G.W.; Slawson, C.; Ramirez-Correa, G.; Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011 , 80 , 825–858. [ Google Scholar ] [ CrossRef ]
- Zochodne, D.W. Diabetic polyneuropathy: An update. Curr. Opin. Neurol. 2008 , 21 , 527–533. [ Google Scholar ] [ CrossRef ]
- Reddy, M.A.; Tak Park, J.; Natarajan, R. Epigenetic modifications in the pathogenesis of diabetic nephropathy. Semin. Nephrol. 2013 , 33 , 341–353. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brasacchio, D.; Okabe, J.; Tikellis, C.; Balcerczyk, A.; George, P.; Baker, E.K.; Calkin, A.C.; Brownlee, M.; Cooper, M.E.; El-Osta, A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 2009 , 58 , 1229–1236. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Feng, B.; Cao, Y.; Chen, S.; Chu, X.; Chu, Y.; Chakrabarti, S. miR-200b Mediates Endothelial-to-Mesenchymal Transition in Diabetic Cardiomyopathy. Diabetes 2016 , 65 , 768–779. [ Google Scholar ] [ CrossRef ]
- Putta, S.; Lanting, L.; Sun, G.; Lawson, G.; Kato, M.; Natarajan, R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2012 , 23 , 458–469. [ Google Scholar ] [ CrossRef ]
- Yan, B.; Tao, Z.F.; Li, X.M.; Zhang, H.; Yao, J.; Jiang, Q. Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014 , 55 , 941–951. [ Google Scholar ] [ CrossRef ]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010 , 107 , 810–817. [ Google Scholar ] [ CrossRef ]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020 , 127 , 553–570. [ Google Scholar ] [ CrossRef ]
- Tang, W.H.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017 , 63 , 297–306. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013 , 11 , 639–647. [ Google Scholar ] [ CrossRef ]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012 , 143 , 913–916.e7. [ Google Scholar ] [ CrossRef ]
- Shah, J.; Cheong, Z.Y.; Tan, B.; Wong, D.; Liu, X.; Chua, J. Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. Nutrients 2022 , 14 , 5021. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018 , 19 , 1816. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rodriguez, M.L.; Perez, S.; Mena-Molla, S.; Desco, M.C.; Ortega, A.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxidative Med. Cell. Longev. 2019 , 2019 , 4940825. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kupis, M.; Samelska, K.; Szaflik, J.; Skopinski, P. Novel therapies for diabetic retinopathy. Cent. Eur. J. Immunol. 2022 , 47 , 102–108. [ Google Scholar ] [ CrossRef ]
- Kastelan, S.; Oreskovic, I.; Biscan, F.; Kastelan, H.; Gverovic Antunica, A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Med. 2020 , 30 , 030502. [ Google Scholar ] [ CrossRef ]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019 , 157 , 107840. [ Google Scholar ] [ CrossRef ]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020 , 11 , 583687. [ Google Scholar ] [ CrossRef ]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011 , 30 , 343–358. [ Google Scholar ] [ CrossRef ]
- Santiago, A.R.; Boia, R.; Aires, I.D.; Ambrosio, A.F.; Fernandes, R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Front. Physiol. 2018 , 9 , 820. [ Google Scholar ] [ CrossRef ]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018 , 19 , 110. [ Google Scholar ] [ CrossRef ]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; Van Wijngaarden, P.; Martin, K.R. Diabetic retinopathy: A complex pathophysiology requiring novel therapeutic strategies. Expert. Opin. Biol. Ther. 2018 , 18 , 1257–1270. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018 , 19 , 942. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kastelan, S.; Tomic, M.; Gverovic Antunica, A.; Salopek Rabatic, J.; Ljubic, S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediat. Inflamm. 2013 , 2013 , 213130. [ Google Scholar ] [ CrossRef ]
- Gouliopoulos, N.S.; Kalogeropoulos, C.; Lavaris, A.; Rouvas, A.; Asproudis, I.; Garmpi, A.; Damaskos, C.; Garmpis, N.; Kostakis, A.; Moschos, M.M. Association of serum inflammatory markers and diabetic retinopathy: A review of literature. Eur. Rev. Med. Pharmacol. Sci. 2018 , 22 , 7113–7128. [ Google Scholar ] [ CrossRef ]
- Yang, J.; Liu, Z. Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy. Front. Endocrinol. 2022 , 13 , 816400. [ Google Scholar ] [ CrossRef ]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017 , 2 , e93751. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fu, Z.; Chen, C.T.; Cagnone, G.; Heckel, E.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Li, Q.; Britton, W.; et al. Dyslipidemia in retinal metabolic disorders. EMBO Mol. Med. 2019 , 11 , e10473. [ Google Scholar ] [ CrossRef ]
- Lu, L.; Jiang, Y.; Jaganathan, R.; Hao, Y. Current Advances in Pharmacotherapy and Technology for Diabetic Retinopathy: A Systematic Review. J. Ophthalmol. 2018 , 2018 , 1694187. [ Google Scholar ] [ CrossRef ]
- Sweeney, M.; Foldes, G. It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front. Cardiovasc. Med. 2018 , 5 , 154. [ Google Scholar ] [ CrossRef ]
- Simo-Servat, O.; Hernandez, C.; Simo, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019 , 62 , 211–217. [ Google Scholar ] [ CrossRef ]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016 , 51 , 156–186. [ Google Scholar ] [ CrossRef ]
- Maniadakis, N.; Konstantakopoulou, E. Cost Effectiveness of Treatments for Diabetic Retinopathy: A Systematic Literature Review. Pharmacoeconomics 2019 , 37 , 995–1010. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017 , 169 , 381–405. [ Google Scholar ] [ CrossRef ]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022 , 13 , 800714. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran. 2017 , 31 , 134. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New Highlights of Resveratrol: A Review of Properties against Ocular Diseases. Int. J. Mol. Sci. 2021 , 22 , 1295. [ Google Scholar ] [ CrossRef ]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell. Longev. 2022 , 2022 , 7836828. [ Google Scholar ] [ CrossRef ]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020 , 37 , 101799. [ Google Scholar ] [ CrossRef ]
- Keegan, G.; Pardhan, S.; Chichger, H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Exp. Eye Res. 2020 , 197 , 108104. [ Google Scholar ] [ CrossRef ]
- Mthembu, S.X.H.; Mazibuko-Mbeje, S.E.; Moetlediwa, M.T.; Muvhulawa, N.; Silvestri, S.; Orlando, P.; Nkambule, B.B.; Muller, C.J.F.; Ndwandwe, D.; Basson, A.K.; et al. Sulforaphane: A nutraceutical against diabetes-related complications. Pharmacol. Res. 2023 , 196 , 106918. [ Google Scholar ] [ CrossRef ]
- Tanito, M.; Masutani, H.; Kim, Y.C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Investig. Ophthalmol. Vis. Sci. 2005 , 46 , 979–987. [ Google Scholar ] [ CrossRef ]
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab. Res. Rev. 2021 , 37 , e3447. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Fernandez-Robredo, P.; Gonzalez-Zamora, J.; Recalde, S.; Bilbao-Malave, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020 , 9 , 838. [ Google Scholar ] [ CrossRef ]
- Putz, Z.; Tordai, D.; Hajdu, N.; Vagi, O.E.; Kempler, M.; Bekeffy, M.; Korei, A.E.; Istenes, I.; Horvath, V.; Stoian, A.P.; et al. Vitamin D in the Prevention and Treatment of Diabetic Neuropathy. Clin. Ther. 2022 , 44 , 813–823. [ Google Scholar ] [ CrossRef ]
- Totolici, G.; Tiutiuca, C.; Jurja, S.; Tutunaru, D.; Patrascu, A.M. The role of vitamin D in the onset and progression of diabetic retinopathy. Rom. J. Ophthalmol. 2022 , 66 , 214–218. [ Google Scholar ] [ CrossRef ]
- Jiang, Y.; Liu, L.; Curtiss, E.; Steinle, J.J. Epac1 Blocks NLRP3 Inflammasome to Reduce IL-1beta in Retinal Endothelial Cells and Mouse Retinal Vasculature. Mediat. Inflamm. 2017 , 2017 , 2860956. [ Google Scholar ] [ CrossRef ]
- Gu, J.; Geng, K.; Guo, M.; Huang, W.; Zhao, T.; Li, X.; Xu, Y.H.; Xu, Y. Targeting Pyroptosis: New Insights into the Treatment of Diabetic Microvascular Complications. Evid.-Based Complement. Alternat Med. 2022 , 2022 , 5277673. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zhang, Y.; Song, Z.; Li, X.; Xu, S.; Zhou, S.; Jin, X.; Zhang, H. Long noncoding RNA KCNQ1OT1 induces pyroptosis in diabetic corneal endothelial keratopathy. Am. J. Physiol.-Cell Physiol. 2023 , 318 , C346–C359, Erratum in Am. J. Physiol.-Cell Physiol. 2023 , 325 , C364. [ Google Scholar ] [ CrossRef ]
- Chen, Y.; He, Y.; Zhou, H. The potential role of lncRNAs in diabetes and diabetic microvascular complications. Endocr. J. 2020 , 67 , 659–668. [ Google Scholar ] [ CrossRef ]
- Zhu, X.; Wu, Y.B.; Zhou, J.; Kang, D.M. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem. Biophys. Res. Commun. 2016 , 469 , 319–325. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- You, L.; Wang, N.; Yin, D.; Wang, L.; Jin, F.; Zhu, Y.; Yuan, Q.; De, W. Downregulation of Long Noncoding RNA Meg3 Affects Insulin Synthesis and Secretion in Mouse Pancreatic Beta Cells. J. Cell. Physiol. 2016 , 231 , 852–862. [ Google Scholar ] [ CrossRef ]
- Akerman, I.; Tu, Z.; Beucher, A.; Rolando, D.M.Y.; Sauty-Colace, C.; Benazra, M.; Nakic, N.; Yang, J.; Wang, H.; Pasquali, L.; et al. Human Pancreatic beta Cell lncRNAs Control Cell-Specific Regulatory Networks. Cell Metab. 2017 , 25 , 400–411. [ Google Scholar ] [ CrossRef ]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017 , 40 , 136–154. [ Google Scholar ] [ CrossRef ]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019 , 5 , 42. [ Google Scholar ] [ CrossRef ]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal Symmetric Polyneuropathy: A Review. JAMA 2015 , 314 , 2172–2181. [ Google Scholar ] [ CrossRef ]
- Callaghan, B.C.; Kerber, K.A.; Lisabeth, L.L.; Morgenstern, L.B.; Longoria, R.; Rodgers, A.; Longwell, P.; Feldman, E.L. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. 2014 , 71 , 1143–1149. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, E.; Iverson, D.J.; Perkins, B.; Russell, J.W.; et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011 , 76 , 1758–1765. [ Google Scholar ] [ CrossRef ]
- Yang, H.; Sloan, G.; Ye, Y.; Wang, S.; Duan, B.; Tesfaye, S.; Gao, L. New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front. Endocrinol. 2019 , 10 , 929. [ Google Scholar ] [ CrossRef ]
- Ling, E.; Lepow, B.; Zhou, H.; Enriquez, A.; Mullen, A.; Najafi, B. The impact of diabetic foot ulcers and unilateral offloading footwear on gait in people with diabetes. Clin. Biomech. 2020 , 73 , 157–161. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jeyam, A.; McGurnaghan, S.J.; Blackbourn, L.A.K.; McKnight, J.M.; Green, F.; Collier, A.; McKeigue, P.M.; Colhoun, H.M.; Investigators, S.B. Diabetic Neuropathy Is a Substantial Burden in People With Type 1 Diabetes and Is Strongly Associated With Socioeconomic Disadvantage: A Population-Representative Study From Scotland. Diabetes Care 2020 , 43 , 734–742. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Miyashita, A.; Kobayashi, M.; Yokota, T.; Zochodne, D.W. Diabetic Polyneuropathy: New Strategies to Target Sensory Neurons in Dorsal Root Ganglia. Int. J. Mol. Sci. 2023 , 24 , 5977. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ishibashi, F.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. Improvement in Neuropathy Outcomes with Normalizing HbA(1c) in Patients with Type 2 Diabetes. Diabetes Care 2019 , 42 , 110–118. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pop-Busui, R.; Lu, J.; Brooks, M.M.; Albert, S.; Althouse, A.D.; Escobedo, J.; Green, J.; Palumbo, P.; Perkins, B.A.; Fred Whitehouse, F.; et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 2013 , 36 , 3208–3215. [ Google Scholar ] [ CrossRef ]
- Hébert, H.L.; Veluchamy, A.; Torrance, N.; Smith, B.H. Risk factors for neuropathic pain in diabetes mellitus. Pain 2017 , 158 , 560–568. [ Google Scholar ] [ CrossRef ]
- Raputova, J.; Srotova, I.; Vlckova, E.; Sommer, C.; Üçeyler, N.; Birklein, F.; Rittner, H.L.; Rebhorn, C.; Adamova, B.; Kovalova, I.; et al. Sensory phenotype and risk factors for painful diabetic neuropathy: A cross-sectional observational study. Pain 2017 , 158 , 2340–2353. [ Google Scholar ] [ CrossRef ]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017 , 93 , 1296–1313. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, H.; Kim, J.J.; Yoon, Y.S. Emerging therapy for diabetic neuropathy: Cell therapy targeting vessels and nerves. Endocr. Metab. Immune Disord. Drug Targets 2012 , 12 , 168–178. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Komai, H. Vascular Disease and Diabetes. Ann. Vasc. Dis. 2024 , 17 , 109–113. [ Google Scholar ] [ CrossRef ]
- Zochodne, D.W. Diabetes and the plasticity of sensory neurons. Neurosci. Lett. 2015 , 596 , 60–65. [ Google Scholar ] [ CrossRef ]
- Sugimoto, K.; Murakawa, Y.; Sima, A.A. Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J. Peripher. Nerv. Syst. 2002 , 7 , 44–53. [ Google Scholar ] [ CrossRef ]
- Zochodne, D.W. Diabetic neuropathies: Features and mechanisms. Brain Pathol. 1999 , 9 , 369–391. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pop-Busui, R.; Ang, L.; Boulton, A.J.M.; Feldman, E.L.; Marcus, R.L.; Mizokami-Stout, K.; Singleton, J.R.; Ziegler, D. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy ; American Diabetes Association: Arlington, VA, USA, 2022. [ Google Scholar ]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 2012 , 6 , CD007543. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Page, N.; Deluca, J.; Crowell, K. Clinical inquiry: What medications are best for diabetic neuropathic pain? J. Fam. Pract. 2012 , 61 , 691–693. [ Google Scholar ]
- Akter, S.; Choubey, M.; Mohib, M.M.; Arbee, S.; Sagor, M.A.T.; Mohiuddin, M.S. Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sci. 2023 , 13 , 255. [ Google Scholar ] [ CrossRef ]
- Voute, M.; Morel, V.; Pickering, G. Topical Lidocaine for Chronic Pain Treatment. Drug Des. Devel Ther. 2021 , 15 , 4091–4103. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016 , 17 , 917. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cernea, S.; Raz, I. Management of diabetic neuropathy. Metabolism 2021 , 123 , 154867. [ Google Scholar ] [ CrossRef ]
- Singh, B.; Singh, V.; Krishnan, A.; Koshy, K.; Martinez, J.A.; Cheng, C.; Almquist, C.; Zochodne, D.W. Regeneration of diabetic axons is enhanced by selective knockdown of the PTEN gene. Brain 2014 , 137 , 1051–1067. [ Google Scholar ] [ CrossRef ]
- de la Hoz, C.L.; Cheng, C.; Fernyhough, P.; Zochodne, D.W. A model of chronic diabetic polyneuropathy: Benefits from intranasal insulin are modified by sex and RAGE deletion. Am. J. Physiol. Endocrinol. Metab. 2017 , 312 , E407–E419. [ Google Scholar ] [ CrossRef ]
- Kobayashi, M.; Chandrasekhar, A.; Cheng, C.; Martinez, J.A.; Ng, H.; de la Hoz, C.; Zochodne, D.W. Diabetic polyneuropathy, sensory neurons, nuclear structure and spliceosome alterations: A role for CWC22. Dis. Model. Mech. 2017 , 10 , 215–224. [ Google Scholar ] [ CrossRef ]
- Chandrasekhar, A.; Komirishetty, P.; Areti, A.; Krishnan, A.; Zochodne, D.W. Dual Specificity Phosphatases Support Axon Plasticity and Viability. Mol. Neurobiol. 2021 , 58 , 391–407. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kaburagi, H.; Nagata, T.; Enomoto, M.; Hirai, T.; Ohyagi, M.; Ihara, K.; Yoshida-Tanaka, K.; Ebihara, S.; Asada, K.; Yokoyama, H.; et al. Systemic DNA/RNA heteroduplex oligonucleotide administration for regulating the gene expression of dorsal root ganglion and sciatic nerve. Mol. Ther. Nucleic Acids 2022 , 28 , 910–919. [ Google Scholar ] [ CrossRef ]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D.A.; Duttagupta, R.; Willingham, A.T.; Stadler, P.F.; Hertel, J.; Hackermuller, J.; Hofacker, I.L.; et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007 , 316 , 1484–1488. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yao, J.; Wang, X.Q.; Li, Y.J.; Shan, K.; Yang, H.; Wang, Y.N.; Yao, M.D.; Liu, C.; Li, X.M.; Shen, Y.; et al. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol. Med. 2022 , 14 , e16660. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, G.; Li, X.; Li, M.; Zhang, Z. Long non-coding RNA MALAT1 promotes the proliferation and migration of Schwann cells by elevating BDNF through sponging miR-129-5p. Exp. Cell Res. 2020 , 390 , 111937. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Radhakrishnan, R.; Kowluru, R.A. Long Noncoding RNA MALAT1 and Regulation of the Antioxidant Defense System in Diabetic Retinopathy. Diabetes 2021 , 70 , 227–239. [ Google Scholar ] [ CrossRef ]
- Arunkumar, G. LncRNAs: The good, the bad, and the unknown. Biochem. Cell Biol. 2024 , 102 , 9–27. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hussein, R.M. Long non-coding RNAs: The hidden players in diabetes mellitus-related complications. Diabetes Metab. Syndr. 2023 , 17 , 102872. [ Google Scholar ] [ CrossRef ]
- Ebrahimi, A.; Ahmadi, H.; Pourfraidon Ghasrodashti, Z.; Tanide, N.; Shahriarirad, R.; Erfani, A.; Ranjbar, K.; Ashkani-Esfahani, S. Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review. Bosn. J. Basic. Med. Sci. 2021 , 21 , 672–701. [ Google Scholar ] [ CrossRef ]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020 , 61 , 449–459. [ Google Scholar ] [ CrossRef ]
- Streckmann, F.; Balke, M.; Cavaletti, G.; Toscanelli, A.; Bloch, W.; Décard, B.F.; Lehmann, H.C.; Faude, O. Exercise and Neuropathy: Systematic Review with Meta-Analysis. Sports Med. 2022 , 52 , 1043–1065. [ Google Scholar ] [ CrossRef ]
- Dagar, N.; Das, P.; Bisht, P.; Taraphdar, A.K.; Velayutham, R.; Arumugam, S. Diabetic nephropathy: A twisted thread to unravel. Life Sci. 2021 , 278 , 119635. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018 , 71 , 884–895. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Papadopoulou-Marketou, N.; Paschou, S.A.; Marketos, N.; Adamidi, S.; Adamidis, S.; Kanaka-Gantenbein, C. Diabetic nephropathy in type 1 diabetes. Minerva Med. 2018 , 109 , 218–228. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Koch, E.A.T.; Nakhoul, R.; Nakhoul, F.; Nakhoul, N. Autophagy in diabetic nephropathy: A review. Int. Urol. Nephrol. 2020 , 52 , 1705–1712. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ying, A.F.; Tang, T.Y.; Jin, A.; Chong, T.T.; Hausenloy, D.J.; Koh, W.P. Diabetes and other vascular risk factors in association with the risk of lower extremity amputation in chronic limb-threatening ischemia: A prospective cohort study. Cardiovasc. Diabetol. 2022 , 21 , 7. [ Google Scholar ] [ CrossRef ]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018 , 107 , 306–328. [ Google Scholar ] [ CrossRef ]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017 , 28 , 1023–1039. [ Google Scholar ] [ CrossRef ]
- Calle, P.; Hotter, G. Macrophage Phenotype and Fibrosis in Diabetic Nephropathy. Int. J. Mol. Sci. 2020 , 21 , 2806. [ Google Scholar ] [ CrossRef ]
- Dalla Vestra, M.; Mussap, M.; Gallina, P.; Bruseghin, M.; Cernigoi, A.M.; Saller, A.; Plebani, M.; Fioretto, P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J. Am. Soc. Nephrol. 2005 , 16 (Suppl. S1), S78–S82. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013 , 124 , 139–152. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lim, A. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renovasc Dis. 2014 , 7 , 361–381. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023 , 107 , 689–705. [ Google Scholar ] [ CrossRef ]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front. Endocrinol. 2021 , 12 , 649525. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural antioxidants in the treatment and prevention of diabetic nephropathy; a potential approach that warrants clinical trials. Redox Rep. 2017 , 22 , 99–118. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jia, Q.; Yang, R.; Liu, X.F.; Ma, S.F.; Wang, L. Genistein attenuates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2019 , 19 , 423–431. [ Google Scholar ] [ CrossRef ]
- Lachin, J.M.; Nathan, D.M.; Group, D.E.R. Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021 , 44 , 2216–2224. [ Google Scholar ] [ CrossRef ]
- Bebu, I.; Braffett, B.H.; Schade, D.; Sivitz, W.; Malone, J.I.; Pop-Busui, R.; Lorenzi, G.M.; Lee, P.; Trapani, V.R.; Wallia, A.; et al. An Observational Study of the Equivalence of Age and Duration of Diabetes to Glycemic Control Relative to the Risk of Complications in the Combined Cohorts of the DCCT/EDIC Study. Diabetes Care 2020 , 43 , 2478–2484. [ Google Scholar ] [ CrossRef ]
- Ahmad, J. Management of diabetic nephropathy: Recent progress and future perspective. Diabetes Metab. Syndr. 2015 , 9 , 343–358. [ Google Scholar ] [ CrossRef ]
- Cha, A.S.; Chen, Y.; Fazioli, K.; Rivara, M.B.; Devine, E.B. Microvascular Benefits of New Antidiabetic Agents: A Systematic Review and Network Meta-Analysis of Kidney Outcomes. J. Clin. Endocrinol. Metab. 2021 , 106 , 1225–1234. [ Google Scholar ] [ CrossRef ]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022 , 12 , 1227. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.M.; Jha, J.C. Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes. Biology 2020 , 10 , 18. [ Google Scholar ] [ CrossRef ]
- Eltablawy, N.; Ashour, H.; Rashed, L.A.; Hamza, W.M. Vitamin D protection from rat diabetic nephropathy is partly mediated through Klotho expression and renin-angiotensin inhibition. Arch. Physiol. Biochem. 2018 , 124 , 461–467. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, T.K.; Kam, K.K.; Yan, B.P.; Lam, Y.Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: Current status. Br. J. Pharmacol. 2010 , 160 , 1273–1292. [ Google Scholar ] [ CrossRef ]
- Mazzieri, A.; Porcellati, F.; Timio, F.; Reboldi, G. Molecular Targets of Novel Therapeutics for Diabetic Kidney Disease: A New Era of Nephroprotection. Int. J. Mol. Sci. 2024 , 25 , 3969. [ Google Scholar ] [ CrossRef ]
- Zhang, Y.; Sun, Y.; Peng, R.; Liu, H.; He, W.; Zhang, L.; Peng, H.; Zhang, Z. The Long Noncoding RNA 150Rik Promotes Mesangial Cell Proliferation via miR-451/IGF1R/p38 MAPK Signaling in Diabetic Nephropathy. Cell Physiol. Biochem. 2018 , 51 , 1410–1428. [ Google Scholar ] [ CrossRef ]
- Huang, S.; Xu, Y.; Ge, X.; Xu, B.; Peng, W.; Jiang, X.; Shen, L.; Xia, L. Long noncoding RNA NEAT1 accelerates the proliferation and fibrosis in diabetic nephropathy through activating Akt/mTOR signaling pathway. J. Cell Physiol. 2019 , 234 , 11200–11207. [ Google Scholar ] [ CrossRef ]
- Mao, Q.; Chen, C.; Liang, H.; Zhong, S.; Cheng, X.; Li, L. Astragaloside IV inhibits excessive mesangial cell proliferation and renal fibrosis caused by diabetic nephropathy via modulation of the TGF-beta1/Smad/miR-192 signaling pathway. Exp. Ther. Med. 2019 , 18 , 3053–3061. [ Google Scholar ] [ CrossRef ]
- Hanson, R.L.; Craig, D.W.; Millis, M.P.; Yeatts, K.A.; Kobes, S.; Pearson, J.V.; Lee, A.M.; Knowler, W.C.; Nelson, R.G.; Wolford, J.K. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes 2007 , 56 , 975–983. [ Google Scholar ] [ CrossRef ]
- Lin, B.; Ma, Y.Y.; Wang, J.W. Nano-Technological Approaches for Targeting Kidney Diseases With Focus on Diabetic Nephropathy: Recent Progress, and Future Perspectives. Front. Bioeng. Biotechnol. 2022 , 10 , 870049. [ Google Scholar ] [ CrossRef ]
- American Diabetes, A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019 , 42 , S90–S102. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ramos, A.M.; Fernandez-Fernandez, B.; Perez-Gomez, M.V.; Carriazo Julio, S.M.; Sanchez-Nino, M.D.; Sanz, A.; Ruiz-Ortega, M.; Ortiz, A. Design and optimization strategies for the development of new drugs that treat chronic kidney disease. Expert. Opin. Drug Discov. 2020 , 15 , 101–115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020 , 12 , 29. [ Google Scholar ] [ CrossRef ]
- Rubin, J.D.; Barry, M.A. Improving Molecular Therapy in the Kidney. Mol. Diagn. Ther. 2020 , 24 , 375–396. [ Google Scholar ] [ CrossRef ]
- van Alem, C.M.A.; Boonstra, M.; Prins, J.; Bezhaeva, T.; van Essen, M.F.; Ruben, J.M.; Vahrmeijer, A.L.; van der Veer, E.P.; de Fijter, J.W.; Reinders, M.E.; et al. Local delivery of liposomal prednisolone leads to an anti-inflammatory profile in renal ischaemia-reperfusion injury in the rat. Nephrol. Dial. Transplant. 2018 , 33 , 44–53. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Tandon, N.; Ali, M.K.; Narayan, K.M. Pharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: Implications of the results of recent clinical trials in type 2 diabetes. Am. J. Cardiovasc. Drugs 2012 , 12 , 7–22. [ Google Scholar ] [ CrossRef ]
- Milligan, S. Combination therapy for the improvement of long-term macrovascular and microvascular outcomes in type 2 diabetes: Rationale and evidence for early initiation. J. Diabetes Complicat. 2016 , 30 , 1177–1185. [ Google Scholar ] [ CrossRef ]
- Tilinca, M.C.; Tiuca, R.A.; Tilea, I.; Varga, A. The SGLT-2 Inhibitors in Personalized Therapy of Diabetes Mellitus Patients. J. Pers. Med. 2021 , 11 , 1249. [ Google Scholar ] [ CrossRef ]
- Hussain, S.; Chowdhury, T.A. The Impact of Comorbidities on the Pharmacological Management of Type 2 Diabetes Mellitus. Drugs 2019 , 79 , 231–242. [ Google Scholar ] [ CrossRef ]
- Tsapas, A.; Avgerinos, I.; Karagiannis, T.; Malandris, K.; Manolopoulos, A.; Andreadis, P.; Liakos, A.; Matthews, D.R.; Bekiari, E. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2020 , 173 , 278–286. [ Google Scholar ] [ CrossRef ]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into the Use of Liraglutide-Impact on Cardiovascular Risk and Microvascular Outcomes. Biomedicines 2023 , 11 , 1159. [ Google Scholar ] [ CrossRef ] [ PubMed ]
Click here to enlarge figure
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Yapislar, H.; Gurler, E.B. Management of Microcomplications of Diabetes Mellitus: Challenges, Current Trends, and Future Perspectives in Treatment. Biomedicines 2024 , 12 , 1958. https://doi.org/10.3390/biomedicines12091958
Yapislar H, Gurler EB. Management of Microcomplications of Diabetes Mellitus: Challenges, Current Trends, and Future Perspectives in Treatment. Biomedicines . 2024; 12(9):1958. https://doi.org/10.3390/biomedicines12091958
Yapislar, Hande, and Esra Bihter Gurler. 2024. "Management of Microcomplications of Diabetes Mellitus: Challenges, Current Trends, and Future Perspectives in Treatment" Biomedicines 12, no. 9: 1958. https://doi.org/10.3390/biomedicines12091958
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Bedtime dosing of glyburide and the treatment of type II diabetes mellitus
Research output : Contribution to journal › Article › peer-review
| Original language | English |
|---|---|
| Pages (from-to) | 234-238 |
| Number of pages | 5 |
| Journal | |
| Volume | 308 |
| Issue number | 4 |
| DOIs | |
| State | Published - 1994 |
| Externally published | Yes |
ASJC Scopus Subject Areas
- General Medicine
Access to Document
- 10.1097/00000441-199410000-00004
Other files and links
- Link to publication in Scopus
- Link to the citations in Scopus
T1 - Bedtime dosing of glyburide and the treatment of type II diabetes mellitus
AU - Hennessey, J. V.
AU - Bustamante, M. A.
AU - Teter, M. L.
AU - Markert, R. J.
AU - McDonald, S. D.
UR - http://www.scopus.com/inward/record.url?scp=0028072544&partnerID=8YFLogxK
UR - http://www.scopus.com/inward/citedby.url?scp=0028072544&partnerID=8YFLogxK
U2 - 10.1097/00000441-199410000-00004
DO - 10.1097/00000441-199410000-00004
M3 - Article
C2 - 7942982
AN - SCOPUS:0028072544
SN - 0002-9629
JO - American Journal of the Medical Sciences
JF - American Journal of the Medical Sciences
- Open access
- Published: 29 August 2024
Associations between type 2 diabetes mellitus and risk of falls among community-dwelling elderly people in Guangzhou, China: a prospective cohort study
- Wei-Quan Lin 1 , 2 na1 ,
- Ying-Xin Liao 3 na1 ,
- Jing-Ya Wang 4 ,
- Li-Ying Luo 1 ,
- Le-Xin Yuan 5 ,
- Si-Yu Sun 6 ,
- Min-Ying Sun 1 ,
- Chang Wang 1 ,
- Qin Zhou 1 ,
- Xiang-Yi Liu 1 &
- Hui Liu 1
BMC Geriatrics volume 24 , Article number: 717 ( 2024 ) Cite this article
Metrics details
Several studies have demonstrated that older adults with type 2 diabetes mellitus (T2DM) have a higher risk of falls compared to those without T2DM, which may lead to disability and a lower quality of life. While, limited prospective studies have quantified the associations in southern China. We conducted a longitudinal cohort study to quantify the associations between T2DM and falls and investigate the risk factors of falls among community-dwelling elderly people in Guangzhou, China.
The population-based study included 8800 residents aged 65 and over in 11 counties of Guangzhou at baseline in 2020 and then prospectively followed up through 2022. Of 6169 participants had complete follow-up and were included in the present study. A fall event was identified by self-reported. The Cox regression was applied to quantify the associations between T2DM and falls, and hazard ratios (HRs) were calculated to the factors associated with falls among participants.
The median follow-up time for participants was 2.42 years. During the follow-up period, the incidence of falls among all participants was 21.96%. After adjusting for covariates in Cox regression models, T2DM remained a significant risk factor for falls, with HR of 1.781 (95% CI: 1.600-1.983) in the unadjusted covariates model and 1.757 (1.577–1.957) in the adjusted covariates model. Female (1.286, 1.136–1.457), older age (≥ 80: 1.448, 1.214–1.729), single marital status (1.239, 1.039–1.477), lower education level (primary school and below: 1.619, 1.004–1.361), hypertension (1.149, 1.026–1.286) and stroke (1.619, 1.176–2.228) were associated with a higher risk of falls, whereas everyday physical exercise (0.793, 0.686–0.918) was associated with a lower risk of falls.
Falls are common, with risks between T2DM and falls quantified and several factors investigated in the longitudinal cohort study among community-dwelling elderly people in Guangzhou, China. Targeted action on the risk factors may reduce the burden of falls in elderly people with T2DM in the future.
Peer Review reports
As a result of increasing longevity, unprecedented numbers of individuals are reaching older ages [ 1 ]. According to the Seventh National Populations Census, of the national population, 1.9 billion individuals, accounting for 13.50%, are aged 65 and over. Of Guangzhou’s population, 1.4 million persons, accounting for 7.82%, are aged 65 and over [ 2 ]. Consequently, a great challenge for the aging population arises, and chronic diseases, which place a heavy burden on society, are expected to increase simultaneously. Diabetes, of which about 90% are type 2 diabetes mellitus (T2DM), is one of the substantial public health issues worldwide [ 2 , 3 ]. As the country with the largest number of people with diabetes, China is expected to reach approximately 174 million diabetic patients by 2045 [ 4 ], of which at least 20% are older adults, seriously threatening the quality of life and life expectancy of elderly patients [ 5 , 6 , 7 ].
Falls are another threat to the elderly, as approximately 30% of people over 65 have at least one fall annually around the world [ 8 , 9 ]. Data from a Chinese longitudinal healthy longevity survey in 2018, the incidence of falls among community-dwelling elderly people is 22.49% in China [ 10 ], and the incidence of falls varied between 10.7% and 22.49% [ 8 , 10 , 11 , 12 , 13 , 14 ]. Falls do have negative effects on the quality of life in old age, leading to a drastic increase in mortality and disability-adjusted life years among elderly people over the past few decades [ 15 , 16 ]. According to the Global Burden of Disease Study 2019 and 2021, falls have been a leading cause of disability, possibly due to fractured bones, which is one of the most costly injuries in China [ 6 , 17 ].
Many studies have found that the risk of falls is higher among people with diabetes comparing those without diabetes, and insulin treatment, retinopathy, neuropathy, hypoglycemia, and cognitive impairment caused by diabetes may contribute to fall events [ 14 , 17 , 18 , 19 ]. Previous studies have revealed that reduced cognitive function is a mediator of the relationship between diabetes and falls [ 20 , 21 ]. However, some studies have different viewpoints [ 22 , 23 ]. A 5-year follow-up study from Australia has revealed that the incidence of falls is similar in men with and without T2DM after adjusting for significant risk factors [ 22 ]. A cohort study from the China Health and Retirement Longitudinal Study has figured that diseases of the metabolic system, such as diabetes and dyslipidemia, are not associated with falls among middle-aged and older [ 23 ]. Therefore, more longitudinal studies are needed to explore and quantify the risk between T2DM and falls.
Meanwhile, several risk factors for falls have been investigated in previous studies, indicating that age, gender, meteorological factors, visual impairment, and functional ability are the primary risk factors for falls [ 14 , 15 , 23 , 24 ]. However, there is limited strong evidence from cohort studies to understudy those risk factors in China, particularly in Guangzhou, a city with a substantial elderly population.
Therefore, we conducted a prospective cohort study and aimed to: (1) assess the incidence of falls, (2) quantify associations between T2DM and falls, and (3) investigate the risk factors of falls among community-dwelling elderly people in Guangzhou, China.
Study design and participants
The Guangzhou Falls and Health Status Tracking Cohort is a longitudinal, population-based study in which participants were recruited from community health centers in 11 counties of Guangzhou, China. In brief, the Guangzhou Falls and Health Status Tracking Cohort study is designed to explore the associations between lifestyle factors, chronic diseases on falls and health outcomes. The present study is a part of the Guangzhou Falls and Health Status Tracking Cohort. Ethical approval for this study was obtained (GZCDC-ECHR-2023P0061).
8800 participants aged 65 and over from 11 counties of Guangzhou were enrolled in the cohort in 2020. Participants who refused to participate, had missing responses, and had incomplete data during the follow-up period were excluded. 6169 community-dwelling elderly people with complete data were included in the final analysis from January 2020 to December 2022 (Fig. 1 ). Among them, there were 1970 participants with T2DM and 4199 participants without T2DM at baseline, and 588 cases and 767 cases, respectively, were reported as fall events during the follow-up period. Additionally, participants in the non-exposure group were also terminated if they were newly diagnosed with T2DM during the follow-up period (Fig. 1 ).
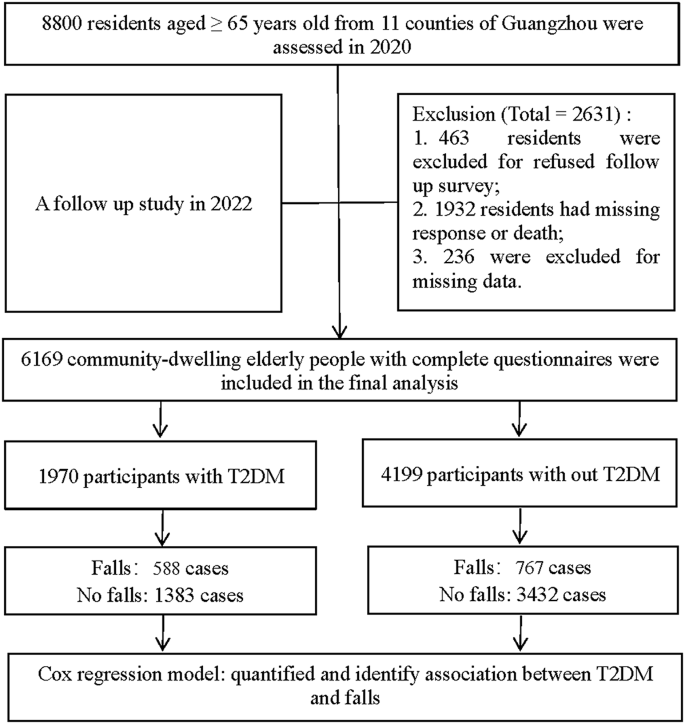
Flow diagram of study participants in Guangzhou, China
Baseline data collection and definitions
Self-designed baseline questionnaire (Supplementary material 1 ) was used to collect information on demographic characteristics, lifestyle behaviors, and chronic diseases by well trained clinic staff following standard procedures.
At baseline, demographic variables were assessed by questionnaire, including age, gender, ethnic groups, marital status, and education. Age was categorized into four groups as follows: 65–69, 70–74, 75–79, and ≥ 80 years. Marital status was categorized as married and single, while single included unmarried, divorced, or widowed. Education was categorized according to the highest level of school the participants completed: primary school and below, secondary school, and college and above. Lifestyle behaviors included cigarette smoking, alcohol drinking, and physical exercise. Chronic diseases, which mainly included hypertension, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD), stroke, and T2DM, were diagnosed by physicians or medical examination at baseline.
In this study, T2DM was defined as anyone of the following standards: those who were previously diagnosed with T2DM by physicians or taking glucose-lowering agents; baseline medical examinations of elevated fasting plasma glucose level (≥ 7.0 mmol/L); 2-hour oral glucose tolerance test or random blood glucose (≥ 11.1mmol/L) [ 25 ].
Follow-up and outcomes assessment
Following up through 2022, physicians at community health centers of Guangzhou conducted health examinations and face-to-face surveys (Supplementary material 1 ) to collect information on falls and the health status of participants. When participants refused to undergo health examinations, physicians conducted telephonic interviews to gather information on their falls and health status. A fall event was defined as an unexpected, unintentional change in position that caused an individual to remain at a lower level [ 26 ]. Falls were assessed during the 2022 follow-up by asking, “Have you ever fallen during the follow-up period?” and “When have you fallen?”
To mitigate the possibility of elderly individuals forgetting about fall events, participants’ occurrences of falls were reported by the Guangzhou Injury Monitoring System during the follow-up period, which was also acknowledged after verifying the accuracy with the participants.
Statistical analysis
Statistical analysis was performed using R (version 4.0.0) and SPSS (version 25.0, SPSS Inc., Chicago, IL, USA). Baseline characteristics of the participants were summarized as frequency and differences in the incidence of falls, which were analyzed by Chi-square analysis for categorical measures. We used Cox regression models, with the time at baseline as the start of follow-up, to investigate the associations of baseline variables, T2DM, and their combination with the risk of fall incidence. Model 1 was adjusted for gender and age groups. Model 2 was adjusted as in Model 1 and for ethnic groups, marital status, education, cigarette smoking, alcohol drinking, physical exercise, and other four chronic diseases. Regarding the use of Cox regression models, we adhered to the methodological requirements and tested the proportional hazards assumption using the Schoenfeld residuals technique, and no violations were observed. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated to quantify associations. To assess the robustness and consistency of our findings, we also performed subgroup analyses stratified by age groups and gender and employed competing Cox regression as sensitivity analyses.
All results were considered statistically significant at P < 0.05.
Baseline characteristics
6169 community-dwelling elderly people with complete data were included in the final analysis, of whom the mean age was 72.53 ± 5.96, and 3138 (50.87%) were female (Table 1 ). Most of the participants (99.72%) were Han, and 91.81% were married. Referring to education, 1954 (31.67%) participants completed primary school and below, 3010 participants (48.79%) completed secondary school, and the remaining 19.53% completed college and above. Our sample was composed of 12.87% cigarette smokers and 12.76% alcohol drinkers. Besides, half of the participants exercised every day, while the others exercised sometimes or even never. At baseline, out of all the participants, 3602 had hypertension (58.39%), 513 had CHD (8.32%), 37 had COPD (0.60%), 121 had suffered a stroke (1.96%), and 1970 had T2DM (31.93%).
Incidence of falls
The median follow-up time was 885 days, namely 2.42 years. All participants reported no fall events at baseline medical examinations in 2020, and during the follow-up period, 1355 participants (21.96%, 95%CI: 20.93-22.99%) reported having experienced at least one fall. Chi-square analysis (Table 1 ) showed that differences between falls and gender, age groups, marital status, education, physical exercise, hypertension, stroke, and T2DM were found (all P < 0.01), while no significant differences were observed among ethnic groups, cigarette smoking, alcohol drinking, CHD, COPD (all P > 0.05).
The incidence trend of falls by gender and age groups is shown in Fig. 2 . An absolute growth in the incidence of falls occurred in both males and females as they aged. Whereas it was intuitively seen that females experienced a higher incidence of falls at first, and the incidence of the two genders became similar among the oldest old.

Incidence of falls by age groups and gender among community-dwelling elderly people
Associations between T2DM and falls, and factors of falls
According to unadjusted covariates in Cox regression Model 0 (Table 2 ; Figs. 3 a, and Fig. 4 ), elderly people with T2DM were at a higher risk for falls (HR: 1.781, 1.600-1.983). After adjusting for gender and age groups in Model 1 (Figs. 3 b and Fig. 4 ), elderly people with T2DM remained at a higher risk for falls (1.756, 1.576–1.955). Adjusting for a combination of gender, age groups, ethnic groups, marital status, education, smoking, alcohol drinking, physical exercise, and four chronic diseases in Model 2 (Table 2 ; Figs. 3 c, and Fig. 4 ), the results remained similar, as the risk of falls in the elderly with T2DM was significantly higher (1.757, 1.577–1.957). Cumulative hazard curves of falls corresponding to all models for T2DM are presented in Fig. 3 .

Cumulative hazard for follow-up time to falls form Cox regression models. a Unadjusted covariates. b Adjusted for gender and age groups. c Adjusted for gender, age groups, ethnic groups, marital status, education, cigarette smoking, alcohol drinking, physical exercise, hypertension, coronary heart disease, chronic obstructive pulmonary disease and stroke

Associations between T2DM and falls, and subgroup analysis of form Cox regression models. HR = Hazard ratio, 95%CI = 95% confidence interval. Subgroup analysis was adjusted for all covariates. Adjusted for all covariates: adjusted for gender, age groups, ethnic groups, marital status, education, cigarette smoking, alcohol drinking, physical exercise, hypertension, coronary heart disease, chronic obstructive pulmonary disease and stroke
The stable and consistent results were found in sensitivity analysis, and Cox regression in subgroups in gender and age groups found that those with T2DM had an elevated risk for falls (HR ranges: 1.608–1.845, all P < 0.001), similarly (Fig. 4 ). The results of the competing Cox regression were similar to those of the previous Cox regression presented in Table 2 , indicating that participants with T2DM had a higher risk for falls (1.757, 1.577–1.957).
Meanwhile, results (Table 2 ) of Cox regression Model 2 showed that female (1.286, 1.136–1.457), older age (≥ 80: 1.448, 1.214–1.729), single marital status (1.239, 1.039–1.477), lower education level (primary school and below: 1.619, 1.004–1.361), hypertension (1.149, 1.026–1.286) and stroke (1.619, 1.176–2.228) were associated with a higher risk of falls, while everyday physical exercise was associated with a decreased risk of falls (0.793, 0.686–0.918) among community-dwelling elderly people in Guangzhou, China.
During the 2.42-year median follow-up time, we discovered that the incidence of falls was 21.96% among community-dwelling elderly people in Guangzhou, southern China. This result is consistent with findings from previous studies, which reported rates of 20.65% in older adult samples in Shantou City, southern China [ 13 ], and 22.49% in Chinese longitudinal healthy longevity survey [ 10 ]. Interestingly, several studies found a lower incidence of falls in southern China specifically, such as 10.7% in Shenzhen City [ 14 ], 15.41% in Guangzhou City [ 12 ], and 11.9% in Guangdong province [ 8 , 27 ], where elderly people experienced at least one fall in the past 12 months. Even in Chinese Longitudinal Survey samples, 14.0% and 17.0% of urban and rural community-dwelling older adults experienced at least one fall in the previous 12 months [ 26 ]. The lower fall incidence was also observed in Western Europe, where 13.84% of older adults sought medical treatment for fall injuries in 2017 [ 15 ]. The differences in the definition of falls may contribute to this phenomenon, such as the difference in falls occurring in the past 12 months and during 2.42-year median follow-up time. However, 36% of older men with T2DM had a fall over 2 years in Australia [ 22 ]. The higher incidence of falls could be attributed to age differences (average age: 77.0 years vs. 72.5 years). Nonetheless, falls are an important public health problem, and it is crucial to urgently investigate risk factors and implement interventions among elderly individuals living in the community in Guangzhou, China.
Our study found that after adjusting for covariates in Cox regression models, T2DM remained a risk factor for falls (Model 1: 1.756, 1.576–1.955. Model 2: 1.757, 1.577–1.957) among community-dwelling elderly people in Guangzhou, China, which was similar to previous study (HR:1.48, 1.12–1.95) [ 19 ]. Chronic diseases that increase the risk of falls have been examined in previous studies [ 23 , 28 ]. Stroke is considered a major diabetes-related complication [ 7 , 29 ], and T2DM is another independent risk factor of falls [ 29 ]. On the one hand, poor balance and weakened muscles due to diabetes contribute to the occurrence for falls. On the other hand, falls associated with diabetes are considered to increase the risk of fractures [ 17 , 30 ]. Older adults with T2DM perform worse on physical function due to a range of long-term complications, such as poor balance, poor grip strength, and poor gait performance, which may explain why participants with diagnosed T2DM are more likely to fall [ 18 , 21 ]. Interestingly, recent evidence has shown that diabetes incidence is decreasing in several high-income countries [ 4 , 31 ], suggesting the high quality and specific interventions implemented in these countries to reduce the number of T2DM patients and potentially prevent falls among T2DM patients. However, it is important to consider any potential confounding factors when analyzing the relationship between T2DM and falls, as diabetic vascular diseases have also been associated with long-term fall incidence [ 32 , 33 , 34 ].
In this study, being female was identified as a risk factor for falls, with the incidence rate 4.91% higher in females than in males. However, this finding is less pronounced than the 6.29-10.98% higher incidence of falls in females compared to males as reported in previous studies [ 12 , 27 , 35 ]. The observed inconsistencies in findings can be ascribed to the diversity in research methodologies, regional variations, and the specific attributes of the study cohorts, including their size and demographic profiles. Additionally, females were associated with higher HRs for falls in all models, which may be attributed to postmenopausal osteoporosis in older women. This condition can lead to a decrease in muscle strength and sensory capacity, ultimately resulting in body imbalance and gait instability [ 36 ]. An overview of falls on the NHS website has also explained that the increased risk of falls in older female is linked to osteoporosis caused by hormonal changes during menopause [ 8 ]. However, the incidence of falls of the two genders became similar among the oldest old. The finding was consistent with a previous study which has acknowledged that the incidence of falls is similar in older male and female [ 16 ].
In this community-based prospective cohort study, aging was a recognized risk factor for falls [ 35 ]. Our study was consistent with the previous study that people aged 80 and older are generally at the highest risk of falls [ 16 ]. We also found that single marital status (unmarried, divorced, or widowed) was a risk factor for falls. Close cooperation may help reduce unintentional falls in those older and living alone [ 9 , 37 , 38 ]. Furthermore, a close relationship plays an important role in encouraging those who are depressed or fearful of falling, as these emotions have been linked in earlier research to fall [ 39 , 40 , 41 ]. Earlier studies have shown that falls are very common in people with cognitive impairments, which also suggests the need for more care in close relationships [ 42 , 43 ].
Previous studies have reported that a lower education background was considered one of the risk factors for falls and fall-related injuries [ 37 , 44 ]. People with better education are more likely to emphasize health and are more willing to put safety education into practice. This might also explain why, with more highly qualified personnel concentrated in the urban areas which are political, economic, and educational centers, there is a higher occurrence of falls in the suburban areas, where there are fewer highly qualified personnel.
Non-smoking has been considered a protective factor against falls in older age [ 9 ]. However, in this study, we noted that cigarette smoking and alcohol drinking were not statistically significant risk factors for falls. A previous study has suggested that former drinkers might have stopped drinking due to poor health status, which predisposes them to risk of falls [ 14 ], and similarly, we speculate that the same may apply to smoking. Whereas, another study has figured out that former drinkers experience a higher risk of falls than those who never drink [ 44 ].
Consistent with our findings, physical exercise was an effective intervention measure to prevent falls for the elderly [ 37 ]. Taking more exercise to strengthen the body may fundamentally curb the trend of falls and fall-related fractures. Besides, as previously described, fear of falling is associated with falls, and exercise also helps reduce the fear of falling in older people living in the community in a way [ 40 ]. Referring to clinical guidelines, the management of T2DM generally includes nutrition management, increasing physical activity, monitoring blood glucose, and controlling health behaviors [ 9 , 45 ]. Considering our findings that older adults with T2DM who lack physical exercise are more likely to fall, more individualized intervention measures on physical activity are required. These are strongly recommended by a global initiative for falls, balance challenges, and functional exercises (e.g., sit-to-stand, stepping), which are suggested to be incorporated into sessions three or more times a week that are individualized and progressive in intensity for at least 12 weeks [ 45 ].
Our study has several advantages. Firstly, we revealed the incidence of falls, relationships between T2DM and falls, and factors associated with falls among community-dwelling elderly people in a prospective cohort study design. Secondly, all hospitalization records were validated and reviewed by trained community service staffs. However, some limitations need to be acknowledged and recognized. First, although our results are similar to previous studies and data from the Guangzhou Injury Monitoring System as a supplement to fall events, fall events were primarily based on the responses to individual perspective questions, which introduced recall bias. The recall bias may be even greater in the participants who refused to undergo health examinations and were only informed about falls and health status by telephonic interviews. Second, we did not identify whether the fall event was severe or injurious. Third, some possible risk factors for falls were neglected, such as history of falls, diabetes complications, cognitive function, depression, medication use, and glycaemic control. Fourth, our findings may not be generalisable to the other population as the present study was conducted among community-dwelling elderly people in Guangzhou, China. Fifth, as with any observational study, our study cannot establish causality. Therefore, to confirm the causal relationships in the present study, a larger, more comprehensive evaluation of influencing factors and prospective cohort studies would be required in future studies.
Falls are common, the risks between T2DM and falls were quantified, the individuals with T2DM are exposed to a greater risk of falls, and several factors are also investigated in the longitudinal cohort study. These findings provide evidence supporting the associations between T2DM and the risk of falls among community-dwelling elderly people, which can serve as a valuable reference for developing targeted interventions to reduce falls and the associated disease burden in this population.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Beard JR, Officer AM, Cassels AK. The World Report on Ageing and Health. Gerontologist. 2016;56(Suppl 2):S163–166.
Article PubMed Google Scholar
Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–18.
Article CAS PubMed Google Scholar
Rodríguez-Almagro J, García-Manzanares Á, Lucendo AJ, Hernández-Martínez A. Health-related quality of life in diabetes mellitus and its social, demographic and clinical determinants: a nationwide cross-sectional survey. J Clin Nurs. 2018;27(21–22):4212–23.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al. Prevalence and ethnic pattern of diabetes and Prediabetes in China in 2013. JAMA. 2017;317(24):2515–23.
Article PubMed PubMed Central Google Scholar
Global regional, national burden of diabetes. From 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
Article Google Scholar
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, et al. Prevalence and Control of Diabetes in Chinese adults. JAMA. 2013;310(9):948–59.
Lin XZ, Meng RL, Peng DD, Li C, Zheng XY, Xu HF, Xu XJ, Lin LF. Cross-sectional study on prevalence and risk factors for falls among the elderly in communities of Guangdong province, China. BMJ Open. 2022;12(11):e062257.
Phelan EA, Ritchey K. Fall Prevention in Community-Dwelling older adults. Ann Intern Med. 2018;169(11):Itc81–96.
Shen FX, Zhang LW, Fang Y. Study on the current Situation and influencing factors of Falls among the Old people in Chinese communities in 2018. Injury Med. 2022;11(01):7–12. (In Chinese).
Google Scholar
Chang NT, Yang NP, Chou P. Incidence, risk factors and consequences of falling injuries among the community-dwelling elderly in Shihpai, Taiwan. Aging Clin Exp Res. 2010;22(1):70–7.
Lin WQ, Lin L, Sun SY, Yuan LX, Sun MY, Wang C, Chen JM, Li YH, Zhou Q, Wu D, et al. Prevalence of falls, injury from falls and associations with chronic diseases among community-dwelling older adults in Guangzhou, China: a cross-sectional study. Front Public Health. 2023;11:1251858.
Chen X, Lin Z, Gao R, Yang Y, Li L. Prevalence and Associated Factors of Falls among older adults between Urban and Rural areas of Shantou City, China. Int J Environ Res Public Health. 2021;18(13):7050.
Zhou H, Peng K, Tiedemann A, Peng J, Sherrington C. Risk factors for falls among older community dwellers in Shenzhen, China. Inj Prev. 2019;25(1):31–5.
Haagsma JA, Olij BF, Majdan M, van Beeck EF, Vos T, Castle CD, Dingels ZV, Fox JT, Hamilton EB, Liu Z, et al. Falls in older aged adults in 22 European countries: incidence, mortality and burden of disease from 1990 to 2017. Inj Prev. 2020;26(Supp 1):i67–74.
Ye P, Er Y, Wang H, Fang L, Li B, Ivers R, Keay L, Duan L, Tian M. Burden of falls among people aged 60 years and older in mainland China, 1990–2019: findings from the global burden of Disease Study 2019. Lancet Public Health. 2021;6(12):e907–18.
Wallander M, Axelsson KF, Nilsson AG, Lundh D, Lorentzon M. Type 2 diabetes and risk of hip fractures and Non-skeletal Fall Injuries in the Elderly: a study from the fractures and Fall Injuries in the Elderly Cohort (FRAILCO). J Bone Min Res. 2017;32(3):449–60.
Article CAS Google Scholar
Lee AK, Juraschek SP, Windham BG, Lee CJ, Sharrett AR, Coresh J, Selvin E. Severe hypoglycemia and risk of falls in type 2 diabetes: the atherosclerosis risk in communities (ARIC) Study. Diabetes Care. 2020;43(9):2060–5.
Yau RK, Strotmeyer ES, Resnick HE, Sellmeyer DE, Feingold KR, Cauley JA, Vittinghoff E, De Rekeneire N, Harris TB, Nevitt MC, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care. 2013;36(12):3985–91.
Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26(7):507–19.
Roman de Mettelinge T, Cambier D, Calders P, Van Den Noortgate N, Delbaere K. Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. PLoS ONE. 2013;8(6):e67055.
Mesinovic J, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Waite LM, Handelsman DJ, Hirani V. Risk factors for Incident Falls and fractures in older men with and without type 2 diabetes Mellitus: the Concord Health and Ageing in Men Project. J Gerontol Biol Sci Med Sci. 2021;76(6):1090–100.
Lin WQ, Lin L, Yuan LX, Pan LL, Huang TY, Sun MY, Qin FJ, Wang C, Li YH, Zhou Q, et al. Association between meteorological factors and elderly falls in injury surveillance from 2014 to 2018 in Guangzhou, China. Heliyon. 2022;8(10):e10863.
Shuyi O, Zheng C, Lin Z, Zhang X, Li H, Fang Y, Hu Y, Yu H, Wu G. Risk factors of falls in elderly patients with visual impairment. Front Public Health. 2022;10:984199.
International Diabetes Federation. IDF DIABETES ATLAS Eighth edition 2017. 2017. https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf Accesed 20 Aug 2023
Zhang L, Ding Z, Qiu L, Li A. Falls and risk factors of falls for urban and rural community-dwelling older adults in China. BMC Geriatr. 2019;19(1):379.
Liao T, Lin L, Lin X, Xu H, Meng R, Zheng X, Peng D, Song X, Li C. Prevalence of falls and their influencing factors and impaired balance among the elderly in Guangdong Province. Chin J Disease Control Prev. 2022;26(07):851–6. (In Chinese).
dos Reis KM, de Jesus CA. Cohort study of institutionalized elderly people: fall risk factors from the nursing diagnosis. Rev Lat Am Enfermagem. 2015;23(6):1130–8.
Lin J, Wei Y, Chen G, Pei L. Chronic diseases and sleep duration in association with falls of different severity among the Chinese elderly. Chin J Disease Control Prev. 2021;25(01):25–31. (In Chinese).
Vergara I, Vrotsou K, Orive M, Garcia-Gutierrez S, Gonzalez N, Las Hayas C, Quintana JM. Wrist fractures and their impact in daily living functionality on elderly people: a prospective cohort study. BMC Geriatr. 2016;16:11.
Magliano DJ, Chen L, Islam RM, Carstensen B, Gregg EW, Pavkov ME, Andes LJ, Balicer R, Baviera M, Boersma-van Dam E, et al. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021;9(4):203–11.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Gebre AK, Sim M, Rodríguez AJ, Hodgson JM, Blekkenhorst LC, Szulc P, Bondonno N, Zhu K, Bondonno C, Kiel DP, et al. Abdominal aortic calcification is associated with a higher risk of injurious fall-related hospitalizations in older Australian women. Atherosclerosis. 2021;328:153–9.
Article CAS PubMed PubMed Central Google Scholar
Dalla Via J, Gebre AK, Smith C, Gilani Z, Suter D, Sharif N, Szulc P, Schousboe JT, Kiel DP, Zhu K, et al. Machine-learning assessed abdominal aortic calcification is Associated with Long-Term fall and fracture risk in Community-Dwelling older Australian women. J Bone Min Res. 2023;38(12):1867–76.
Lin W, Liu H, Li Y, Huang T, Yang Y, Sun M, Qin F, Liu L, Shen J, Liu H. Epidemiological characteristics of injury caused by fall in the elderly aged ≥ 60 years in Guangzhou in 2014–2018. Chin J Disease Control Prev. 2020;24(03):269–73. (In Chinese).
Liu P, Yin D. Research progression of related factors with falling down during hospotalization in women elderly patients and nursing countermeasures. Hebei Med J. 2020;42(05):769–73. (In Chinese).
Wang Z, Hu Y, Peng F. Long-term trends in unintentional fall mortality in China: a Population-based age-period-cohort study. Front Public Health. 2021;9:749295.
Phillips DR, Feng Z. Challenges for the Aging Family in the people’s Republic of China. Can J Aging. 2015;34(3):290–304.
Sai AJ, Gallagher JC, Smith LM, Logsdon S. Fall predictors in the community dwelling elderly: a cross sectional and prospective cohort study. J Musculoskelet Neuronal Interact. 2010;10(2):142–50.
CAS PubMed Google Scholar
Kendrick D, Kumar A, Carpenter H, Zijlstra GA, Skelton DA, Cook JR, Stevens Z, Belcher CM, Haworth D, Gawler SJ, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;2014(11):Cd009848.
PubMed PubMed Central Google Scholar
Gazibara T, Kurtagic I, Kisic-Tepavcevic D, Nurkovic S, Kovacevic N, Gazibara T, Pekmezovic T. Falls, risk factors and fear of falling among persons older than 65 years of age. Psychogeriatrics. 2017;17(4):215–23.
Montero-Odasso M, Speechley M. Falls in cognitively impaired older adults: implications for Risk Assessment and Prevention. J Am Geriatr Soc. 2018;66(2):367–75.
Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012;41(3):299–308.
Wen Y, Liao J, Yin Y, Liu C, Gong R, Wu D. Risk of falls in 4 years of follow-up among Chinese adults with diabetes: findings from the China Health and Retirement Longitudinal Study. BMJ Open. 2021;11(6):e043349.
Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, Aguilar-Navarro S, Alexander NB, Becker C, Blain H, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51(9):afac205.
Download references
Acknowledgements
We would like to also express our gratitude to all medical staff of community health centers for help with data collection in the follow-up period.
This study was supported by National Natural Science Foundation of China (72104061); Science and Technology Plan Project of Guangzhou (202201010022); The Key Project of Medicine Discipline of Guangzhou (No.2021-2023-12); Basic Research Project of Key Laboratory of Guangzhou (No.202102100001), Basic and Applied Research Project of Guangzhou (SL2022A03J01446), and The Science Technology Project of Guangzhou Municipal Health Commission (20241A011055).
Author information
Wei-Quan Lin and Ying-Xin Liao contributed equally to this work.
Authors and Affiliations
Department of Basic Public Health, Center for Disease Control and Prevention of Guangzhou, Guangzhou, 510440, China
Wei-Quan Lin, Li-Ying Luo, Yue Xu, Min-Ying Sun, Chang Wang, Qin Zhou, Xiang-Yi Liu & Hui Liu
Institute of Public Health, Guangzhou Medical University & Guangzhou Center for Disease Control and Prevention, Guangzhou, 510440, China
Wei-Quan Lin
School of Public Health, Guangzhou Medical University, Guangzhou, 511436, China
Ying-Xin Liao
Institute of Applied Health Research, University of Birmingham, Birmingham, B152TT, UK
Jing-Ya Wang
Brain Hospital of Guangzhou Medical University, Guangzhou Huiai Hospital, Guangzhou, 510370, China
Le-Xin Yuan
School of Public Health, Southern Medical University, Guangzhou, 510515, China
You can also search for this author in PubMed Google Scholar
Contributions
WQL supervised the study data collection and quality control, conducted the literature review, conducted the data analyses, drafted the manuscript, and finalized the manuscript with inputs from all authors. YXL conducted the data analyses, and drafted the manuscript. JYW conducted the literature review. LXY drafted the manuscript. SYS and YX conducted the literature review. CW supervised the study data collection and quality control. QZ supervised the study data collection and quality control. XYL supervised the study data collection and quality control. MYS supervised the study data collection and quality control. LYL supervised the study data collection and quality control. HL supervised the study data collection and quality control, and finalized the manuscript with inputs from all authors.
Corresponding author
Correspondence to Hui Liu .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval for the Guangzhou Falls and Health Status Tracking Cohort was obtained from the Ethics Committee of the Center for Disease Control and Prevention of Guangzhou (GZCDC-ECHR-2023P0061), and informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lin, WQ., Liao, YX., Wang, JY. et al. Associations between type 2 diabetes mellitus and risk of falls among community-dwelling elderly people in Guangzhou, China: a prospective cohort study. BMC Geriatr 24 , 717 (2024). https://doi.org/10.1186/s12877-024-05314-5
Download citation
Received : 11 January 2024
Accepted : 19 August 2024
Published : 29 August 2024
DOI : https://doi.org/10.1186/s12877-024-05314-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 2 diabetes mellitus
- Risk factors
- Elderly people
BMC Geriatrics
ISSN: 1471-2318
- General enquiries: [email protected]
Annals of Sports Medicine and Research

The Relationship Between the Type 2 Diabetes Mellitus: Insulin Resistance Loses Relevance
- 1. Teaching Hospital, OLVG, 1090 HA Amsterdam, The Netherlands

Subscribe to Newsletters
And stay informed about our news and events
Recent Advances
ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it. In 2018 alone, ADA-funded scientists published over 200 articles related to their awards!
Identification of a new player in type 1 diabetes risk
Type 1 diabetes is caused by an autoimmune attack of insulin-producing beta-cells. While genetics and the environment are known to play important roles, the underlying factors explaining why the immune system mistakenly recognize beta-cells as foreign is not known. Now, Dr. Delong has discovered a potential explanation. He found that proteins called Hybrid Insulin Peptides (HIPs) are found on beta-cells of people with type 1 diabetes and are recognized as foreign by their immune cells. Even after diabetes onset, immune cells are still present in the blood that attack these HIPs.
Next, Dr. Delong wants to determine if HIPs can serve as a biomarker or possibly even targeted to prevent or treat type 1 diabetes. Baker, R. L., Rihanek, M., Hohenstein, A. C., Nakayama, M., Michels, A., Gottlieb, P. A., Haskins, K., & Delong, T. (2019). Hybrid Insulin Peptides Are Autoantigens in Type 1 Diabetes. Diabetes , 68 (9), 1830–1840.
Understanding the biology of body-weight regulation in children
Determining the biological mechanisms regulating body-weight is important for preventing type 2 diabetes. The rise in childhood obesity has made this even more urgent. Behavioral studies have demonstrated that responses to food consumption are altered in children with obesity, but the underlying biological mechanisms are unknown. This year, Dr. Schur tested changes in brain and hormonal responses to a meal in normal-weight and obese children. Results from her study show that hormonal responses in obese children are normal following a meal, but responses within the brain are reduced. The lack of response within the brain may predispose them to overconsumption of food or difficulty with weight-loss.
With this information at hand, Dr. Schur wants to investigate how this information can be used to treat obesity in children and reduce diabetes.
Roth, C. L., Melhorn, S. J., Elfers, C. T., Scholz, K., De Leon, M. R. B., Rowland, M., Kearns, S., Aylward, E., Grabowski, T. J., Saelens, B. E., & Schur, E. A. (2019). Central Nervous System and Peripheral Hormone Responses to a Meal in Children. The Journal of Clinical Endocrinology and Metabolism , 104 (5), 1471–1483.
A novel molecule to improve continuous glucose monitoring
To create a fully automated artificial pancreas, it is critical to be able to quantify blood glucose in an accurate and stable manner. Current ways of continuously monitoring glucose are dependent on the activity of an enzyme which can change over time, meaning the potential for inaccurate readings and need for frequent replacement or calibration. Dr. Wang has developed a novel molecule that uses a different, non-enzymatic approach to continuously monitor glucose levels in the blood. This new molecule is stable over long periods of time and can be easily integrated into miniaturized systems.
Now, Dr. Wang is in the process of patenting his invention and intends to continue research on this new molecule so that it can eventually benefit people living with diabetes.
Wang, B. , Chou, K.-H., Queenan, B. N., Pennathur, S., & Bazan, G. C. (2019). Molecular Design of a New Diboronic Acid for the Electrohydrodynamic Monitoring of Glucose. Angewandte Chemie (International Ed. in English) , 58 (31), 10612–10615.
Addressing the legacy effect of diabetes
Several large clinical trials have demonstrated the importance of tight glucose control for reducing diabetes complications. However, few studies to date have tested this in the real-world, outside of a controlled clinical setting. In a study published this year, Dr. Laiteerapong found that indeed in a real-world setting, people with lower hemoglobin A1C levels after diagnosis had significantly lower vascular complications later on, a phenomenon known as the ‘legacy effect’ of glucose control. Her research noted the importance of early intervention for the best outcomes, as those with the low A1C levels just one-year after diagnosis had significantly lower vascular disease risk compared to people with higher A1C levels.
With these findings in hand, physicians and policymakers will have more material to debate and determine the best course of action for improving outcomes in people newly diagnosed with diabetes.
Laiteerapong, N. , Ham, S. A., Gao, Y., Moffet, H. H., Liu, J. Y., Huang, E. S., & Karter, A. J. (2019). The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care , 42 (3), 416–426.
A new way to prevent immune cells from attacking insulin-producing beta-cells
Replacing insulin-producing beta-cells that have been lost in people with type 1 diabetes is a promising strategy to restore control of glucose levels. However, because the autoimmune disease is a continuous process, replacing beta-cells results in another immune attack if immunosorbent drugs are not used, which carry significant side-effects. This year, Dr. Song reported on the potential of an immunotherapy he developed that prevents immune cells from attacking beta-cells and reduces inflammatory processes. This immunotherapy offers several potential benefits, including eliminating the need for immunosuppression, long-lasting effects, and the ability to customize the treatment to each patient.
The ability to suppress autoimmunity has implications for both prevention of type 1 diabetes and improving success rates of islet transplantation.
Haque, M., Lei, F., Xiong, X., Das, J. K., Ren, X., Fang, D., Salek-Ardakani, S., Yang, J.-M., & Song, J . (2019). Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes. JCI Insight , 4 (7).
A new target to improve insulin sensitivity
The hormone insulin normally acts like a ‘key’, traveling through the blood and opening the cellular ‘lock’ to enable the entry of glucose into muscle and fat cells. However, in people with type 2 diabetes, the lock on the cellular door has, in effect, been changed, meaning insulin isn’t as effective. This phenomenon is called insulin resistance. Scientists have long sought to understand what causes insulin resistance and develop therapies to enable insulin to work correctly again. This year, Dr. Summers determined an essential role for a molecule called ceramides as a driver of insulin resistance in mice. He also presented a new therapeutic strategy for lowering ceramides and reversing insulin resistance. His findings were published in one of the most prestigious scientific journals, Science .
Soon, Dr. Summers and his team will attempt to validate these findings in humans, with the ultimate goal of developing a new medication to help improve outcomes in people with diabetes.
Chaurasia, B., Tippetts, T. S., Mayoral Monibas, R., Liu, J., Li, Y., Wang, L., Wilkerson, J. L., Sweeney, C. R., Pereira, R. F., Sumida, D. H., Maschek, J. A., Cox, J. E., Kaddai, V., Lancaster, G. I., Siddique, M. M., Poss, A., Pearson, M., Satapati, S., Zhou, H., … Summers, S. A. (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science (New York, N.Y.) , 365 (6451), 386–392.
Determining the role of BPA in type 2 diabetes risk
Many synthetic chemicals have infiltrated our food system during the period in which rates of diabetes has surged. Data has suggested that one particular synthetic chemical, bisphenol A (BPA), may be associated with increased risk for developing type 2 diabetes. However, no study to date has determined whether consumption of BPA alters the progression to type 2 diabetes in humans. Results reported this year by Dr. Hagobian demonstrated that indeed when BPA is administered to humans in a controlled manner, there is an immediate, direct effect on glucose and insulin levels.
Now, Dr. Hagobian wants to conduct a larger clinical trial including exposure to BPA over a longer period of time to determine precisely how BPA influences glucose and insulin. Such results are important to ensure the removal of chemicals contributing to chronic diseases, including diabetes.
Hagobian, T. A. , Bird, A., Stanelle, S., Williams, D., Schaffner, A., & Phelan, S. (2019). Pilot Study on the Effect of Orally Administered Bisphenol A on Glucose and Insulin Response in Nonobese Adults. Journal of the Endocrine Society , 3 (3), 643–654.
Investigating the loss of postmenopausal protection from cardiovascular disease in women with type 1 diabetes
On average, women have a lower risk of developing heart disease compared to men. However, research has shown that this protection is lost in women with type 1 diabetes. The process of menopause increases rates of heart disease in women, but it is not known how menopause affects women with type 1 diabetes in regard to risk for developing heart disease. In a study published this year, Dr. Snell-Bergeon found that menopause increased risk markers for heart disease in women with type 1 diabetes more than women without diabetes.
Research has led to improved treatments and significant gains in life expectancy for people with diabetes and, as a result, many more women are reaching the age of menopause. Future research is needed to address prevention and treatment options.
Keshawarz, A., Pyle, L., Alman, A., Sassano, C., Westfeldt, E., Sippl, R., & Snell-Bergeon, J. (2019). Type 1 Diabetes Accelerates Progression of Coronary Artery Calcium Over the Menopausal Transition: The CACTI Study. Diabetes Care , 42 (12), 2315–2321.
Identification of a potential therapy for diabetic neuropathy related to type 1 and type 2 diabetes
Diabetic neuropathy is a type of nerve damage that is one of the most common complications affecting people with diabetes. For some, neuropathy can be mild, but for others, it can be painful and debilitating. Additionally, neuropathy can affect the spinal cord and the brain. Effective clinical treatments for neuropathy are currently lacking. Recently, Dr. Calcutt reported results of a new potential therapy that could bring hope to the millions of people living with diabetic neuropathy. His study found that a molecule currently in clinical trials for the treatment of depression may be valuable for diabetic neuropathy, particularly the type affecting the brain.
Because the molecule is already in clinical trials, there is the potential that it can benefit patients sooner than later.
Jolivalt, C. G., Marquez, A., Quach, D., Navarro Diaz, M. C., Anaya, C., Kifle, B., Muttalib, N., Sanchez, G., Guernsey, L., Hefferan, M., Smith, D. R., Fernyhough, P., Johe, K., & Calcutt, N. A. (2019). Amelioration of Both Central and Peripheral Neuropathy in Mouse Models of Type 1 and Type 2 Diabetes by the Neurogenic Molecule NSI-189. Diabetes , 68 (11), 2143–2154.
ADA-funded researcher studying link between ageing and type 2 diabetes
One of the most important risk factors for developing type 2 diabetes is age. As a person gets older, their risk for developing type 2 diabetes increases. Scientists want to better understand the relationship between ageing and diabetes in order to determine out how to best prevent and treat type 2 diabetes. ADA-funded researcher Rafael Arrojo e Drigo, PhD, from the Salk Institute for Biological Studies, is one of those scientists working hard to solve this puzzle.
Recently, Dr. Arrojo e Drigo published results from his research in the journal Cell Metabolism . The goal of this specific study was to use high-powered microscopes and novel cellular imaging tools to determine the ‘age’ of different cells that reside in organs that control glucose levels, including the brain, liver and pancreas. He found that, in mice, the cells that make insulin in the pancreas – called beta-cells – were a mosaic of both old and young cells. Some beta-cells appeared to be as old as the animal itself, and some were determined to be much younger, indicating they recently underwent cell division.
Insufficient insulin production by beta-cells is known to be a cause of type 2 diabetes. One reason for this is thought to be fewer numbers of functional beta-cells. Dr. Arrojo e Drigo believes that people with or at risk for diabetes may have fewer ‘young’ beta-cells, which are likely to function better than old ones. Alternatively, if we can figure out how to induce the production of younger, high-functioning beta-cells in the pancreas, it could be a potential treatment for people with diabetes.
In the near future, Dr. Arrojo e Drigo’s wants to figure out how to apply this research to humans. “The next step is to look for molecular or morphological features that would allow us to distinguish a young cell from and old cell,” Dr. Arrojo e Drigo said.
The results from this research are expected to provide a unique insight into the life-cycle of beta-cells and pave the way to novel therapeutic avenues for type 2 diabetes.
Watch a video of Dr. Arrojo e Drigo explaining his research!
Arrojo E Drigo, R. , Lev-Ram, V., Tyagi, S., Ramachandra, R., Deerinck, T., Bushong, E., … Hetzer, M. W. (2019). Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metabolism , 30 (2), 343-351.e3.
Researcher identifies potential underlying cause of type 1 diabetes
Type 1 diabetes occurs when the immune system mistakenly recognizes insulin-producing beta-cells as foreign and attacks them. The result is insulin deficiency due to the destruction of the beta-cells. Thankfully, this previously life-threatening condition can be managed through glucose monitoring and insulin administration. Still, therapies designed to address the underlying immunological cause of type 1 diabetes remain unavailable.
Conventional approaches have focused on suppressing the immune system, which has serious side effects and has been mostly unsuccessful. The American Diabetes Association recently awarded a grant to Dr. Kenneth Brayman, who proposed to take a different approach. What if instead of suppressing the whole immune system, we boost regulatory aspects that already exist in the system, thereby reigning in inappropriate immune cell activation and preventing beta-cell destruction? His idea focused on a molecule called immunoglobulin M (IgM), which is responsible for limiting inflammation and regulating immune cell development.
In a paper published in the journal Diabetes , Dr. Brayman and a team of researchers reported exciting findings related to this approach. They found that supplementing IgM obtained from healthy mice into mice with type 1 diabetes selectively reduced the amount of autoreactive immune cells known to target beta-cells for destruction. Amazingly, this resulted in reversal of new-onset diabetes. Importantly, the authors of the study determined this therapy is translatable to humans. IgM isolated from healthy human donors also prevented the development of type 1 diabetes in a humanized mouse model of type 1 diabetes.
The scientists tweaked the original experiment by isolating IgM from mice prone to developing type 1 diabetes, but before it actually occurred. When mice with newly onset diabetes were supplemented with this IgM, their diabetes was not reversed. This finding suggests that in type 1 diabetes, IgM loses its capacity to serve as a regulator of immune cells, which may be contribute to the underlying cause of the disease.
Future studies will determine exactly how IgM changes its regulatory properties to enable diabetes development. Identification of the most biologically optimal IgM will facilitate transition to clinical applications of IgM as a potential therapeutic for people with type 1 diabetes. Wilson, C. S., Chhabra, P., Marshall, A. F., Morr, C. V., Stocks, B. T., Hoopes, E. M., Bonami, R.H., Poffenberger, G., Brayman, K.L. , Moore, D. J. (2018). Healthy Donor Polyclonal IgM’s Diminish B Lymphocyte Autoreactivity, Enhance Treg Generation, and Reverse T1D in NOD Mice. Diabetes .
ADA-funded researcher designs community program to help all people tackle diabetes
Diabetes self-management and support programs are important adjuncts to traditional physician directed treatment. These community-based programs aim to give people with diabetes the knowledge and skills necessary to effectively self-manage their condition. While several clinical trials have demonstrated the value of diabetes self-management programs in terms of improving glucose control and reducing health-care costs, whether this also occurs in implemented programs outside a controlled setting is unclear, particularly in socially and economically disadvantaged groups.
Lack of infrastructure and manpower are often cited as barriers to implementation of these programs in socioeconomically disadvantaged communities. ADA-funded researcher Dr. Briana Mezuk addressed this challenge in a study recently published in The Diabetes Educator . Dr. Mezuk partnered with the YMCA to evaluate the impact of the Diabetes Control Program in Richmond, Virginia. This community-academic partnership enabled both implementation and evaluation of the Diabetes Control Program in socially disadvantaged communities, who are at higher risk for developing diabetes and the complications that accompany it.
Dr. Mezuk had two primary research questions: (1) What is the geographic and demographic reach of the program? and (2) Is the program effective at improving diabetes management and health outcomes in participants? Over a 12-week study period, Dr. Mezuk found that there was broad geographic and demographic participation in the program. The program had participants from urban, suburban and rural areas, most of which came from lower-income zip codes. HbA1C, mental health and self-management behaviors all improved in people taking part in the Greater Richmond Diabetes Control Program. Results from this study demonstrate the value of diabetes self-management programs and their potential to broadly improve health outcomes in socioeconomically diverse communities. Potential exists for community-based programs to address the widespread issue of outcome disparities related to diabetes. Mezuk, B. , Thornton, W., Sealy-Jefferson, S., Montgomery, J., Smith, J., Lexima, E., … Concha, J. B. (2018). Successfully Managing Diabetes in a Community Setting: Evidence from the YMCA of Greater Richmond Diabetes Control Program. The Diabetes Educator , 44 (4), 383–394.
Using incentives to stimulate behavior changes in youth at risk for developing diabetes
Once referred to as ‘adult-onset diabetes’, incidence of type 2 diabetes is now rapidly increasing in America’s youth. Unfortunately, children often do not have the ability to understand how everyday choices impact their health. Could there be a way to change a child’s eating behaviors? Davene Wright, PhD, of Seattle Children’s Hospital was granted an Innovative Clinical or Translational Science award to determine whether using incentives, directed by parents, can improve behaviors related to diabetes risk. A study published this year in Preventive Medicine Reports outlined what incentives were most desirable and feasible to implement. A key finding was that incentives should be tied to behavior changes and not to changes in body-weight.
With this information in hand, Dr. Wright now wants to see if incentives do indeed change a child’s eating habits and risk for developing type 2 diabetes. She is also planning to test whether an incentive program can improve behavior related to diabetes management in youth with type 1 diabetes. Jacob-Files, E., Powell, J., & Wright, D. R. (2018). Exploring parent attitudes around using incentives to promote engagement in family-based weight management programs. Preventive Medicine Reports , 10 , 278–284.
Determining the genetic risk for gestational diabetes
Research has identified more than 100 genetic variants linked to risk for developing type 2 diabetes in humans. However, the extent to which these same genetic variants might affect a woman’s probability for getting gestational diabetes has not been investigated.
Pathway to Stop Diabetes ® Accelerator awardee Marie-France Hivert, MD, of Harvard University set out to answer this critical question. Dr. Hivert found that indeed genetic determinants of type 2 diabetes outside of pregnancy are also strong risk factors for gestational diabetes. This study was published in the journal Diabetes .
The implications? Because of this finding, doctors in the clinic may soon be able to identify women at risk for getting gestational diabetes and take proactive steps to prevent it. Powe, C. E., Nodzenski, M., Talbot, O., Allard, C., Briggs, C., Leya, M. V., … Hivert, M.-F. (2018). Genetic Determinants of Glycemic Traits and the Risk of Gestational Diabetes Mellitus. Diabetes , 67 (12), 2703–2709.

Give Today and Change lives!
With your support, the American Diabetes Association® can continue our lifesaving work to make breakthroughs in research and provide people with the resources they need to fight diabetes.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Providing Care
- Living with Diabetes
- Clinical Guidance
- DSMES for Health Care Providers
- Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change
- Employers and Insurers
- Community-based Organizations (CBOs)
- Toolkits for Diabetes Educators and Community Health Workers
- National Diabetes Statistics Report
- Reports and Publications
- Data and Statistics
- Current Research Projects
Related Topics:
- View All Home
- National Diabetes Prevention Program
- State, Local, and National Partner Diabetes Programs for Public Health
- Diabetes Self-Management Education and Support (DSMES) Toolkit
- About the Division of Diabetes Translation
At a glance
- The National Diabetes Statistics Report provides up-to-date information on the prevalence and incidence of diabetes and prediabetes, risk factors for complications, acute and long-term complications, deaths, and costs.
- Data in the report can help focus efforts to prevent and control diabetes across the United States. This report is continually updated as data become available. This report fulfills the requirement mandated by the Catalyst to Better Diabetes Care Act of 2009 (Section 10407 of Public Law 111-148).
Fast Facts on Diabetes
- Total: 38.4 million people have diabetes (11.6% of the U.S. population)
- Diagnosed: 29.7 million people, including 29.4 million adults
- Undiagnosed: 8.7 million people (22.8% of adults with diabetes are undiagnosed)
Prediabetes
- Total: 97.6 million people aged 18 years or older have prediabetes (38.0% of the adult U.S. population)
- 65 years or older: 27.2 million people aged 65 years or older (48.8%) have prediabetes
Methods and tables
Prevalence of both diagnosed and undiagnosed diabetes.
Among the U.S. population overall, crude estimates for 2021 were:
- 38.4 million people of all ages—or 11.6% of the U.S. population—had diabetes.
- 38.1 million adults aged 18 years or older—or 14.7% of all U.S. adults—had diabetes (Table 1a; Table 1b).
- 8.7 million adults aged 18 years or older who met laboratory criteria for diabetes were not aware of or did not report having diabetes (undiagnosed diabetes, Table 1b). This number represents 3.4% of all U.S. adults (Table 1a) and 22.8% of all U.S. adults with diabetes.
- The percentage of adults with diabetes increased with age, reaching 29.2% among those aged 65 years or older (Table 1a).
Table 1a. Estimated crude prevalence of diagnosed diabetes, undiagnosed diabetes, and total diabetes among adults aged 18 years or older, United States, 2017–2020
| Characteristic | Diagnosed diabetes Percentage (95% CI) | Undiagnosed diabetes Percentage (95% CI) | Total diabetes Percentage (95% CI) |
|---|---|---|---|
| Age in years | |||
| 18–44 | 3.0 (2.4–3.7) | 1.9 (1.3–2.7) | 4.8 (4.0–5.9) |
| 45–64 | 14.5 (12.2–17.0) | 4.5 (3.3–6.0) | 18.9 (16.1–22.1) |
| ≥65 | 24.4 (22.1–27.0) | 4.7 (3.0–7.4) | 29.2 (26.4–32.1) |
| Sex | |||
| Men | 12.6 (11.1–14.3) | 2.8 (2.0–3.9) | 15.4 (13.5–17.5) |
| Women | 10.2 (8.8–11.7) | 3.9 (2.7–5.5) | 14.1 (11.8–16.7) |
| Race-Ethnicity | |||
| White, non-Hispanic | 11.0 (9.4–12.8) | 2.7 (1.7–4.2) | 13.6 (11.4–16.2) |
| Black, non-Hispanic | 12.7 (10.7–15.0) | 4.7 (3.3–6.5) | 17.4 (15.2–19.8) |
| Asian, non-Hispanic | 11.3 (9.7–13.1) | 5.4 (3.5–8.3) | 16.7 (14.0–19.8) |
| Hispanic | 11.1 (9.5–13.0) | 4.4 (3.3–5.8) | 15.5 (13.8–17.3) |
Notes: CI = confidence interval. Time period 2017–2020 covers January 2017 through March 2020 only. Diagnosed diabetes was based on self-report. Undiagnosed diabetes was based on fasting plasma glucose and A1C levels among people self-reporting no diabetes. Numbers for subgroups may not add up to the total because of rounding. Age-adjusted estimates are presented in Appendix Table 1 . Data source: 2017–March 2020 National Health and Nutrition Examination Survey.
Table 1b. Estimated number of adults aged 18 years or older with diagnosed diabetes, undiagnosed diabetes, and total diabetes, United States, 2021
| Characteristic | Diagnosed diabetes Number in Millions (95% CI) | Undiagnosed diabetes Number in Millions (95% CI) | Total diabetes Number in Millions (95% CI) |
|---|---|---|---|
| Age in years | |||
| 18–44 | 3.5 (2.8–4.2) | 2.2 (1.5–3.0) | 5.8 (4.7–6.8) |
| 45–64 | 12.0 (10.1–13.9) | 3.8 (2.7–4.8) | 15.8 (13.4–18.2) |
| ≥65 | 13.8 (12.5–15.1) | 2.7 (1.6–3.8) | 16.5 (15.0–18.1) |
| Sex | |||
| Men | 16.1 (14.1–18.0) | 3.7 (2.6–4.8) | 19.8 (17.4–22.1) |
| Women | 13.3 (11.5–15.1) | 5.0 (3.3–6.7) | 18.3 (15.3–21.3) |
| Race-Ethnicity | |||
| White, non-Hispanic | 17.8 (15.2–20.4) | 4.3 (2.4–6.1) | 22.1 (18.5–25.7) |
| Black, non-Hispanic | 4.0 (3.3–4.6) | 1.4 (1.0–1.9) | 5.4 (4.7–6.1) |
| Asian, non-Hispanic | 1.8 (1.5–2.1) | 0.9 (0.5–1.2) | 2.7 (2.2–3.1) |
| Hispanic | 5.0 (4.3–5.7) | 1.9 (1.4–2.4) | 6.9 (6.2–7.6) |
Notes: CI = confidence interval. Estimated numbers for 2021 were derived from percentages for 2017–March 2020 applied to July 1, 2021, U.S. resident population estimates from the U.S. Census Bureau (See detailed methods and data sources ). Diagnosed diabetes was based on self-report. Undiagnosed diabetes was based on fasting plasma glucose and A1C levels among people self-reporting no diabetes. Numbers for subgroups may not add up to the total because of rounding.
Data sources: 2017–March 2020 National Health and Nutrition Examination Survey; 2021 U.S. Census Bureau data.
Trends in prevalence of diagnosed diabetes, undiagnosed diabetes, and total diabetes
- During 2001–2020, the age-adjusted prevalence of total diabetes significantly increased among adults aged 18 years or older (Figure 1).
- Prevalence estimates for total diabetes were 10.3% in 2001–2004 and 13.2% in 2017–2020 ( Appendix Table 2 ).
- During this period, the age-adjusted prevalence significantly increased for diagnosed diabetes. No significant change in undiagnosed diabetes prevalence was found (Figure 1; Appendix Table 2 ).
Figure 1. Trends in age-adjusted prevalence of diagnosed diabetes, undiagnosed diabetes, and total diabetes among adults aged 18 years or older, United States, 2001–2020
Notes: Diagnosed diabetes was based on self-report. Undiagnosed diabetes was based on fasting plasma glucose and A1C levels among people self-reporting no diabetes. Time period 2017–2020 covers January 2017 through March 2020 only.
Prevalence of diagnosed diabetes
- 29.7 million people of all ages—or 8.9% of the U.S. population—had diagnosed diabetes.
- 352,000 children and adolescents younger than age 20 years—or 35 per 10,000 U.S. youths—had diagnosed diabetes. This includes 304,000 with type 1 diabetes.
- 1.7 million adults aged 20 years or older—or 5.7% of all U.S. adults with diagnosed diabetes—reported both having type 1 diabetes and using insulin.
- 3.6 million adults aged 20 years or older—or 12.3% of all U.S. adults with diagnosed diabetes—started using insulin within a year of their diagnosis.
Among U.S. adults aged 18 years or older, age-adjusted data for 2019–2021 indicated the following:
- For both men and women, prevalence of diagnosed diabetes was highest among American Indian and Alaska Native adults (13.6%), followed by non-Hispanic Black adults (12.1%), adults of Hispanic origin (11.7%), non-Hispanic Asian adults (9.1%) and non-Hispanic White adults (6.9%) ( Appendix Table 3 ).
- Prevalence varied significantly by education level, which is an indicator of socioeconomic status. Specifically, 13.1% of adults with less than a high school education had diagnosed diabetes versus 9.1% of those with a high school education and 6.9% of those with more than a high school education ( Appendix Table 3 ).
- Adults with family income above 500% of the federal poverty level had the lowest prevalence for both men (6.3%) and women (3.9%) ( Appendix Table 3 ).
- For both men and women, prevalence was higher among adults living in nonmetropolitan areas compared to those in metropolitan areas (Figure 2; Appendix Table 3 ).
Figure 2. Age-adjusted estimated prevalence of diagnosed diabetes by metropolitan residence and sex for adults aged 18 years or older, United States, 2019–2021
Note: Error bars represent upper and lower bounds of the 95% confidence interval.
Table 2. Crude prevalence of diagnosed diabetes by detailed race and ethnicity among adults aged 18 years or older, United States, 2019–2021
| American Indian or Alaska Native, non-Hispanic | 16.0 (12.1–20.6) |
|---|---|
| Black, non-Hispanic | 12.5 (11.6–13.4) |
| Native Hawaiian or Other Pacific Islander, non-Hispanic | 11.7 (7.4–17.2) |
| Asian, non-Hispanic | 9.2 (8.2–10.4) |
| Asian Indian, non-Hispanic | 10.8 (8.3–13.7) |
| Chinese, non-Hispanic | 7.1 (5.2–9.3) |
| Filipino, non-Hispanic | 12.2 (9.4–15.6) |
| Japanese, non-Hispanic | 6.8 (4.1–10.5) |
| Korean, non-Hispanic | 6.1 (3.8–9.1) |
| Vietnamese, non-Hispanic | 6.4 (3.7–10.0) |
| Other Asian, non-Hispanic | 8.9 (5.9–12.8) |
| Hispanic | 10.3 (9.4–11.1) |
| Mexican or Mexican American | 11.1 (9.9–12.3) |
| Central American | 7.3 (5.6–9.4) |
| South American | 5.0 (3.3–7.1) |
| Puerto Rican | 13.3 (11.0–15.9) |
| Cuban | 9.0 (6.5–12.1) |
| Dominican | 9.4 (5.9–14.2) |
| Other Hispanic, Latino, or Spanish | 7.2 (5.5–9.2) |
| White, non-Hispanic | 8.5 (8.2–8.8) |
Note: CI = confidence interval. Data sources: National Center for Health Statistics; 2019–2021 National Health Interview Survey.
County-level prevalence among adults
Among U.S. adults aged 20 years or older, age-adjusted, county-level data indicated:
- In 2021, estimates of diagnosed diabetes prevalence varied across U.S. counties, ranging from 4.4% to 17.9% (Figure 3).
- Median county-level prevalence of diagnosed diabetes increased from 6.3% in 2004 to 8.3% in 2021.
Figure 3. Age-adjusted, county-level prevalence of diagnosed diabetes among adults aged 20 years or older, United States, 2004 and 2021
Incidence of newly diagnosed diabetes
Incidence among adults.
Among U.S. adults aged 18 years or older, crude estimates for 2021 were:
- 1.2 million new cases of diabetes—or 5.9 per 1,000 people—were diagnosed (Table 3).
- Compared to adults aged 18 to 44 years, incidence rates of diagnosed diabetes were higher among adults aged 45 to 64 years and those aged 65 years and older (Table 3).
Among U.S. adults aged 18 years or older, age-adjusted data for 2019–2021 indicated:
- Compared to non-Hispanic White adults and Asian adults, incidence estimates were higher for non-Hispanic Black adults and Hispanic adults ( Appendix Table 4 ).
- Incidence rates of diagnosed diabetes were higher among those with less than high school education and those with high school education only compared to adults with more than high school education ( Appendix Table 4 ).
- Incidence was similar among adults living in metropolitan and nonmetropolitan areas ( Appendix Table 4 ).
Table 3. Estimated crude incidence of diagnosed diabetes among adults aged 18 years or older, United States, 2019–2021
| Characteristic | Population Estimates, 2021 Number in Thousands (95% CI) | Incidence Estimates, 2019–2021 Rate per 1,000 (95% CI) | |
|---|---|---|---|
| Age in years | |||
| 18–44 | 305 (241–369) | 3.0 (2.1–4.2) | |
| 45–64 | 633 (550–716) | 10.1 (8.2–12.4) | |
| ≥65 | 273 (222–325) | 6.8 (5.1–8.9) | |
| Sex | |||
| Men | 620 (536–704) | 6.4 (5.2–7.9) | |
| Women | 591 (510–672) | 5.5 (4.4–6.9) | |
| Race/ethnicity | |||
| White, non-Hispanic | 721 (633–809) | 5.1 (4.5–5.8) | |
| Black, non-Hispanic | 185 (139–232) | 6.8 (5.3–8.7) | |
| Asian, non-Hispanic | 52 (29–76) | 3.8 (2.4–5.9) | |
| Hispanic | 233 (178–289) | 6.1 (4.8–7.7) | |
CI = confidence interval.
a Population estimates for 2021 were derived from rates for 2019–2021 applied to July 1, 2021 U.S. resident population estimates from the U.S. Census Bureau (See Appendix B: Detailed Methods ).
b Rates were calculated using 2021 data only.
Data sources: 2019–2021 National Health Interview Survey and 2021 U.S. Census Bureau data.
Trends in incidence among adults
- Among adults aged 18 years or older, the age-adjusted incidence of diagnosed diabetes was similar in 2000 (6.2 per 1,000 adults) and 2021 (5.8 per 1,000 adults). A significant decreasing trend in incidence was detected after 2008 (8.4 per 1,000 adults) through 2021. (Figure 4).
Figure 4. Trends in age-adjusted incidence of diagnosed diabetes among adults aged 18 years or older, United States, 2000–2021
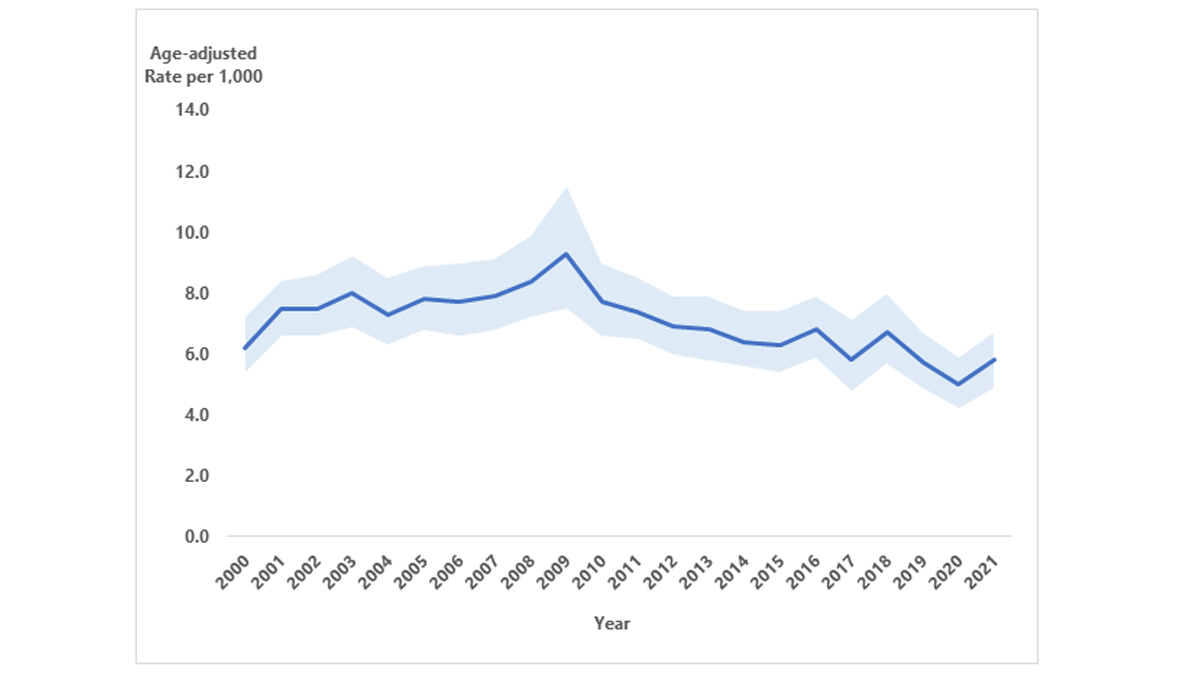
Notes: Data shown are estimated incidence rates (solid blue line) and 95% confidence intervals (shaded). Joinpoint identified in 2008 (see Appendix B: Detailed Methods and Data Sources ). Because of changes to the survey design and survey instruments after 2018, comparisons of the 2000–2018 and 2019–2021 data should be examined with caution.
County-level incidence among adults
Among US adults aged 20 years or older, age-adjusted, county-level data indicated:
- Estimates of diagnosed diabetes incidence varied across U.S. counties, ranging from 2.2 to 53.5 per 1,000 people in 2020 (for more detail, see U.S. Diabetes Surveillance System ).
- Median county-level incidence of diagnosed diabetes was 9.7 and 9.0 per 1,000 people in 2004 and 2020, respectively (for more detail, see U.S. Diabetes Surveillance System ).
Incidence among children and adolescents
Data from the SEARCH for Diabetes in Youth study indicated that, during 2017–2018, the estimated annual number of newly diagnosed cases in the United States included:
- 18,169 children and adolescents younger than age 20 years with type 1 diabetes.
- 5,293 children and adolescents aged 10 to 19 years with type 2 diabetes.
Trends in incidence among children and adolescents
Among U.S. children and adolescents aged younger than 20 years, modeled data in Figure 5 showed:
- For the period 2002–2018, overall incidence of type 1 diabetes significantly increased.
- Non-Hispanic Asian or Pacific Islander children and adolescents had the largest significant increases in incidence of type 1 diabetes, followed by Hispanic and non-Hispanic Black children and adolescents.
- Non-Hispanic White children and adolescents had the highest incidence of type 1 diabetes across all years.
Among U.S. children and adolescents aged 10 to 19 years, modeled data in Figure 5 showed:
- For the entire period 2002–2018, overall incidence of type 2 diabetes significantly increased.
- Incidence of type 2 diabetes significantly increased for all racial and ethnic groups, especially Asian or Pacific Islander, Hispanic, and non-Hispanic Black children and adolescents.
- Non-Hispanic Black children and adolescents had the highest incidence of type 2 diabetes across all years.
Figure 5. Trends in incidence of type 1 and type 2 diabetes in children and adolescents, overall and by race and ethnicity, 2002–2018
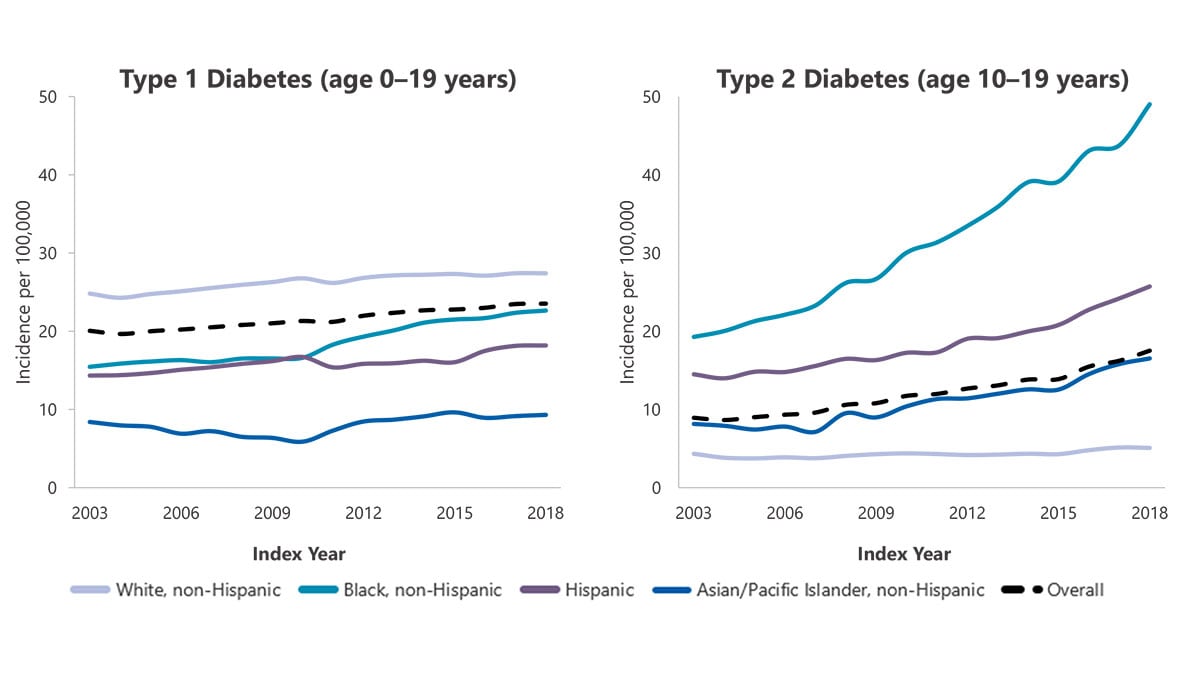
Note: Adapted from Wagenknecht LE et al 1 . Data are model-adjusted incidence estimates (see Appendix B: Detailed Methods and Data Sources ).
Prevalence of prediabetes among adults
- An estimated 97.6 million adults aged 18 years or older had prediabetes in 2021 (Table 4).
- 38.0% of all U.S. adults had prediabetes, based on their fasting glucose or A1C level (Table 4).
- 19.0% of adults with prediabetes reported being told by a health professional that they had this condition (Table 4).
Among U.S. adults aged 18 years or older, age-adjusted data for 2017–2020 indicated:
- 10.8% of adults had prediabetes, based on both elevated fasting plasma glucose and A1C levels ( Appendix Table 5 ).
- A higher percentage of men (41.0%) than women (32.0%) had prediabetes, based on their fasting glucose or A1C level ( Appendix Table 6 ).
- Prevalence of prediabetes (based on fasting glucose or A1C level) was similar among all racial and ethnic groups and education levels ( Appendix Table 6 ).
Table 4. Estimated number, percentage, and awareness of prediabetes a among adults aged 18 years or older, United States, 2017–2020 and 2021
| Characteristic | 2021 Estimates Number in Millions (95% CI) | 2017–2020 Estimates Percentage (95% CI) | 2017–2020 Estimates Percentage (95% CI) |
|---|---|---|---|
| Age in years | |||
| 18–44 | 32.8 (28.2–37.4) | 27.8 (24.0-32.0) | 13.8 (9.8–18.9) |
| 45–64 | 37.5 (35.1–40.0) | 44.8 (41.7–47.9) | 20.6 (14.3–28.9) |
| ≥65 | 27.2 (24.9–29.6) | 48.8 (44.3–53.2) | 23.0 (16.9–30.4) |
| Sex | |||
| Men | 53.2 (48.9–57.6) | 41.9 (38.4–45.6) | 17.4 (13.4–22.2) |
| Women | 44.3 (40.4–48.3) | 34.3 (31.2–37.5) | 20.9 (15.5–27.5) |
| Race-Ethnicity | |||
| White, non-Hispanic | 61.8 (59.6–66.7) | 38.7 (35.5–41.9) | 17.3 (11.8–24.7) |
| Black, non-Hispanic | 12.3 (11.3–13.3) | 39.2 (35.8–42.6) | 21.9 (18.0–26.5) |
| Asian, non-Hispanic | 5.8 (5.1–6.6) | 37.3 (32.6–42.3) | 30.1 (21.0–41.1) |
| Hispanic | 15.0 (13.7–16.3) | 34.5 (31.3–37.7) | 20.9 (15.3–27.9) |
Notes: CI = confidence interval. Data are crude estimates (see Appendix B: Detailed Methods and Data Sources ). Time period 2017–2020 covers January 2017 through March 2020 only.
a Prediabetes was defined as fasting plasma glucose values of 100 to 125 mg/dL or A1C values of 5.7% to 6.4%.
b Prediabetes awareness was based on self-report and estimated only among adults with prediabetes.
Trends in prevalence of prediabetes among adults
- There were no significant changes in age-adjusted prevalence of prediabetes from 2005–2008 to 2017–2020 ( Appendix Table 7 ). About one-third of U.S. adults had prediabetes over the entire period.
- Among adults with prediabetes, the age-adjusted percentage aware that they had this condition increased from 6.5% in 2005–2008 to 17.4% in 2017–2020 ( Appendix Table 7 ).
Risk factors for diabetes-related complications
Among U.S. adults aged 18 years or older with diagnosed diabetes, crude estimates for 2017–2020 shown in Appendix Table 8 were:
- 22.1% were tobacco users based on self-report or levels of serum cotinine.
- 14.6% reported current cigarette smoking.
- 36.0% had quit smoking but had a history of smoking at least 100 cigarettes in their lifetime.
Overweight and obesity
- 26.9% were overweight (BMI of 25.0 to 29.9 kg/m 2 ).
- 47.1% had obesity (BMI of 30.0 to 39.9 kg/m 2 ).
- 15.7% had extreme obesity (BMI of 40.0 kg/m 2 or higher).
Physical inactivity
- 31.9% were physically inactive, defined as getting less than 10 minutes a week of moderate or vigorous activity in each physical activity category of work, leisure time, and transportation.
- 22.9% had an A1C value of 7.0% to 7.9%.
- 11.5% had an A1C value of 8.0% to 9.0%.
- 13.0% had an A1C value higher than 9.0%.
- 10.4% of adults aged 18–44 years had A1C levels of 10% or higher, compared to 9.4% of those aged 45–64 years and 2.6% of those aged 65 years or older ( Appendix Table 9 ).
High blood pressure
- 80.6% had a systolic blood pressure of 130 mmHg or higher or diastolic blood pressure of 80 mmHg or higher or were on prescription medication for their high blood pressure ( Appendix Table 8 ).
- 70.8% had a systolic blood pressure of 140 mmHg or higher or diastolic blood pressure of 90 mmHg or higher or were on prescription medication for their high blood pressure ( Appendix Table 8 ).
High cholesterol*
- 19.9% had a non-HDL level of 130 to 159 mg/dL.
- 11.5% had a non-HDL level of 160 to 189 mg/dL.
- 8.0% had a non-HDL level of 190 mg/dL or higher.
* Non-high-density lipoprotein cholesterol (non-HDL) contains all the atherogenic lipoproteins, including low-density lipoprotein cholesterol (LDL), very-low-density lipoprotein, lipoprotein(a), and others. Growing evidence supports non-HDL as a better predictor of cardiovascular disease risk than LDL 2 .
Preventing diabetes-related complications
Among U.S. adults aged 18 years or older with diagnosed diabetes, crude data for 2017–2020 shown in Appendix Table 10 indicated:
Usual source for diabetes care
- 78.8% reported having at least one usual source of diabetes care, such as a doctor or other health care professional.
Physical activity
- 24.1% met the recommended goal of at least 150 minutes per week of leisure-time physical activity.
Weight management
- 73.1% reported managing or losing weight to lower their risk for developing certain diseases.
Statin treatment
- 57.8% of adults aged 40–75 years were on statin therapy.
A1C, blood pressure, cholesterol, and smoking (ABCs)
- 11.1% met all these criteria: A1C value <7.0%, blood pressure <130/80 mmHg, non-HDL cholesterol <130 mg/dL, and being a nonsmoker (Table 5).
- 36.8% met all these criteria: A1C value <8.0%, blood pressure <140/90 mmHg, non-HDL cholesterol <160 mg/dL, and being a nonsmoker (Table 5).
Table 5. Crude percentage of adults aged 18 years or older with diagnosed diabetes meeting all ABCs goals , United States, 2017–2020 3 4
| Risk Factor | ABCs goals for many adults | Less stringent ABCs goals |
|---|---|---|
| A1C | <7.0% | <8.0% |
| Blood Pressure | <130/80 mmHg | <140/90 mmHg |
| Cholesterol, non-HDL | <130 mg/dL | <160 mg/dL |
| Smoking, current | Nonsmoker | Nonsmoker |
| Percentage meeting all ABCs goals |
Notes: ABCs = A1C, blood pressure, cholesterol, and smoking. CI = confidence interval. Estimates are crude percentages and 95% confidence intervals.
Data source: 2017–2020 National Health and Nutrition Examination Survey.
Among U.S. adults aged 18 years or older with diagnosed diabetes, crude data for 2021 indicated:
- 94.2% (95% CI, 93.0–95.2) received a blood test for A1C.
- 96.8% (95% CI, 95.8–97.5) had their blood pressure checked.
- 93.0% (95% CI, 91.8–94.1) had their cholesterol checked.
Vaccinations
- 65.9% (95% CI, 63.8–68.0) had received an influenza vaccination in the past year.
- 8.9% (95% CI, 7.6–10.4) had received one COVID-19 vaccination.
- 63.8% (95% CI, 61.5–66.1) had received two COVID-19 vaccinations.
- 8.7% (95% CI, 7.6–10.1) had received more than two COVID-19 vaccinations.
- 35.9% (95% CI, 32.2–39.8) of adults aged 18–59 years had ever received a hepatitis B vaccination.
- 50.7% (95% CI, 48.6–52.9) had ever received a pneumococcal vaccination.
Coexisting conditions and complications
Emergency department visits.
In 2020, about 16.8 million emergency department visits were reported with diabetes as any listed diagnosis among adults aged 18 years or older (Table 6), including:
- 267,000 for hyperglycemic crisis (11.4 per 1,000 adults with diabetes).
- 202,000 for hypoglycemia (8.6 per 1,000 adults with diabetes).
Table 6. Number and rate of emergency department visits per 1,000 adults aged 18 years or older with diabetes for selected causes, United States, 2019 and 2020
| Risk factor | 2019 Number | 2019 Crude rate per 1,000 (95% CI) | ||
|---|---|---|---|---|
| Diabetes as any listed diagnosis | ||||
| 255,000 | 10.9 (10.1–11.7) | 267,000 | 11.4 (10.5–12.3) | |
| Diabetic ketoacidosis | 229,000 | 9.8 (9.1–10.5) | 240,000 | 10.2 (9.4–11.0) |
| Hyperosmolar hyperglycemic syndrome | 26,000 | 1.1 (1.0–1.2) | 27,000 | 1.2 (1.1–1.3) |
| Hypoglycemia | 246,000 | 10.5 (9.7–11.2) | 202,000 | 8.6 (8.0–9.3) |
Note: CI = confidence interval. Numbers rounded to the nearest thousand. Data sources: 2019 and 2020 National Emergency Department Sample; 2019 and 2020 National Health Interview Survey.
In 2020, of the emergency department visits with diabetes as any listed diagnosis among U.S. adults aged 18 years or older, disposition data (see Appendix B: Detailed Methods and Data Sources ) indicated:
- 54.9% were treated and released; 38.4% were admitted to the hospital; 2.5% were transferred to another hospital; 2.6% were transferred to a skilled nursing facility, an intermediate care facility, or home with home health care; 1.3% left against medical advice; 0.3% died; and <0.1% had unknown disposition but were not admitted to a hospital.
- Of those ED visits involving hypoglycemia, 66.8% were treated and released, 25.1% were admitted to the hospital, and <0.1% died.
- Of the ED visits involving hyperglycemic crisis, 8.4% were treated and released, 84.4% were admitted to the hospital, and <0.1% died.
Hospitalizations
In 2020, a total of 7.86 million hospital discharges were reported with diabetes as any listed diagnosis among U.S. adults aged 18 years or older (335.4 per 1,000 adults with diabetes) (Table 7). These discharges included:
- 368,000 for ischemic heart disease (15.7 per 1,000 adults with diabetes).
- 321,000 for stroke (13.7 per 1,000 adults with diabetes).
- 160,000 for a lower-extremity amputation (6.8 per 1,000 adults with diabetes).
- 232,000 for hyperglycemic crisis (9.9 per 1,000 adults with diabetes).
- 51,000 for hypoglycemia (2.2 per 1,000 adults with diabetes).
Table 7. Number and rate of hospitalizations per 1,000 adults aged 18 years or older with diabetes for selected causes, United States, 2019 and 2020
| Risk factor | 2019 Number | 2019 Crude rate per 1,000 (95% CI) | ||
|---|---|---|---|---|
| Diabetes as any listed diagnosis | ||||
| 1,920,000 | 82.0 (77.4–86.5) | 1,677,000 | 71.6 (67.4–75.8) | |
| Ischemic heart disease | 443,000 | 18.9 (17.8–20.0) | 368,000 | 15.7 (14.7–16.7) |
| Stoke | 346,000 | 14.8 (13.9–15.6) | 321,000 | 13.7 (12.9–14.5) |
| 162,000 | 6.9 (6.5–7.3) | 160,000 | 6.8 (6.4–7.2) | |
| 231,000 | 9.9 (9.3–10.4) | 232,000 | 9.9 (9.3–10.5) | |
| Diabetic ketoacidosis | 205,000 | 8.8 (8.3–9.2) | 206,000 | 8.8 (8.3–9.3) |
| Hyperosmolar hyperglycemic syndrome | 26,000 | 1.1 (1.0–1.2) | 26,000 | 1.1 (1.1–1.2) |
| 60,000 | 2.5 (2.4–2.7) | 51,000 | 2.2 (2.1–2.3) |
Notes: CI = confidence interval. Numbers rounded to the nearest thousand. Data sources: 2019 and 2020 National Inpatient Sample; 2019 and 2020 National Health Interview Survey.
Kidney disease
Among U.S. adults aged 18 years or older with diagnosed diabetes, crude data for 2017–2020 shown in Appendix Table 11 indicated:
- 15.7% had moderate to severe CKD (stage 3 or 4).
- 23.1% of non-Hispanic Black adults, 17.2% of non-Hispanic White adults, and 8.9% of Hispanic adults had moderate to severe CKD (stage 3 or 4).
- 32.5% with moderate to severe CKD (stage 3 or 4) were aware of their kidney disease.
- 40.9% had chronic kidney disease (CKD, stages 1–4), based on the 2009 CKD-EPI eGFR equation, which included a factor for non-Hispanic Black race.
- A total of 61,522 people developed end-stage kidney disease with diabetes as the primary cause.
- Crude incidence of end-stage kidney disease with diabetes as the primary cause was 192.7 per 1 million population (61,522 new cases). Adjusted for age group, sex, and racial or ethnic group, the rate was 179.5 per 1 million people.
- The proportion of end-stage kidney disease with diabetes listed as the primary cause was 39.2% (307,385 out of 783,594 people). As a result, diabetes was the leading cause of end-stage kidney disease, followed by high blood pressure (26.7%), glomerulonephritis (14.6%), and cystic kidney disease (5.0%).
Vision disability
- Diabetes is the leading cause of new cases of blindness among adults aged 18–64 years.
- 10.1% (95% CI, 9.6%–11.3%) reported severe vision difficulty or blindness.
- In 2021, diabetes was the eighth leading cause of death in the United States. This finding is based on 103,294 death certificates in which diabetes was listed as the underlying cause of death (crude rate, 31.1 per 100,000 people).
- In 2021, there were 399,401 death certificates with diabetes listed as the underlying or contributing cause of death (crude rate, 120.3 per 100,000 people).
- The total direct and indirect estimated costs* of diagnosed diabetes in the United States in 2022 was $413 billion.
- Total direct estimated costs of diagnosed diabetes increased from $227 billion in 2012 to $307 billion in 2022 (2022 dollars). Total indirect costs increased from $89 billion to $106 billion in the same period (2022 dollars).
- From 2012 to 2022, excess medical costs per person associated with diabetes increased from $10,179 to $12,022 (2022 dollars).
* Direct costs = medical costs; indirect costs = lost productivity from work-related absenteeism, reduced productivity at work and at home, unemployment from chronic disability, and premature mortality.
- Wagenknecht LE, Lawrence JM, Isom S, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol . 2023;11(4):242–250. doi: 10.1016/S2213-8587(23)00025-6
- Su X, Kong Y, Peng D. Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis . 2019;18(1):134.
- American Diabetes Association. Standards of medical care in diabetes—2023. Diabetes Care . 2023;46 (suppl 1).
- American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for the management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract . 2017;23(suppl 2).
- Centers for Disease Control and Prevention. CDC WONDER. About Underlying Cause of Death 1999–2020. Accessed April 25, 2023. https://wonder.cdc.gov/ucd-icd10.html
- Parker ED, Lin J, Mahoney T, Ume N, Yang G, Gabbay RA, ElSayed NA, Bannuru RR. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care. 2024;47(1):26–43. doi: 10.2337/dci23-0085. Online ahead of print.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report website. https://www.cdc.gov/diabetes/php/data-research/index.html Accessed [date].
Diabetes is a chronic disease that affects how your body turns food into energy. About 1 in 10 Americans has diabetes.
For Everyone
Health care providers, public health.

History of the Diabetes Control and Complications Trial and Its Follow-up Epidemiology of Diabetes Interventions and Complications Study: Studies That Changed the Treatment of Type 1 Diabetes
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Cite Icon Cite
- Get Permissions
David M. Nathan , John M. Lachin; History of the Diabetes Control and Complications Trial and Its Follow-up Epidemiology of Diabetes Interventions and Complications Study: Studies That Changed the Treatment of Type 1 Diabetes. Diabetes Care 27 August 2024; 47 (9): 1511–1517. https://doi.org/10.2337/dci24-0063
Download citation file:
- Ris (Zotero)
- Reference Manager
This article is part of a special article collection available at diabetesjournals.org/collection/2296/DCCT-EDIC-40th-Anniversary-Collection.
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
This article is part of a special article collection available at diabetesjournals.org/collection/2296/DCCT-EDIC-40th-Anniversary-Collection .
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air .

Sign in via ADA
Sign in via your institution, email alerts.
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Diabetes mellitus: an overview of the types, symptoms, complications and management
Affiliation.
- 1 School of Nursing, Avondale University, Wahroonga, Sydney NSW, Australia.
- PMID: 34708622
- DOI: 10.7748/ns.2021.e11709
The incidence of diabetes mellitus is rapidly increasing, and this condition often results in significant metabolic disease and severe complications. Nurses have a crucial role in monitoring, educating and supporting people with diabetes, as well as their families and significant others. This article provides an overview of the main types and common symptoms of diabetes, its acute and long-term complications and its management. It also outlines the nurse's role in diabetes care, which frequently includes assessing and empowering patients.
Keywords: blood glucose; clinical; diabetes; diabetic foot ulcers; diabetic ketoacidosis; glycaemic control; hyperglycaemia; hypoglycaemia; insulin; type 1 diabetes; type 2 diabetes.
© 2021 RCN Publishing Company Ltd. All rights reserved. Not to be copied, transmitted or recorded in any way, in whole or part, without prior permission of the publishers.
PubMed Disclaimer
Conflict of interest statement
None declared
Similar articles
- Perioperative care of the adult diabetic patient. Lloyd H. Lloyd H. J Perioper Pract. 2020 Dec;30(12):372-377. doi: 10.1177/1750458920915660. Epub 2020 Apr 17. J Perioper Pract. 2020. PMID: 32301384
- [Diabetes mellitus in children]. Körner A. Körner A. Orv Hetil. 2005 Jun 19;146(25):1335-43. Orv Hetil. 2005. PMID: 16106756 Review. Hungarian.
- Children and young people with diabetes: recognition and management. Hamilton H, Knudsen G, Vaina CL, Smith M, Paul SP. Hamilton H, et al. Br J Nurs. 2017 Mar 23;26(6):340-347. doi: 10.12968/bjon.2017.26.6.340. Br J Nurs. 2017. PMID: 28345986
- Understanding the principles of insulin use in type 1 and type 2 diabetes management. Smyth T. Smyth T. Nurs Stand. 2021 Jan 13;36(1):61-66. doi: 10.7748/ns.2020.e11677. Epub 2020 Dec 30. Nurs Stand. 2021. PMID: 33377355
- Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus. Plosker GL, Figgitt DP. Plosker GL, et al. Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
- Bergenin mitigates neuroinflammatory damage induced by high glucose: insights from Zebrafish, murine microbial cell line, and rat models. Yu W, Luo R, He C, Li Z, Yang M, Zhou J, He J, Chen Q, Song Z, Cheng S. Yu W, et al. Front Pharmacol. 2024 Aug 1;15:1339178. doi: 10.3389/fphar.2024.1339178. eCollection 2024. Front Pharmacol. 2024. PMID: 39148536 Free PMC article.
- Anethole Prevents the Alterations Produced by Diabetes Mellitus in the Sciatic Nerve of Rats. Barbosa-Ferreira BS, Silva FERD, Gomes-Vasconcelos YA, Joca HC, Coelho-de-Souza AN, Ferreira-da-Silva FW, Leal-Cardoso JH, Silva-Alves KSD. Barbosa-Ferreira BS, et al. Int J Mol Sci. 2024 Jul 25;25(15):8133. doi: 10.3390/ijms25158133. Int J Mol Sci. 2024. PMID: 39125701 Free PMC article.
- Association between weight-adjusted waist index and risk of diabetes mellitus type 2 in United States adults and the predictive value of obesity indicators. Li X, Zhao D, Wang H. Li X, et al. BMC Public Health. 2024 Jul 29;24(1):2025. doi: 10.1186/s12889-024-19576-6. BMC Public Health. 2024. PMID: 39075353 Free PMC article.
- Sequential Impact of Diabetes Mellitus on Deep Neck Infections: Comparison of the Clinical Characteristics of Patients with and without Diabetes Mellitus. Liao TI, Ho CY, Chin SC, Wang YC, Chan KC, Chen SL. Liao TI, et al. Healthcare (Basel). 2024 Jul 10;12(14):1383. doi: 10.3390/healthcare12141383. Healthcare (Basel). 2024. PMID: 39057526 Free PMC article.
- The effect of socioeconomic status, depression, and diabetes symptoms severity on diabetes patient's life satisfaction in India. Ranjan S, Thakur R. Ranjan S, et al. Sci Rep. 2024 May 28;14(1):12210. doi: 10.1038/s41598-024-62814-5. Sci Rep. 2024. PMID: 38806560 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
- Genetic Alliance
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 June 2022
The burden and risks of emerging complications of diabetes mellitus
- Dunya Tomic ORCID: orcid.org/0000-0003-2471-2523 1 , 2 ,
- Jonathan E. Shaw ORCID: orcid.org/0000-0002-6187-2203 1 , 2 na1 &
- Dianna J. Magliano ORCID: orcid.org/0000-0002-9507-6096 1 , 2 na1
Nature Reviews Endocrinology volume 18 , pages 525–539 ( 2022 ) Cite this article
56k Accesses
307 Citations
55 Altmetric
Metrics details
- Diabetes complications
- Type 1 diabetes
- Type 2 diabetes
The traditional complications of diabetes mellitus are well known and continue to pose a considerable burden on millions of people living with diabetes mellitus. However, advances in the management of diabetes mellitus and, consequently, longer life expectancies, have resulted in the emergence of evidence of the existence of a different set of lesser-acknowledged diabetes mellitus complications. With declining mortality from vascular disease, which once accounted for more than 50% of deaths amongst people with diabetes mellitus, cancer and dementia now comprise the leading causes of death in people with diabetes mellitus in some countries or regions. Additionally, studies have demonstrated notable links between diabetes mellitus and a broad range of comorbidities, including cognitive decline, functional disability, affective disorders, obstructive sleep apnoea and liver disease, and have refined our understanding of the association between diabetes mellitus and infection. However, no published review currently synthesizes this evidence to provide an in-depth discussion of the burden and risks of these emerging complications. This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations, highlight gaps and discrepancies in the evidence, and consider implications for the future management of diabetes mellitus.
With advances in the management of diabetes mellitus, evidence is emerging of an increased risk and burden of a different set of lesser-known complications of diabetes mellitus.
As mortality from vascular diseases has declined, cancer and dementia have become leading causes of death amongst people with diabetes mellitus.
Diabetes mellitus is associated with an increased risk of various cancers, especially gastrointestinal cancers and female-specific cancers.
Hospitalization and mortality from various infections, including COVID-19, pneumonia, foot and kidney infections, are increased in people with diabetes mellitus.
Cognitive and functional disability, nonalcoholic fatty liver disease, obstructive sleep apnoea and depression are also common in people with diabetes mellitus.
As new complications of diabetes mellitus continue to emerge, the management of this disorder should be viewed holistically, and screening guidelines should consider conditions such as cancer, liver disease and depression.
Similar content being viewed by others
Type 2 diabetes mellitus in older adults: clinical considerations and management
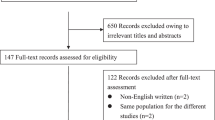
The cross-sectional and longitudinal relationship of diabetic retinopathy to cognitive impairment: a systematic review and meta-analysis

Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025
Introduction.
Diabetes mellitus is a common, albeit potentially devastating, medical condition that has increased in prevalence over the past few decades to constitute a major public health challenge of the twenty-first century 1 . Complications that have traditionally been associated with diabetes mellitus include macrovascular conditions, such as coronary heart disease, stroke and peripheral arterial disease, and microvascular conditions, including diabetic kidney disease, retinopathy and peripheral neuropathy 2 (Fig. 1 ). Heart failure is also a common initial manifestation of cardiovascular disease in patients with type 2 diabetes mellitus (T2DM) 3 and confers a high risk of mortality in those with T1DM or T2DM 4 . Although a great burden of disease associated with these traditional complications of diabetes mellitus still exists, rates of these conditions are declining with improvements in the management of diabetes mellitus 5 . Instead, as people with diabetes mellitus are living longer, they are becoming susceptible to a different set of complications 6 . Population-based studies 7 , 8 , 9 show that vascular disease no longer accounts for most deaths among people with diabetes mellitus, as was previously the case 10 . Cancer is now the leading cause of death in people with diabetes mellitus in some countries or regions (hereafter ‘countries/regions’) 9 , and the proportion of deaths due to dementia has risen since the turn of the century 11 . In England, traditional complications accounted for more than 50% of hospitalizations in people with diabetes mellitus in 2003, but for only 30% in 2018, highlighting the shift in the nature of complications of this disorder over this corresponding period 12 .
The traditional complications of diabetes mellitus include stroke, coronary heart disease and heart failure, peripheral neuropathy, retinopathy, diabetic kidney disease and peripheral vascular disease, as represented on the left-hand side of the diagram. With advances in the management of diabetes mellitus, associations between diabetes mellitus and cancer, infections, functional and cognitive disability, liver disease and affective disorders are instead emerging, as depicted in the right-hand side of the diagram. This is not an exhaustive list of complications associated with diabetes mellitus.
Cohort studies have reported associations of diabetes mellitus with various cancers, functional and cognitive disability, liver disease, affective disorders and sleep disturbance, and have provided new insights into infection-related complications of diabetes mellitus 13 , 14 , 15 , 16 , 17 . Although emerging complications have been briefly acknowledged in reviews of diabetes mellitus morbidity and mortality 11 , 17 , no comprehensive review currently specifically provides an analysis of the evidence for the association of these complications with diabetes mellitus. In this Review, we synthesize information published since the year 2000 on the risks and burden of emerging complications associated with T1DM and T2DM.
Diabetes mellitus and cancer
The burden of cancer mortality.
With the rates of cardiovascular mortality declining amongst people with diabetes mellitus, cancer deaths now constitute a larger proportion of deaths among this population in some countries/regions 8 , 9 . Although the proportion of deaths due to cancer appears to be stable, at around 16–20%, in the population with diabetes mellitus in the USA 7 , in England it increased from 22% to 28% between 2001 and 2018 (ref. 9 ), with a similar increase reported in Australia 8 . Notably, in England, cancer has overtaken vascular disease as the leading cause of death in people with diabetes mellitus and it is the leading contributor to excess mortality in those with diabetes mellitus compared with those without 9 . These findings are likely to be due to a substantial decline in the proportion of deaths from vascular diseases, from 44% to 24% between 2001 and 2018, which is thought to reflect the targeting of prevention measures in people with diabetes mellitus 18 . Over the same time period, cancer mortality rates fell by much less in the population with diabetes mellitus than in that without diabetes 9 , suggesting that clinical approaches for diabetes mellitus might focus too narrowly on vascular complications and might require revision 19 . In addition, several studies have reported that female patients with diabetes mellitus receive less-aggressive treatment for breast cancer compared with patients without diabetes mellitus, particularly with regard to chemotherapy 20 , 21 , 22 , suggesting that this treatment approach might result in increased cancer mortality rates in women with diabetes mellitus compared with those without diabetes mellitus. Although substantial investigation of cancer mortality in people with diabetes mellitus has been undertaken in high-income countries/regions, there is a paucity of evidence from low-income and middle-income countries/regions. It is important to understand the potential effect of diabetes mellitus on cancer mortality in these countries/regions owing to the reduced capacity of health-care systems in these countries/regions to cope with the combination of a rising prevalence of diabetes mellitus and rising cancer mortality rates in those with diabetes mellitus. One study in Mauritius showed a significantly increased risk of all-cause cancer mortality in patients with T2DM 23 , but this study has yet to be replicated in other low-income and middle-income countries/regions.
Gastrointestinal cancers
Of the reported associations between diabetes mellitus and cancer (Table 1 ), some of the strongest have been demonstrated for gastrointestinal cancers.
Hepatocellular carcinoma
In the case of hepatocellular carcinoma, the most rigorous systematic review on the topic — comprising 18 cohort studies with a combined total of more than 3.5 million individuals — reported a summary relative risk (SRR) of 2.01 (95% confidence interval (CI) 1.61–2.51) for an association with diabetes mellitus 24 . This increased risk of hepatocellular carcinoma with diabetes mellitus is supported by the results of another systematic review that included case–control studies 25 . Another review also found that diabetes mellitus independently increased the risk of hepatocellular carcinoma in the setting of hepatitis C virus infection 26 .
Pancreatic cancer
The risk of pancreatic cancer appears to be approximately doubled in patients with T2DM compared with patients without T2DM. A meta-analysis of 36 studies found an adjusted odds ratio (OR) of 1.82 (95% CI 1.66–1.89) for pancreatic cancer among people with T2DM compared with patients without T2DM 27 (Table 1 ). However, it is possible that these findings are influenced by reverse causality — in this scenario, diabetes mellitus is triggered by undiagnosed pancreatic cancer 28 , with pancreatic cancer subsequently being clinically diagnosed only after the diagnosis of diabetes mellitus. Nevertheless, although the greatest risk (OR 2.05, 95% CI 1.87–2.25) of pancreatic cancer was seen in people diagnosed with T2DM 1–4 years previously compared with people without T2DM, those with a diagnosis of T2DM of more than 10 years remained at increased risk of pancreatic cancer (OR 1.51, 95% CI 1.16–1.96) 27 , suggesting that reverse causality can explain only part of the association between T2DM and pancreatic cancer. Although T2DM accounts for ~90% of all cases of diabetes mellitus 29 , a study incorporating data from five nationwide diabetes registries also reported an increased risk of pancreatic cancer amongst both male patients (HR 1.53, 95% CI 1.30–1.79) and female patients (HR 1.25, 95% CI 1.02–1.53) with T1DM 30 .
Colorectal cancer
For colorectal cancer, three systematic reviews have shown a consistent 20–30% increased risk associated with diabetes mellitus 31 , 32 , 33 . One systematic review, which included more than eight million people across 30 cohort studies, reported an incidence SRR of 1.27 (95% CI 1.21–1.34) of colorectal cancer 31 , independent of sex and family history (Table 1 ). Similar increases in colorectal cancer incidence in patients with diabetes mellitus were reported in a meta-analysis of randomized controlled trials (RCTs) and cohort studies 32 and in a systematic review that included cross-sectional studies 33 .
Female-specific cancers
Endometrial, breast and ovarian cancers all occur more frequently in women with diabetes mellitus than in women without diabetes mellitus.
Endometrial cancer
For endometrial cancer, one systematic review of 29 cohort studies and a combined total of 5,302,259 women reported a SRR of 1.89 (95% CI 1.46–2.45) and summary incidence rate ratio (IRR) of 1.61 (95% CI 1.51–1.71) 34 (Table 1 ). Similar increased risks were found in two systematic reviews incorporating cross-sectional studies 35 , 36 , one of which found a particularly strong association of T1DM (relative risk (RR) 3.15, 95% CI 1.07–9.29) with endometrial cancer.
Breast cancer
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR) for the incidence of breast cancer in women with diabetes mellitus compared with women without diabetes mellitus was 1.23 (95% CI 1.12–1.34) 32 (Table 1 ). Two further systematic reviews have also shown this increased association 37 , 38 .
The association of diabetes mellitus with breast cancer appears to vary according to menopausal status. In a meta-analysis of studies of premenopausal women with diabetes mellitus, no significant association with breast cancer was found 39 , whereas in 11 studies that included only postmenopausal women, the SRR was 1.15 (95% CI 1.07–1.24). The difference in breast cancer risk between premenopausal and postmenopausal women with diabetes mellitus was statistically significant. The increased risk of breast cancer after menopause in women with diabetes mellitus compared with women without diabetes mellitus might result from the elevated concentrations and increased bioavailability of oestrogen that are associated with adiposity 40 , which is a common comorbidity in those with T2DM; oestrogen synthesis occurs in adipose tissue in postmenopausal women, while it is primarily gonadal in premenopausal women 41 . Notably, however, there is evidence that hormone-receptor-negative breast cancers, which typically carry a poor prognosis, occur more frequently in women with breast cancer and diabetes mellitus than in women with breast cancer and no diabetes mellitus 42 , indicating that non-hormonal mechanisms also occur.
Ovarian cancer
Diabetes mellitus also appears to increase the risk of ovarian cancer, with consistent results from across four systematic reviews. A pooled RR of 1.32 (95% CI 1.14–1.52) was reported across 15 cohort studies and a total of more than 2.3 million women 43 (Table 1 ). A SRR of 1.19 (95% CI 1.06–1.34) was found across 14 cohort studies and 3,708,313 women 44 . Similar risks were reported in meta-analyses that included cross-sectional studies 45 , 46 .
Male-specific cancers: prostate cancer
An inverse association between diabetes mellitus and prostate cancer has been observed in a systematic review (RR 0.91, 95% CI 0.86–0.96) 47 , and is probably due to reduced testosterone levels that occur secondary to the low levels of sex hormone-binding globulin that are commonly seen in men with T2DM and obesity 48 . Notably, however, the systematic review that showed the inverse association involved mostly white men (Table 1 ), whereas a systematic review of more than 1.7 million men from Taiwan, Japan, South Korea and India found that diabetes mellitus increased prostate cancer risk 49 , suggesting that ethnicity might be an effect modifier of the diabetes mellitus–prostate cancer relationship. The mechanisms behind this increased risk in men in regions of Asia such as Taiwan and Japan, where most study participants came from, remain unclear. Perhaps, as Asian men develop diabetes mellitus at lower levels of total adiposity than do white men 50 , the adiposity associated with diabetes mellitus in Asian men might have a lesser impact on sex hormone-binding globulin and testosterone than it does in white men. Despite the reported inverse association between diabetes mellitus and prostate cancer in white men, however, evidence suggests that prostate cancers that do develop in men with T2DM are typically more aggressive, conferring higher rates of disease-specific mortality than prostate cancers in men without diabetes mellitus 51 .
An assessment of cancer associations
As outlined above, a wealth of data has shown that diabetes mellitus is associated with an increased risk of various cancers. It has been argued, however, that some of these associations could be due to detection bias resulting from increased surveillance of people with diabetes mellitus in the immediate period after diagnosis 52 , or reverse causality, particularly in the case of pancreatic cancer 53 . However, neither phenomenon can account for the excess risks seen in the longer term. An Australian study exploring detection bias and reverse causality found that standardized mortality ratios (SMRs) for several cancer types in people with diabetes mellitus compared with the general population fell over time, but remained elevated beyond 2 years for pancreatic and liver cancers 54 , suggesting that diabetes mellitus is a genuine risk factor for these cancer types.
A limitation of the evidence that surrounds diabetes mellitus and cancer risk is high clinical and methodological heterogeneity across several of the large systematic reviews, which makes it difficult to be certain of the effect size in different demographic groups. Additionally, many of the studies exploring a potential association between diabetes mellitus and cancer were unable to adjust for BMI, which is a major confounder. However, a modelling study that accounted for BMI found that although 2.1% of cancers worldwide in 2012 were attributable to diabetes mellitus as an independent risk factor, twice as many cancers were attributable to high BMI 55 , so it is likely that effect sizes for cancer risk associated with diabetes mellitus would be attenuated after adjustment for BMI. Notably, however, low-income and middle-income countries/regions had the largest increase in the numbers of cases of cancer attributable to diabetes mellitus both alone and in combination with BMI 55 , highlighting the need for public health intervention, given that these countries/regions are less equipped than high-income countries/regions to manage a growing burden of cancer.
As well as the cancer types outlined above, diabetes mellitus has also been linked to various other types of cancer, including kidney cancer 56 , bladder cancer 57 and haematological malignancies; however, the evidence for these associations is not as strong as for the cancers discussed above 58 . Diabetes mellitus might also be associated with other cancer types such as small intestine cancer, but the rarity of some of these types makes it difficult to obtain sufficient statistical power in analyses of any potential association.
Potential aetiological mechanisms
Several aetiological mechanisms that might be involved in linking diabetes mellitus to cancer have been proposed, including hyperinsulinaemia, hyperglycaemia, inflammation and cellular signalling mechanisms.
Hyperinsulinaemia
Most cancer cells express insulin receptors, through which hyperinsulinaemia is thought to stimulate cancer cell proliferation and metastasis 59 . Hyperinsulinaemia might also promote carcinogenesis through increased local levels of insulin-like growth factor 1 (IGF1), which has potent mitogenic and anti-apoptotic activities 60 , owing to decreased levels of insulin-like growth factor binding proteins. As outlined above, people with diabetes mellitus show a strong risk of pancreatic and liver cancers; this increased risk might occur because insulin is produced by pancreatic β-cells and transported to the liver via the portal vein 61 , thereby exposing the liver and pancreas to high levels of endogenous insulin 59 .
Hyperglycaemia and inflammation
Hyperglycaemia can induce DNA damage 62 , increase the generation of reactive oxygen species 63 and downregulate antioxidant expression 64 , all of which are associated with cancer development. Inflammatory markers, including cytokines such as IL-6, appear to have an important role in the association between diabetes and cancer 65 .
Cellular signalling mechanisms
Several cellular signalling components are common to the pathogenesis of T2DM and cancer. These include the mechanistic target of rapamycin (mTOR), a central controller of cell growth and proliferation; AMP-activated protein kinase, a cellular energy sensor and signal transducer 66 ; and the phosphatidylinositol 3-kinase (PI3K)–AKT pathway, which transduces growth factor signals during organismal growth, glucose homeostasis and cell proliferation 67 . Dysregulation of any of these cellular signalling components or pathways could contribute to the development of cancer and metabolic disorders, including T2DM, and glucose-lowering drugs such as metformin have been associated with a reduction in cancer cell proliferation through effective inhibition of some of these components 68 .
Diabetes mellitus and infections
Infection-related complications.
Although infection has long been recognized as a complication of diabetes mellitus, an association between diabetes mellitus and infection has not been well documented in epidemiological studies 69 . Only in the past decade have major studies quantified the burden of infection-related complications in people with diabetes mellitus and explored the specific infections accounting for this burden. In a US cohort of 12,379 participants, diabetes mellitus conferred a significant risk of infection-related hospitalization, with an adjusted HR of 1.67 (95% CI 1.52–1.83) compared with people without diabetes mellitus 70 (Table 2 ). The association was most pronounced for foot infections (HR 5.99, 95% CI 4.38–8.19), with significant associations also observed for respiratory infection, urinary tract infection, sepsis and post-operative infection, but not for gastrointestinal infection, a category that included appendicitis and gastrointestinal abscesses but not viral or bacterial gastroenteritis. Interestingly, a report from Taiwan demonstrated an association between the use of metformin and a lower risk of appendicitis 71 .
In an analysis of the entire Hong Kong population over the period 2001–2016, rates of hospitalization for all types of infection remained consistently higher in people with diabetes mellitus than in those without diabetes mellitus 72 . The strongest association was seen for hospitalization due to kidney infections, for which the adjusted RR was 4.9 (95% CI 3.9–6.2) in men and 3.2 (95% CI 2.8–3.7) in women with diabetes mellitus compared with those without diabetes mellitus in 2016 (Table 2 ). Diabetes mellitus roughly doubled the risk of hospitalization from tuberculosis or sepsis. The most common cause of infection-related hospitalization was pneumonia, which accounted for 39% of infections across the study period, while no other single cause accounted for more than 25% of infections across the same period. Pneumonia-related hospitalization rates increased substantially from 2001 to 2005, probably as a result of the 2003 severe acute respiratory syndrome (SARS) epidemic and the decreased threshold for pneumonia hospitalization in the immediate post-epidemic period. Rates for hospitalization for influenza increased from 2002 to 2016, possibly because of changes in the virus and increased testing for influenza. Declining rates of hospitalization for tuberculosis, urinary tract infections, foot infections and sepsis could be due to improvements in the management of diabetes mellitus.
Infection-related mortality rates were found to be significantly elevated among 1,108,982 Australians with diabetes mellitus studied over the period 2000–2010 compared with rates in people without diabetes mellitus 73 . For overall infection-related mortality, SMRs were 4.42 (95% CI 3.68–5.34) for T1DM and 1.47 (95% CI 1.42–1.53) for people with T2DM compared with those without diabetes mellitus (Table 2 ). Substantially higher infection-related mortality rates were seen in people with T1DM compared with those with T2DM for all infection types, even after accounting for age. Hyperglycaemia is thought to be a driver of infection amongst people with diabetes mellitus (see below) 73 , which might explain the higher SMRs amongst people with T1DM, in whom hyperglycaemia is typically more severe, than in those with T2DM. The highest SMRs were seen for osteomyelitis, and SMRs for septicaemia and pneumonia were also greater than 1.0 for both types of diabetes mellitus compared with those without diabetes mellitus.
Post-operative infection
Post-operative infection is also an important complication of diabetes mellitus. In a meta-analysis, diabetes mellitus was found to be associated with an OR of 1.77 (95% CI 1.13–2.78) for surgical site infection across studies that adjusted for confounding factors 74 (Table 2 ). The effect size appears to be greatest after cardiac procedures, and one US study of patients undergoing coronary artery bypass grafting found diabetes mellitus to be an independent predictor of surgical site infection, with an OR of 4.71 (95% CI 2.39–9.28) compared with those without diabetes mellitus 75 . Risks of infection of more than threefold were reported in some studies of gynaecological 76 and spinal surgery 77 in people with diabetes mellitus compared with those without diabetes mellitus. Increased risks of infection among people with diabetes mellitus were also observed in studies of colorectal and breast surgery and arthroplasty, suggesting that the association between diabetes mellitus and post-operative infection is present across a wide range of types of surgery 74 .
Respiratory infections
The incidence of hospitalizations due to respiratory infections among people with diabetes mellitus was increasing substantially even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, probably owing to increased life expectancy in these patients as well as an increased likelihood of them being hospitalized for conditions such as respiratory infections, which occur mostly in older age 12 . This rising burden of respiratory infection, in combination with the rising prevalence of diabetes mellitus, highlights the importance of addressing the emerging complications of diabetes mellitus to minimize impacts on health-care systems in current and future global epidemics.
Although diabetes mellitus does not appear to increase the risk of becoming infected with COVID-19 (ref. 78 ), various population-based studies have reported increased risks of COVID-19 complications among people with diabetes mellitus. In a study of the total Scottish population, people with diabetes mellitus were found to have an increased risk of fatal or critical care unit-treated COVID-19, with an adjusted OR of 1.40 (95% CI 1.30–1.50) compared with those without diabetes mellitus 79 (Table 2 ). The risk was particularly high for those with T1DM (OR 2.40, 95% CI 1.82–3.16) 79 . Both T1DM and T2DM have been linked to a more than twofold increased risk of hospitalization with COVID-19 in a large Swedish cohort study 80 . In South Korean studies, T2DM was linked to intensive care unit admission among patients with COVID-19 infection 81 , and diabetes mellitus (either T1DM or T2DM) was linked to a requirement for ventilation and oxygen therapy 82 in patients with COVID-19. Diabetes mellitus appears to be the primary predisposing factor for opportunistic infection with mucormycosis in individuals with COVID-19 (ref. 83 ). The evidence for diabetes mellitus as a risk factor for post-COVID-19 syndrome is inconclusive 84 , 85 . Interestingly, an increase in the incidence of T1DM during the COVID-19 pandemic has been reported in several countries/regions 86 , and some data suggest an increased risk of T1DM after COVID-19 infection 87 , but the evidence regarding a causal effect is inconclusive.
Pneumonia, MERS, SARS and H1N1 influenza
The data regarding diabetes mellitus and COVID-19 are consistent with the published literature regarding other respiratory infections, such as pneumonia, for which diabetes mellitus has been shown to increase the risk of hospitalization 88 and mortality 88 , with similar effect sizes to those seen for COVID-19, compared with no diabetes mellitus. Diabetes mellitus has also been also linked to adverse outcomes in people with Middle East respiratory syndrome (MERS), SARS and H1N1 influenza 89 , 90 , 91 , 92 , suggesting that mechanisms specific to COVID-19 are unlikely to be responsible for the relationship between diabetes mellitus and COVID-19. Unlike the case for COVID-19, there is evidence that people with diabetes mellitus are at increased risk of developing certain other respiratory infections, namely pneumonia 93 and possibly also MERS 94 .
The mechanisms that might link diabetes mellitus and infection include a reduced T cell response, reduced neutrophil function and disorders of humoral immunity.
Mononuclear cells and monocytes of individuals with diabetes mellitus secrete less IL-1 and IL-6 than the same cells from people without diabetes mellitus 95 . The release of IL-1 and IL-6 by T cells and other cell types in response to infection has been implicated in the response to several viral infections 96 . Thus, the reduced secretion of these cytokines in patients with diabetes mellitus might be associated with the poorer responses to infection observed among these patients compared with people without diabetes mellitus.
In the context of neutrophil function, hyperglycaemic states might give rise to reductions in the mobilization of polymorphonuclear leukocytes, phagocytic activity and chemotaxis 97 , resulting in a decreased immune response to infection. Additionally, increased levels of glucose in monocytes isolated from patients with obesity and/or diabetes mellitus have been found to promote viral replication in these cells, as well as to enhance the expression of several cytokines, including pro-inflammatory cytokines that are associated with the COVID-19 ‘cytokine storm’; furthermore, glycolysis was found to sustain the SARS coronavirus 2 (SARS-CoV-2)-induced monocyte response and viral replication 98 .
Elevated glucose levels in people with diabetes mellitus are also associated with an increase in glycation, which, by promoting a change in the structure and/or function of several proteins and lipids, is responsible for many of the complications of diabetes mellitus 99 . In people with diabetes mellitus, antibodies can become glycated, a process that is thought to impair their biological function 100 . Although the clinical relevance of this impairment is not clear, it could potentially explain the results of an Israeli study that reported reduced COVID-19 vaccine effectiveness among people with T2DM compared with those without T2DM 101 .
Diabetes mellitus and liver disease
Nonalcoholic fatty liver disease.
The consequences of nonalcoholic fatty liver disease (NAFLD) make it important to recognize the burden of this disease among people with diabetes mellitus. NAFLD and nonalcoholic steatohepatitis (NASH; an advanced form of NAFLD) are major causes of liver transplantation in the general population. In the USA, NASH accounted for 19% of liver transplantations in 2016 — second only to alcoholic liver disease, which was the cause of 24% of transplantations 102 . In Australia and New Zealand, NAFLD was the primary diagnosis in 9% of liver transplant recipients in 2019, only slightly below the figure for alcoholic cirrhosis of 13% 103 . In Europe, NASH increased as the reason for transplantations from 1% in 2002 to more than 8% in 2016, in parallel with the rising prevalence of diabetes mellitus 104 .
NAFLD is highly prevalent among people with T2DM. In a systematic review of 80 studies across 20 countries/regions, the prevalence of NAFLD among 49,419 people with T2DM was 56% 105 , while the global prevalence of NAFLD in the general population is estimated to be 25% 106 . In a Chinese cohort study of 512,891 adults, diabetes mellitus was associated with an adjusted HR of 1.76 (95% CI 1.47–2.16) for NAFLD compared with no diabetes mellitus 107 (Table 3 ). Another smaller longitudinal Chinese study also reported an increased risk of developing NAFLD among those with T2DM compared with those without T2DM 108 . However, most evidence regarding the association between NAFLD and diabetes mellitus is from cross-sectional studies 109 , 110 , 111 .
NASH and fibrosis
Diabetes mellitus appears to enhance the risk of NAFLD complications, including NASH and fibrosis. An analysis of 892 people with NAFLD and T2DM across 10 studies showed that the prevalence of NASH was 37% (ref. 105 ); figures for the prevalence of NASH in the general population with NAFLD vary greatly across different study populations, ranging from 16% to 68% 112 . Amongst 439 people with T2DM and NAFLD in seven studies, 17% had advanced fibrosis 105 . An analysis of 1,069 people with NAFLD in a US study found that diabetes mellitus was an independent predictor for NASH (OR 1.93, 95% CI 1.37–2.73) and fibrosis (3.31, 95% CI 2.26–4.85) 113 .
Bidirectional relationship between diabetes mellitus and liver disease
The relationship between diabetes mellitus and NAFLD is bidirectional, as NAFLD is associated with an increased risk of developing T2DM 114 . There is also a notable bidirectional relationship between diabetes mellitus and liver cirrhosis. The prevalence of diabetes mellitus in people with liver cirrhosis has been reported as 20–63%, depending on the severity of liver damage, aetiology and diagnostic criteria 115 . In an Italian study of 401 participants with cirrhosis, 63% of those with decompensated liver disease had diabetes mellitus compared with 10% of those with well-compensated liver disease 116 , suggesting that diabetes mellitus is more common in severe cases of liver damage. The association between diabetes mellitus and cirrhosis also varies according to the cause of liver disease. In a US study of 204 people with cirrhosis, the prevalence of diabetes mellitus was 25% among those with cirrhosis caused by hepatitis C virus, 19% among those with cirrhosis from alcoholic liver disease and only 1% among those with cirrhosis due to cholestatic liver disease 117 . Among the causes of cirrhosis, haemochromatosis has the strongest association with diabetes mellitus, with diabetes mellitus mainly resulting from the iron deposition that is characteristic of haemochromatosis 118 .
Several factors have been implicated in the aetiology of liver disease in people with diabetes mellitus, with insulin resistance being the most notable 119 .
Insulin resistance
Insulin resistance causes lipolysis, thereby increasing the circulating levels of free fatty acids, which are then taken up by the liver as an energy source 120 . These fatty acids overload the mitochondrial β-oxidation system in the liver, resulting in the accumulation of fatty acids and, consequently, NAFLD 121 . Of those individuals with NAFLD, 2–3% develop hepatic inflammation, necrosis and fibrosis, which are the hallmarks of NASH 122 . The exact mechanisms leading to steatohepatitis are unclear, although dysregulated peripheral lipid metabolism appears to be important 14 .
Ectopic adipose deposition
Excessive or ectopic deposition of adipose tissue around the viscera and in the liver might be an important mechanism underlying both T2DM and liver disease, particularly NAFLD 123 . Dysfunction of long-term adipose storage in white adipose tissue is known to lead to ectopic adipose deposition in the liver. In this state, increased levels of fatty acyl-coenzyme As, the activated form of fatty acids, might lead to organ dysfunction, including NAFLD 124 . Ectopic adipose deposition leading to organ-specific insulin resistance has emerged as a major hypothesis for the pathophysiological basis of T2DM, and ectopic adipose in the pancreas could contribute to β-cell dysfunction and, thus, the development of T2DM 125 .
Diabetes mellitus and affective disorders
The prevalence of depression appears to be high among people with diabetes mellitus. The strongest evidence for an association comes from a systematic review of 147 studies among people with T2DM, which revealed a mean prevalence of depression of 28% 126 , while the global prevalence of depression in the general population is estimated at around 13% 127 . For T1DM, a systematic review reported a pooled prevalence of depression of 12% compared with only 3% in those without T1DM 128 . The risk of depression among people with diabetes mellitus appears to be roughly 25% greater than the risk in the general population, with consistent findings across several meta-analyses (Table 4 ). A 2013 study found an adjusted RR of 1.25 (95% CI 1.10–1.44) for incident depression among people with diabetes mellitus compared with those without diabetes mellitus 129 . Another systematic review of people with T2DM reported a near identical effect size 130 .
Anxiety and eating disorders
Evidence exists for an association of diabetes mellitus with anxiety, and of T1DM with eating disorders. In a systematic review involving 2,584 individuals with diabetes mellitus, a prevalence of 14% was found for generalized anxiety disorder and 40% for anxiety symptoms, whereas the prevalence of generalized anxiety disorder in the general population is estimated as only 3–4% 131 . People with diabetes mellitus had an increased risk of anxiety disorders (OR 1.20, 95% CI 1.10–1.31) and anxiety symptoms (OR 1.48, 95% CI 1.02–1.93) compared with those without diabetes mellitus in a meta-analysis 132 (Table 4 ), although these findings were based on cross-sectional data. Across 13 studies, 7% of adolescents with T1DM were found to have eating disorders, compared with 3% of peers without diabetes mellitus 133 .
Broader psychological impacts
There is a substantial literature on a broad range of psychological impacts of diabetes mellitus. Social stigma 134 can have profound impacts on the quality of life of not only people with diabetes mellitus, but their families and carers, too 135 . In a systematic review, diabetes mellitus distress was found to affect around one-third of adolescents with T1DM, which was consistent with the results of studies of adults with diabetes mellitus 136 . Diabetes mellitus burnout appears to be a distinct concept, and is characterized by exhaustion and detachment, accompanied by the experience of a loss of control over diabetes mellitus 137 .
Diabetes mellitus and depression appear to have common biological origins. Activation of the innate immune system and acute-phase inflammation contribute to the pathogenesis of T2DM — increased levels of inflammatory cytokines predict the onset of T2DM 138 — and there is growing evidence implicating cytokine-mediated inflammation in people with depression in the absence of diabetes mellitus 139 . Dysregulation of the hypothalamic–pituitary–adrenal axis is another potential biological mechanism linking depression and diabetes mellitus 140 . There have been numerous reports of hippocampal atrophy, which might contribute to chronic activation of the hypothalamic–pituitary–adrenal axis, in individuals with T2DM as well as those with depression 141 , 142 . A meta-analysis found that, although hypertension modified global cerebral atrophy in those with T2DM, it had no effect on hippocampal atrophy 143 . This suggests that, although global cerebral atrophy in individuals with T2DM might be driven by atherosclerotic disease, hippocampal atrophy is an independent effect that provides a common neuropathological aetiology for the comorbidity of T2DM with depression. There is a lack of relevant information regarding the potential aetiological mechanisms that link diabetes to other affective disorders.
Diabetes mellitus and sleep disturbance
Obstructive sleep apnoea.
Obstructive sleep apnoea (OSA) is highly prevalent among people with diabetes mellitus. In a systematic review of 41 studies of adults with diabetes mellitus, the prevalence of OSA was found to be 60% 144 , whereas reports for OSA prevalence in the general population range from 9% to 38% 145 . In a UK study of 1,656,739 participants, T2DM was associated with an IRR for OSA of 1.48 (95% CI 1.42–1.55) compared with no T2DM 146 . A population-based US study reported a HR of 1.53 (95% CI 1.32–1.77) for OSA in people with T2DM compared with those without diabetes mellitus 147 . However, the association in this latter report was attenuated after adjustment for BMI and waist circumference (1.08, 95% CI 1.00–1.16), suggesting that the excess risk of OSA among people with diabetes mellitus might be mainly explained by the comorbidity of obesity. Although most studies on OSA have focused on T2DM, a meta-analysis of people with T1DM revealed a similar prevalence of 52% 148 ; however, this meta-analysis was limited to small studies. The association between T2DM and OSA is bidirectional: the severity of OSA was shown to be positively associated with the incidence of T2DM, independent of adiposity, in a large US cohort study 149 .
The mechanism by which T2DM might increase the risk of developing OSA is thought to involve dysregulation of the autonomic nervous system leading to sleep-disordered breathing 150 . Conversely, the specific mechanism behind OSA as a causative factor for T2DM remains poorly understood. It has been suggested that OSA is able to induce insulin resistance 151 , 152 and is a risk factor for the development of glucose intolerance 152 . However, once T2DM has developed, there is no clear evidence that OSA worsens glycaemic control, as an RCT of people with T2DM found that treating OSA had no effect on glycaemic control 153 .
Diabetes mellitus and cognitive disability
Dementia and cognitive impairment.
Dementia is emerging as a major cause of mortality in both individuals with diabetes mellitus and the general population, and is now the leading cause of death in some countries/regions 9 . However, compared with the general population, diabetes mellitus increases the risk of dementia, particularly vascular dementia. The association is supported by several systematic reviews, including one of eight population-based studies with more than 23,000 people, which found SRRs of 2.38 (95% CI 1.79–3.18) for vascular dementia and 1.39 (95% CI 1.16–1.66) for Alzheimer disease comparing people with diabetes mellitus with those without diabetes mellitus 154 (Table 4 ). Similar results, as well as a RR of 1.21 (95% CI 1.02–1.45) for mild cognitive impairment (MCI), were reported across 19 population-based studies of 44,714 people, 6,184 of whom had diabetes mellitus 155 . Two meta-analyses of prospective cohort studies have shown increased risks of all-cause dementia in people with diabetes mellitus compared with those without diabetes mellitus 156 , 157 , and T2DM has been shown to increase progression to dementia in people with MCI 158 .
The boundaries between Alzheimer disease and vascular dementia remain controversial, and these conditions are often difficult to differentiate clinically 159 . Consequently, vascular dementia might have been misdiagnosed as Alzheimer disease in some studies investigating diabetes mellitus and dementia, resulting in an overestimation of the effect size of the association between diabetes mellitus and Alzheimer disease. Although a cohort study found a significant association between diabetes mellitus and Alzheimer disease using imaging 160 , autopsy studies have failed to uncover an association between diabetes mellitus and Alzheimer disease pathology 161 , 162 , suggesting that vascular mechanisms are the key driver of cognitive decline in people with diabetes mellitus.
Another important finding is a 45% prevalence of MCI among people with T2DM in a meta-analysis, compared with a prevalence of 3–22% reported for the general population 163 . Notably, however, the prevalence of MCI in individuals with T2DM was similar in people younger than 60 years (46%) and those older than 60 years (44%), which is at odds with previous research suggesting that MCI is most common in older people, particularly those aged more than 65 years 164 However, another meta-analysis found cognitive decline in people with T2DM who are younger than 65 years 165 , suggesting that a burden of cognitive disease exists among younger people with diabetes mellitus.
Although there is solid evidence that links diabetes mellitus to cognitive disability, our understanding of the underlying mechanisms is incomplete. Mouse models suggest a strong association between hyperglycaemia, the advanced glycation end products glyoxal and methylglyoxal, enhanced blood–brain barrier (BBB) permeability and cognitive dysfunction in both T1DM and T2DM 166 . The BBB reduces the access of neurotoxic compounds and pathogens to the brain and sustains brain homeostasis, so disruption to the BBB can result in cognitive dysfunction through dysregulation of transport of molecules between the peripheral circulation and the brain 167 . There appears to be a continuous relationship between glycaemia and cognition, with associations found between even high-normal blood levels of glucose and cognitive decline 168 . Another hypothetical mechanism involves a key role for impaired insulin signalling in the pathogenesis of Alzheimer disease. Brain tissue obtained post mortem from individuals with Alzheimer disease showed extensive abnormalities in insulin and insulin-like growth factor signalling mechanisms compared with control brain tissue 169 . Although the synthesis of insulin-like growth factors occurred normally in people with Alzheimer disease, their expression levels were markedly reduced, which led to the subsequent proposal of the term ‘type 3 diabetes’ to characterize Alzheimer disease.
Diabetes mellitus and disability
Functional disability.
Disability (defined as a difficulty in functioning in one or more life domains as experienced by an individual with a health condition in interaction with contextual factors) 170 is highly prevalent in people with diabetes mellitus. In a systematic review, lower-body functional limitation was found to be the most prevalent disability (47–84%) among people with diabetes mellitus 171 The prevalence of difficulties with activities of daily living among people with diabetes mellitus ranged from 12% to 55%, although most studies were conducted exclusively in individuals aged 60 years and above, so the results are not generalizable to younger age groups. A systematic review showed a significant association between diabetes mellitus and falls in adults aged 60 years and above 172 . A 2013 meta-analysis 173 showed an increased risk of mobility disability, activities of daily living disability and independent activities of daily living disability among people with diabetes mellitus compared with those without diabetes mellitus (Table 4 ). Although this analysis included cross-sectional data, results were consistent across longitudinal and cross-sectional studies, suggesting little effect of reverse causality. However, people with functional disabilities that limit mobility (for example, people with osteoarthritis or who have had a stroke) might be more prone to developing diabetes mellitus owing to physical inactivity 174 .
Workplace productivity
Decreased productivity while at work, increased time off work and early dropout from the workforce 175 are all associated with diabetes mellitus, probably partly due to functional disability, and possibly also to comorbidities such as obesity and physical inactivity 176 . Given that young-onset diabetes is becoming more common, and most people with diabetes mellitus in middle-income countries/regions are less than 65 years old 177 , a pandemic of diabetes mellitus-related work disability among a middle-aged population does not bode well for the economies of these regions.
The mechanisms by which diabetes mellitus leads to functional disability remain unclear. One suggestion is that hyperglycaemia leads to systemic inflammation, which is one component of a multifactorial process that results in disability 154 . The rapid loss of skeletal muscle strength and quality seen among people with diabetes mellitus might be another cause of functional disability 178 (Box 1 ). In addition, complications of diabetes mellitus, including stroke, peripheral neuropathy and cardiac dysfunction, can obviously directly cause disability 179 .
Box 1 Diabetes mellitus and skeletal muscle atrophy
Individuals with diabetes mellitus exhibit skeletal muscle atrophy that is typically mild in middle age and becomes more substantial with increasing age.
This muscle loss leads to reduced strength and functional capacity and, ultimately, increased mortality.
Skeletal muscle atrophy results from a negative balance between the rate of synthesis and degradation of contractile proteins, which occurs in response to disuse, ageing and chronic diseases such as diabetes mellitus.
Degradation of muscle proteins is more rapid in diabetes mellitus, and muscle protein synthesis has also been reported to be decreased.
Proposed mechanisms underlying skeletal muscle atrophy include systemic inflammation (affecting both protein synthesis and degradation), dysregulation of muscle protein anabolism and lipotoxicity.
Mouse models have also revealed a key role for the WWP1/KLF15 pathway, mediated by hyperglycaemia, in the pathogenesis of muscle atrophy.
See refs 195 , 196 , 197 , 198 .
Diabetes management and control
Although a detailed discussion of the impacts of anti-diabetes mellitus medications and glucose control on emerging complications is beyond the scope of this Review, their potential effect on these complications must be acknowledged.
Medications
Anti-diabetes mellitus medications and cancer.
In the case of cancer as an emerging complication, the use of medications for diabetes mellitus was not controlled for in most studies of diabetes mellitus and cancer and might therefore be a confounding factor. People taking metformin have a lower cancer risk than those not taking metformin 180 . However, this association is mainly accounted for by other factors. For example, metformin is less likely to be administered to people with diabetes mellitus who have kidney disease 181 , who typically have longer duration diabetes mellitus, which increases cancer risk. A review of observational studies into the association between metformin and cancer found that many studies reporting significant reductions in cancer incidence or mortality associated with metformin were affected by immortal time bias and other time-related biases, casting doubt on the ability of metformin to reduce cancer mortality 182 . Notably, the use of insulin was associated with an increased risk of several cancers in a meta-analysis 183 . However, in an RCT of more than 12,000 people with dysglycaemia, randomization to insulin glargine (compared with standard care) did not increase cancer incidence 184 . Furthermore, cancer rates in people with T1DM and T2DM do not appear to vary greatly, despite substantial differences in insulin use between people with these types of diabetes mellitus.
Anti-diabetes mellitus medications and other emerging complications
Anti-diabetes medications appear to affect the onset and development of some other emerging complications of diabetes mellitus. Results from RCTs suggest that metformin might confer therapeutic effects against depression 185 , and its use was associated with reduced dementia incidence in a systematic review 186 . In an RCT investigating a potential association between metformin and NAFLD, no improvement in NAFLD histology was found among people using metformin compared with those given placebo 187 . An RCT reported benefits of treatment with the glucagon-like peptide 1 receptor agonist dulaglutide on cognitive function in a post hoc analysis 188 , suggesting that trials designed specifically to test the effects of dulaglutide on cognitive function should be undertaken.
Glucose control
Another important consideration is glycaemic control, which appears to have variable effects on emerging complications. A meta-analysis found no association of glycaemic control with cancer risk among those with diabetes mellitus 189 , and an RCT found no effect of intensive glucose lowering on cognitive function in people with T2DM 190 . However, glycaemic control has been associated with improved physical function 191 , decreased COVID-19 mortality 192 and a decreased risk of NAFLD 193 in observational studies of patients with diabetes mellitus; notably, no RCTs have yet confirmed these associations.
Conclusions
With advances in the management of diabetes mellitus and associated increased life expectancy, the face of diabetes mellitus complications is changing. As the management of glycaemia and traditional complications of diabetes mellitus is optimized, we are beginning instead to see deleterious effects of diabetes mellitus on the liver, brain and other organs. Given the substantial burden and risk of these emerging complications, future clinical and public health strategies should be updated accordingly. There is a need to increase the awareness of emerging complications among primary care physicians at the frontline of diabetes mellitus care, and a place for screening for conditions such as depression, liver disease and cancers in diabetes mellitus guidelines should be considered. Clinical care for older people with diabetes mellitus should target physical activity, particularly strength-based activity, to reduce the risk of functional disability in ageing populations. Ongoing high-quality surveillance of diabetes mellitus outcomes is imperative to ensure we know where the main burdens lie. Given the growing burden of these emerging complications, the traditional management of diabetes mellitus might need to broaden its horizons.
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414 , 782–787 (2001).
Article CAS PubMed Google Scholar
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 26 , 77–82 (2008).
Article Google Scholar
Shah, A. D. et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 3 , 105–113 (2015).
Article PubMed PubMed Central Google Scholar
Bertoni, A. G. et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27 , 699–703 (2004).
Article PubMed Google Scholar
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 370 , 1514–1523 (2014).
Gregg, E. W., Sattar, N. & Ali, M. K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4 , 537–547 (2016).
Gregg, E. W. et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391 , 2430–2440 (2018).
Harding, J. L., Shaw, J. E., Peeters, A., Davidson, S. & Magliano, D. J. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 39 , 1018–1026 (2016).
Pearson-Stuttard, J. et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 9 , 165–173 (2021).
Einarson, T. R., Acs, A., Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17 , 83 (2018).
Pearson-Stuttard, J., Buckley, J., Cicek, M. & Gregg, E. W. The changing nature of mortality and morbidity in patients with diabetes. Endocrinol. Metab. Clin. North Am. 50 , 357–368 (2021).
Pearson-Stuttard, J. et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10 , 46–57 (2022).
Pearson-Stuttard, J., Blundell, S., Harris, T., Cook, D. G. & Critchley, J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 4 , 148–158 (2016).
Tolman, K. G., Fonseca, V., Dalpiaz, A. & Tan, M. H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30 , 734–743 (2007).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E. & Ioannidis, J. P. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350 , g7607 (2015).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62 , 3–16 (2019).
Unal, B., Critchley, J. A. & Capewell, S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 109 , 1101–1107 (2004).
Pearson-Stuttard, J., Ezzati, M. & Gregg, E. W. Multimorbidity — a defining challenge for health systems. Lancet Public. Health 4 , e599–e600 (2019).
Lee, L., Cheung, W. Y., Atkinson, E. & Krzyzanowska, M. K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J. Clin. Oncol. 29 , 106–117 (2011).
Srokowski, T. P., Fang, S., Hortobagyi, G. N. & Giordano, S. H. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J. Clin. Oncol. 27 , 2170–2176 (2009).
Gross, C. P., McAvay, G. J., Guo, Z. & Tinetti, M. E. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 109 , 2410–2419 (2007).
Harding, J. L. et al. All-cause cancer mortality over 15 years in multi-ethnic Mauritius: the impact of diabetes and intermediate forms of glucose tolerance. Int. J. Cancer 131 , 2385–2393 (2012).
Wang, C. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 130 , 1639–1648 (2012).
El-Serag, H. B., Hampel, H. & Javadi, F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 4 , 369–380 (2006).
Desbois, A. C. & Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J. Gastroenterol. 23 , 1697–1711 (2017).
Article CAS PubMed PubMed Central Google Scholar
Huxley, R., Ansary-Moghaddam, A., Berrington De González, A., Barzi, F. & Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92 , 2076–2083 (2005).
Gullo, L. et al. Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 331 , 81–84 (1994).
Gershell, L. Type 2 diabetes market. Nat. Rev. Drug Discov. 4 , 367–368 (2005).
Carstensen, B. et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 59 , 980–988 (2016).
Jiang, Y. et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 26 , 863–876 (2011).
De Bruijn, K. M. J. et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 100 , 1421–1429 (2013).
Deng, L., Gui, Z., Zhao, L., Wang, J. & Shen, L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci. 57 , 1576–1585 (2012).
Liao, C., Zhang, D., Mungo, C., Andrew Tompkins, D. & Zeidan, A. M. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol. Oncol. 135 , 163–171 (2014).
Saed, L. et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 19 , 527 (2019).
Friberg, E., Orsini, N., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 50 , 1365–1374 (2007).
Anothaisintawee, T. et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia-Pac. J. Public Health 25 , 368–387 (2013).
Larsson, S. C., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer 121 , 856–862 (2007).
Boyle, P. et al. Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer 107 , 1608–1617 (2012).
Rinaldi, S. et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int. J. Cancer 118 , 2832–2839 (2006).
Michels, K. B. et al. Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care 26 , 1752–1758 (2003).
Bronsveld, H. K. et al. Diabetes and breast cancer subtypes. PLoS ONE 12 , e0170084 (2017).
Article PubMed PubMed Central CAS Google Scholar
Zhang, D., Li, N., Xi, Y., Zhao, Y. & Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 130 , 43–52 (2017).
Weng, L., Wang, L., Zhang, J., Wang, B. & Liu, H. Association between diabetes mellitus and subsequent ovarian cancer in women: a systematic review and meta-analysis of cohort studies. Medicine 96 , e6396 (2017).
Wang, L., Zhong, L., Xu, B., Chen, M. & Huang, H. Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open 10 , e040137 (2020).
Lee, J. Y. et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 23 , 402–412 (2013).
Bonovas, S., Filioussi, K. & Tsantes, A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47 , 1071–1078 (2004).
Shikata, K., Ninomiya, T. & Kiyohara, Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 104 , 9–14 (2013).
Long, X. J., Lin, S., Sun, Y. N. & Zheng, Z. F. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac. J. Cancer Preven. 13 , 4097–4100 (2012).
Rhee, E. J. Diabetes in Asians. Endocrinol. Metab. 30 , 263–269 (2015).
Article CAS Google Scholar
Bensimon, L., Yin, H., Suissa, S., Pollak, M. N. & Azoulay, L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 25 , 329–338 (2014).
Johnson, J. A., Bowker, S. L., Richardson, K. & Marra, C. A. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 54 , 2263–2271 (2011).
Johnson, J. A. et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55 , 1607–1618 (2012).
Harding, J. L., Shaw, J. E., Peeters, A., Cartensen, B. & Magliano, D. J. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 38 , 264–270 (2015).
Pearson-Stuttard, J. et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 6 , e6–e15 (2018).
Larsson, S. C. & Wolk, A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 54 , 1013–1018 (2011).
Xu, X. et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE 8 , e58079 (2013).
Gong, I. Y. et al. Association between diabetes and haematological malignancies: a population-based study. Diabetologia 64 , 540–551 (2021).
Giovannucci, E. et al. Diabetes and cancer: a consensus report. Diabetes Care 33 , 1674–1685 (2010).
Weinstein, D., Simon, M., Yehezkel, E., Laron, Z. & Werner, H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev. 25 , 41–49 (2009).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology 34 , 198–215 (2019).
Lorenzi, M., Montisano, D. F., Toledo, S. & Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Invest. 77 , 322–325 (1986).
Robertson, R., Zhou, H., Zhang, T. & Harmon, J. S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 48 , 139–146 (2007).
Turturro, F., Friday, E. & Welbourne, T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer 7 , 96 (2007).
Wu, Y., Liu, Y., Dong, Y. & Vadgama, J. Diabetes-associated dysregulated cytokines and cancer. Integr. Cancer Sci. Ther. 3 , 370–378 (2016).
PubMed PubMed Central Google Scholar
Inoki, K., Kim, J. & Guan, K. L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52 , 381–400 (2012).
Huang, X., Liu, G., Guo, J. & Su, Z. Q. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14 , 1483–1496 (2018).
Zhao, Y. et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol. Endocrinol. 34 , 428–432 (2018).
Knapp, S. Diabetes and infection: is there a link?-A mini-review. Gerontology 59 , 99–104 (2013).
Fang, M. et al. Diabetes and the risk of hospitalisation for infection: the atherosclerosis risk in communities (ARIC) study. Diabetologia 64 , 2458–2465 (2021).
Tseng, C.-H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 11 , 12400 (2021).
Luk, A. O. Y. et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia 64 , 109–118 (2021).
Magliano, D. J. et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 38 , 1274–1280 (2015).
Martin, E. T. et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 37 , 88–99 (2016).
Trussell, J. et al. Impact of a patient care pathway protocol on surgical site infection rates in cardiothoracic surgery patients. Am. J. Surg. 196 , 883–889 (2008).
Coleman, J. S. et al. Surgical site infections after hysterectomy among HIV-infected women in the HAART era: a single institution’s experience from 1999–2012. Am. J. Obstet. Gynecol. 210 , 117.e111–117.e117 (2014).
Friedman, N. D., Sexton, D. J., Connelly, S. M. & Kaye, K. S. Risk factors for surgical site infection complicating laminectomy. Infect. Control. Hosp. Epidemiol. 28 , 1060–1065 (2007).
Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8 , 782–792 (2020).
McGurnaghan, S. J. et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 9 , 82–93 (2021).
Rawshani, A. et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 4 , 100105 (2021).
You, J. H. et al. Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol. Metab. 35 , 901–908 (2020).
Moon, S. J. et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes Metab. J. 44 , 737–746 (2020).
Aranjani, J. M., Manuel, A., Razack, H. I. A. & Mathew, S. T. Covid-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS. Negl. Trop. Dis. 15 , e0009921 (2021).
Crankson, S., Pokhrel, S. & Anokye, N. K. Determinants of COVID-19-related length of hospital stays and long COVID in Ghana: a cross-sectional analysis. Int. J. Environ. Res. Public Health 19 , 527 (2022).
Bellan, M. et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw. Open 4 , e2036142 (2021).
Gottesman, B. L., Yu, J., Tanaka, C., Longhurst, C. A. & Kim, J. J. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 176 , 414–415 (2022).
Barrett, C. E. et al. Risk for newly diagnosed diabetes <30 days after SARS-CoV-2 infection among persons aged >18 years-United States, March 1, 2020-June 28, 2021. Morb. Mortal. Wkly. Rep. 71 , 59–65 (2022).
Kornum, J. B. et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31 , 1541–1545 (2008).
Matsuyama, R., Nishiura, H., Kutsuna, S., Hayakawa, K. & Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health 16 , 1203 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 49 , 129–133 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the middle east respiratory syndrome coronavirus: a systematic review and meta-analysis. J. Public. Health Res. 5 , 130–138 (2016).
Yang, J. K. et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 23 , 623–628 (2006).
Ehrlich, S. F., Quesenberry, C. P. Jr, Van Den Eeden, S. K., Shan, J. & Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 33 , 55–60 (2010).
Alraddadi, B. M. et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 22 , 49–55 (2016).
Geerlings, S. E. & Hoepelman, A. I. M. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26 , 259–265 (1999).
Velazquez-Salinas, L., Verdugo-Rodriguez, A., Rodriguez, L. L. & Borca, M. V. The role of interleukin 6 during viral infections. Front. Microbiol. 10 , 1057 (2019).
Joshi, N., Caputo, G. M., Weitekamp, M. R. & Karchmer, A. W. Infections in patients with diabetes mellitus. N. Engl. J. Med. 341 , 1906–1912 (1999).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 32 , 437–446.e435 (2020).
Miyazawa, T., Nakagawa, K., Shimasaki, S. & Nagai, R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids 42 , 1163–1170 (2012).
Peleg, A. Y., Weerarathna, T., McCarthy, J. S. & Davis, T. M. E. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 23 , 3–13 (2007).
Barda, N., Dagan, N. & Balicer, R. D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. reply. N. Engl. J. Med. 384 , 1970 (2021).
PubMed Google Scholar
Cholankeril, G. & Ahmed, A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. 16 , 1356–1358 (2018).
Fink, M. & Byrne, M. Australia and New Zealand Liver and Intestinal Transplant Registry Annual Report 2019 (Melbourne, Victoria, Australia, 2019).
Haldar, D. et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J. Hepatol. 71 , 313–322 (2019).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71 , 793–801 (2019).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 , 73–84 (2016).
Pang, Y. et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 68 , 1308–1318 (2018).
Li, Y. et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS ONE 12 , e0174291 (2017).
Mansour-Ghanaei, F. et al. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in north of Iran. Diabetes Metab. Syndr. 13 , 1591–1596 (2019).
Leite, N. C., Salles, G. F., Araujo, A. L., Villela-Nogueira, C. A. & Cardoso, C. R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 29 , 113–119 (2009).
Singh, S. P. et al. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case-control study. J. Clin. Exp. Hepatol. 5 , 295–302 (2015).
Dufour, J.-F. et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–a targeted literature review. Endocr. Metab. Sci. 3 , 100089 (2021).
Loomba, R. et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56 , 943–951 (2012).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10 , 330–344 (2013).
Holstein, A., Hinze, S., Thießen, E., Plaschke, A. & Egberts, E. H. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 17 , 677–681 (2002).
Del Vecchio Blanco, C., Gentile, S., Marmo, R., Carbone, L. & Coltorti, M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res. Clin. Pract. 8 , 29–36 (1990).
Zein, N. N., Abdulkarim, A. S., Wiesner, R. H., Egan, K. S. & Persing, D. H. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J. Hepatol. 32 , 209–217 (2000).
Niederau, C. et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 313 , 1256–1262 (1985).
Larter, C. Z. & Farrell, G. C. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat? J. Hepatol. 44 , 253–261 (2006).
Marchesini, G. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 107 , 450–455 (1999).
Angulo, P. Medical progress: nonalcoholic fatty liver disease. N. Engl. J. Med. 346 , 1221–1231 (2002).
Porepa, L., Ray, J. G., Sanchez-Romeu, P. & Booth, G. L. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ 182 , E526–E531 (2010).
Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 8 , 616–627 (2020).
Stefan, N., Häring, H. U. & Cusi, K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 7 , 313–324 (2019).
Sattar, N. & Gill, J. M. R. Type 2 diabetes as a disease of ectopic fat? BMC Med. 12 , 123 (2014).
Harding, K. A. et al. Depression prevalence in type 2 diabetes is not related to diabetes–depression symptom overlap but is related to symptom dimensions within patient self-report measures: a meta-analysis. Diabet. Med. 36 , 1600–1611 (2019).
Lim, G. Y. et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8 , 2861 (2018).
Roy, T. & Lloyd, C. E. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 142 , S8–S21 (2012).
Rotella, F. & Mannucci, E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res. Clin. Pract. 99 , 98–104 (2013).
Nouwen, A. et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 53 , 2480–2486 (2010).
Grigsby, A. B., Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. Prevalence of anxiety in adults with diabetes a systematic review. J. Psychosom. Res. 53 , 1053–1060 (2002).
Smith, K. J. et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J. Psychosom. Res. 74 , 89–99 (2013).
Young, V. et al. Eating problems in adolescents with type1 diabetes: a systematic review with meta-analysis. Diabet. Med. 30 , 189–198 (2013).
Schabert, J., Browne, J. L., Mosely, K. & Speight, J. Social stigma in diabetes: a framework to understand a growing problem for an increasing epidemic. Patient 6 , 1–10 (2013).
Barnard, K. D., Speight, J. & Skinner, T. C. Quality of life and impact of continuous subcutaneous insulin infusion for children and their parents. Pract. Diabetes Int. 25 , 278–283 (2008).
Hagger, V., Hendrieckx, C., Sturt, J., Skinner, T. C. & Speight, J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr. Diabetes Rep. 16 , 1–14 (2016).
Abdoli, S. et al. New insights into diabetes burnout and its distinction from diabetes distress and depressive symptoms: a qualitative study. Diabetes Res. Clin. Pract. 169 , 108446 (2020).
Pickup, J. C. & Crook, M. A. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41 , 1241–1248 (1998).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Prestele, S., Aldenhoff, J. & Reiff, J. [The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction]. Fortschr. Neurol. Psychiatr. 71 , 24–36 (2003).
Cole, J., Costafreda, S. G., McGuffin, P. & Fu, C. H. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 134 , 483–487 (2011).
Gold, S. M. et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50 , 711–719 (2007).
Moulton, C. D., Costafreda, S. G., Horton, P., Ismail, K. & Fu, C. H. Y. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 9 , 651–662 (2015).
Khalil, M., Power, N., Graham, E., Deschênes, S. S. & Schmitz, N. The association between sleep and diabetes outcomes – systematic review. Diabetes Res. Clin. Pract. 161 , 108035 (2020).
Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 34 , 70–81 (2017).
Subramanian, A. et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care 42 , 954–963 (2019).
Huang, T. et al. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. Cohorts. Diabetes Care 41 , 2111–2119 (2018).
Reutrakul, S. et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep. Med. 23 , 26–45 (2016).
Nagayoshi, M. et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep. Med. 25 , 156–161 (2016).
Ficker, J. H. et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur. Respir. J. 11 , 14–19 (1998).
Young, T., Peppard, P. E. & Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 99 , 1592–1599 (2005).
Ip, M. S. M. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165 , 670–676 (2002).
Shaw, J. E. et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 194 , 486–492 (2016).
Lu, F. P., Lin, K. P. & Kuo, H. K. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS ONE 4 , e4144 (2009).
Cheng, G., Huang, C., Deng, H. & Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J. 42 , 484–491 (2012).
Li, X. Y. et al. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr. Alzheimer Res. 16 , 1254–1268 (2019).
Article PubMed CAS Google Scholar
Xue, M. et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 55 , 100944 (2019).
Pal, K., Mukadam, N., Petersen, I. & Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 53 , 1149–1160 (2018).
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C. & Scheltens, P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 , 64–74 (2006).
Peila, R., Rodriguez, B. L. & Launer, L. J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 51 , 1256–1262 (2002).
Abner, E. L. et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dement. 12 , 882–889 (2016).
Matioli, M. N. P. S. et al. Association between diabetes and causes of dementia: evidence from a clinicopathological study. Dement. Neuropsychol. 11 , 406–412 (2017).
You, Y. et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 58 , 671–685 (2021).
Langa, K. M. & Levine, D. A. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312 , 2551–2561 (2014).
Pelimanni, E. & Jehkonen, M. Type 2 diabetes and cognitive functions in middle age: a meta-analysis. J. Int. Neuropsychol. Soc. 25 , 215–229 (2019).
Rom, S. et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 10 , 7274 (2020).
Hussain, B., Fang, C. & Chang, J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 15 , 688090 (2021).
Anstey, K. J., Sargent-Cox, K., Eramudugolla, R., Magliano, D. J. & Shaw, J. E. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res. Ther. 7 , 48 (2015).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - Is this type 3 diabetes? J. Alzheimer’s Dis. 7 , 63–80 (2005).
Leonardi, M., Bickenbach, J., Ustun, T. B., Kostanjsek, N. & Chatterji, S. The definition of disability: what is in a name? Lancet 368 , 1219–1221 (2006).
Lisy, K., Campbell, J. M., Tufanaru, C., Moola, S. & Lockwood, C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: a systematic review. Int. J. Evid. Based Healthc. 16 , 154–166 (2018).
Yang, Y., Hu, X., Zhang, Q. & Zou, R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing 45 , 761–767 (2016).
Wong, E. et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 1 , 106–114 (2013).
Havercamp, S. M., Scandlin, D. & Roth, M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public. Health Rep. 119 , 418–426 (2004).
Herquelot, E., Guéguen, A., Bonenfant, S. & Dray-Spira, R. Impact of diabetes on work cessation: data from the GAZEL cohort study. Diabetes Care 34 , 1344–1349 (2011).
Virtanen, M. et al. Work disability among employees with diabetes: latent class analysis of risk factors in three prospective cohort studies. PLoS ONE 10 , e0143184 (2015).
Cho, N. H. et al. IDF Diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138 , 271–281 (2018).
Seok, W. P. et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 30 , 1507–1512 (2007).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br. Med. J. 321 , 405–412 (2000).
DeCensi, A. et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 3 , 1451–1461 (2010).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patientswith type 2 diabetes and kidney disease a systematic review. JAMA 312 , 2668–2675 (2014).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35 , 2665–2673 (2012).
Karlstad, Ø. et al. Use of insulin and insulin analogs and risk of cancer-systematic review and meta-analysis of observational studies. Curr. Drug. Saf. 8 , 333–348 (2013).
Bordeleau, L. et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 37 , 1360–1366 (2014).
Guo, M. et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 41 , 650–656 (2014).
CAS PubMed Google Scholar
Campbell, J. M. et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J. Alzheimer’s Dis. 65 , 1225–1236 (2018).
Haukeland, J. W. et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 44 , 853–860 (2009).
Cukierman-Yaffe, T. et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19 , 582–590 (2020).
Johnson, J. A. & Bowker, S. L. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 54 , 25–31 (2011).
Launer, L. J. et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 10 , 969–977 (2011).
Jia, Y. et al. Associations of the glycaemic control of diabetes with dementia and physical function in rural-dwelling older Chinese adults: a population-based study. Clin. Interv. Aging 16 , 1503–1513 (2021).
Lesniak, C. et al. Inpatient glycemic control and outcome of COVID-19 patients: a retrospective cohort. SAGE Open. Med. 9 , 20503121211039105 (2021).
Afolabi, B. I. et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J. Natl Med. Assoc. 110 , 256–264 (2018).
Nouwen, A. et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 36 , 1562–1572 (2019).
Perry, B. D. et al. Muscle atrophy in patients with Type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 22 , 94–109 (2016).
Hirata, Y. et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 4 , e124952 (2019).
Article PubMed Central Google Scholar
Bassil, M. S. & Gougeon, R. Muscle protein anabolism in type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 16 , 83–88 (2013).
Meex, R. C. R., Blaak, E. E. & van Loon, L. J. C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 20 , 1205–1217 (2019).
Download references
Acknowledgements
D.T. is supported by an Australian Government Research Training Program (RTP) Scholarship and Monash Graduate Excellence Scholarship. J.E.S. is supported by a National Health and Medical Research Council Investigator Grant. D.J.M. is supported by a National Health and Medical Research Council Senior Research Fellowship.
Author information
These authors jointly supervised this work: Jonathan E. Shaw and Dianna J. Magliano.
Authors and Affiliations
Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia
Dunya Tomic, Jonathan E. Shaw & Dianna J. Magliano
School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Contributions
D.T. researched data for the article and wrote the article. J.E.S and D.J.M. contributed substantially to discussion of the content. D.T., J.E.S. and D.J.M reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Dianna J. Magliano .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Endocrinology thanks Emily Gallagher, Norbert Stefan and Assaad Eid for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fundamental skills required to independently care for oneself such as eating, bathing and mobility.
Activities that allow an individual to live independently in a community.
The error in estimating the association between an exposure and an outcome that results from misclassification or exclusion of time intervals.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Tomic, D., Shaw, J.E. & Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol 18 , 525–539 (2022). https://doi.org/10.1038/s41574-022-00690-7
Download citation
Accepted : 06 May 2022
Published : 06 June 2022
Issue Date : September 2022
DOI : https://doi.org/10.1038/s41574-022-00690-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Hyperglycemia enhances brain susceptibility to lipopolysaccharide-induced neuroinflammation via astrocyte reprogramming.
- Kyung-Seo Lee
- Sung-Hyun Yoon
Journal of Neuroinflammation (2024)
Application of the path analysis model to evaluate the role of distress, mental health literacy and burnout in predicting self-care behaviors among patients with type 2 diabetes
- Alireza Jafari
- Mahdi Moshki
- Hassan Alizadeh
Diabetology & Metabolic Syndrome (2024)
Gender-specific accuracy of lipid accumulation product index for the screening of metabolic syndrome in general adults: a meta-analysis and comparative analysis with other adiposity indicators
- Bendix Samarta Witarto
- Andro Pramana Witarto
- Delvac Oceandy
Lipids in Health and Disease (2024)
The relationship between diabetes and the dementia risk: a meta-analysis
- Fushuang Yang
- Wenfeng Zhang
Implications of cognitive and daily living capabilities on early type 2 diabetes management: a preliminary case–control study
- Romina Mahmoudi
- Farzin Kamari
- Vahideh Sadra
European Journal of Medical Research (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Biomedicines

Current Advances in the Management of Diabetes Mellitus
Chinyere aloke.
1 Protein Structure-Function and Research Unit, School of Molecular and Cell Biology, Faculty of Science, University of the Witwatersrand, Braamfontein, Johannesburg 2050, South Africa
2 Department of Medical Biochemistry, Alex Ekwueme Federal University Ndufu-Alike, Abakaliki PMB 1010, Nigeria
Chinedu Ogbonnia Egwu
Patrick maduabuchi aja.
3 Department of Biochemistry, Faculty of Biological Sciences, Ebonyi State University, Abakaliki PMB 53, Nigeria
Nwogo Ajuka Obasi
Jennifer chukwu.
4 John Hopkins Program on International Education in Gynaecology and Obstetrics, Abuja 900281, Nigeria
Blessing Oluebube Akumadu
Patience nkemjika ogbu, ikechukwu achilonu, associated data.
Not applicable.
Diabetes mellitus (DM) underscores a rising epidemic orchestrating critical socio-economic burden on countries globally. Different treatment options for the management of DM are evolving rapidly because the usual methods of treatment have not completely tackled the primary causes of the disease and are laden with critical adverse effects. Thus, this narrative review explores different treatment regimens in DM management and the associated challenges. A literature search for published articles on recent advances in DM management was completed with search engines including Web of Science, Pubmed/Medline, Scopus, using keywords such as DM, management of DM, and gene therapy. Our findings indicate that substantial progress has been made in DM management with promising results using different treatment regimens, including nanotechnology, gene therapy, stem cell, medical nutrition therapy, and lifestyle modification. However, a lot of challenges have been encountered using these techniques, including their optimization to ensure optimal glycemic, lipid, and blood pressure modulation to minimize complications, improvement of patients’ compliance to lifestyle and pharmacologic interventions, safety, ethical issues, as well as an effective delivery system among others. In conclusion, lifestyle management alongside pharmacological approaches and the optimization of these techniques is critical for an effective and safe clinical treatment plan.
1. Introduction
Diabetes mellitus (DM) is a long-standing, complicated, and non-transmissible endocrine ailment that is growing rapidly and has posed clinical challenges globally, often linked with threats related to complicated metabolic development in patients. It is marked by elevated glucose and lipids in the blood as well as oxidative stress, which culminate in chronic complications involving diverse organs, mainly the kidneys, eyes, nerves, and blood vessels, among others, in the body. As reported by World Health Organization (WHO), DM is an outbreak prone to high malaise and death. Globally, approximately 387 million persons are affected by this disorder and it is estimated to be more than 640 million by 2040 [ 1 ].
According to a report in 2017 by International Diabetes Federation (IDF), 425 million persons suffer from diabetes mellitus out of which more than 90 percent are adults and 352 million had impaired glucose tolerance (IGT) [ 2 ]. In individuals suffering from type II diabetes mellitus (T2DM), hyperglycemia is not the only characteristic; it also involves multiple complications such as kidney failure, blindness, heart attack, stroke, and amputations of the lower limb [ 3 ]. Mounting evidence obtained from epidemiological studies has shown that T2DM is an ailment with numerous causes associated with both polygenic and various environmental factors [ 4 ]. T2DM is thus too complicated to cure due to genetic polymorphism and other numerous risk factors.
Despite the fact that most cases are a result of obesity-linked T2DM, the annual prevalence of T1DM is on the rise [ 5 ]. It has been reported that about 10 percent of people suffering from diabetes have T1DM. However, the two forms are linked with a prolonged risk of circulatory system complexities [ 6 ] and the threat of lowered blood glucose. Ample proof suggests that normoglycemia accomplishment will mitigate the risk of complications linked with DM [ 7 ]. However, hypoglycemia occurrences limit the attainment of near normoglycemia in subjects with T1DM. Diabetic individuals who are not aware of their hypoglycemic status are vulnerable to T1DM which then limits them from the attainment of the needed glycemic control. Globally, DM health centers have several individuals with T1DM who have recurrent low blood glucose and the idea of hypoglycemic unconsciousness poses critical clinical challenges. Providentially, many favorable and interesting gain ground exist in the perspective for subjects with the problem of DM, including gene therapy, as reported by Bosch and colleagues [ 8 ].
Currently, the main therapeutic regimens for T2DM are injection of insulin-like agents and oral administration of hypoglycaemic agents. However, these agents play crucial functions in T2DM treatment but are laden with side effects [ 9 , 10 ]. Insulin has taken the centerpiece for the management of unrestrained insulin-deficient DM since its invention [ 11 ]. Admittedly, due to the severe lack of beta cells, the injection of exogenous insulin is vital for survival. Notwithstanding the advances made in comprehending the etiology, effects, and continuance of DM, including the progress made in insulin development and its analogues, ensuring tight glycaemic modulation without negative side effects such as low blood glucose and gain in weight still poses significant problems [ 7 , 12 , 13 ]. Hence, this further accentuates the importance of alternative techniques or adjuncts to insulin [ 14 ].
Consequently, this narrative review exploits different alternative therapeutic regimen for the management of two forms of DM, including nanotechnology, stem cell technology, gene therapy, medical nutrition therapy, lifestyle modification and the challenges associated with these techniques.
To identify published works on recent advances in the management of DM, the literature search for this narrative review was carried out using different search engines including Scopus, Google Scholar, Pubmed/Medline and Web of Science databases. Keywords and subject headings employed include diabetes mellitus, hyperglyceamia, management of DM, T2DM, nanotechnology in diabetes, gene therapy in DM management and current treatment, etc. The titles and abstracts of the results after the search were painstakingly screened to select eligible articles for full-text reading. Articles that were found to be eligible were retrieved and full-text screening was performed independently by three of the authors to select studies for inclusion in the final analysis. Original research and review articles published between 1993 and 2022 (in English) were included. Unpublished articles and thesis were excluded. All authors confirmed the validity of the selected papers.
3. Risk Factors of Diabetes
There are several risk factors associated with diabetes. These risk factors contribute significantly to the progression of diabetes. They include but not limited to age; weight; family history of diabetes; smoking and race/ethnicity [ 15 , 16 ] (Asiimwe et al., 2020; Noh et al., 2018). While T1DM is mostly found in the young, T2DM is an adult-related condition. The risk of T2DM increases with age which is due to the deficiency of insulin secretion which develops with age, and growing insulin resistance caused by a change in body composition [ 17 ]. Increase in body weight which leads to obesity is closely associated with diabetes in a condition termed diabesity. This is because increase in body weight leads to increased insulin resistance [ 18 ].
According to the FDA, smokers are 30 to 40% more likely to come down with T2DM than nonsmokers. Smoking can also increase insulin resistance which makes the patients require more insulin for the control of their sugar level [ 19 ]. Diabetes is hereditary. Those with the family history are advised to adhere to lifestyles that reduce the risk of developing diabetes.
4. Management of Diabetes
There are several modern approaches involved in the management of diabetes. However, early diagnosis is central to achieving any targets set in DM management [ 20 ]. Each patient is treated with the aim of achieving a particular outcome. These outcomes are set out from the first day of clinic visit to ensure an individualized approach in the management of diabetes.
4.1. Internet Intervention for Lifestyle Modification in Diabetes
Lifestyle modification is an integral part of diabetes management. It is recommended for both patients in pre-diabetic and diabetic conditions, respectively. Reduced sedentary lifestyle, increased physical activities, and healthy diets are among the recommended lifestyle modifications. The right exercise may depend on the state of the patient. The exercise helps to bring down the plasma glucose level. For a healthy diet, it is recommended that diabetic subjects take a lot of vegetables, fruits, and whole grains; choose nonfat dairy and lean meats; and limit foods that are high in sugar and fat. Other lifestyle changes include stopping smoking and reduction in alcohol intake [ 21 , 22 ]. The lifestyle changes are usually individualized.
Even though the above strategies help in the effective management of diabetes, communicating or constantly reminding the subjects to complete them could be challenging. Web or internet-based program have been deployed to improve adherence to the lifestyle changes. These web-based strategies provide a viable option for facilitating diabetes self-management [ 23 ].
4.2. Nanotechnology and Diabetes
Nanotechnology involves the use of nanoparticles (<100 nm). These nanoparticles are developed through the manipulation of individual atoms or molecules in a substance. The application of nanotechnology in medicine is termed nanomedicine. Nanomedicine involves the combination of the knowledge of nanotechnology in the application of drugs or diagnostic molecules which generally improves their ability to target specific cells or tissues. Nanotechnology in diabetes research has played several roles in improving the outcome of diabetic management in diabetics through the deployment of novel nanotechnology-based glucose measurement and insulin delivery techniques [ 24 , 25 ]. Nanotechnology employs non-invasive approaches for insulin delivery and the development of a more efficacious vaccine including cell-based and gene-based therapies for T1DM [ 24 ]. The importance of nanotechnology in diabetes includes, but is not limited to, inventive diabetes diagnosis, detection of immune cell activity and beta-cell mass, monitoring of glucose level, and non-invasive insulin delivery, etc.
Early and accurate diagnosis of a disease may be as important as the treatment of the disease itself. Prompt diagnosis may prevent dysglycaemia and reduce the time to onset of diabetes [ 26 ]. Conventional approaches have been utilized in the different diagnostic needs in diabetes, such as detection of immune destruction that precedes T1DM and/or measurement of plasma glucose levels. However, the shortcomings of the conventional approaches which include, but are not limited to, non-early detection of the disease progression necessitate the need for a novel technology that can improve the diagnostic outcome.
The mass of the beta cell is an indication of the functionality of the beta-cell in secreting insulin. The progressive loss of the beta cells precipitates T1DM [ 27 ]. Prompt detection of the stage of beta cell loss through nanotechnology can allow for the immediate application of clinical interventions for its arrest. Magnetic nanoparticles (MNPs), for instance, have distinctive physical properties qualifying them as outstanding contrast media for magnetic resonance imaging (MRI). This can enable the early detection of the stages of beta-cell loss.
Glycaemic fluctuation should be avoided during diabetic management. Individuals have treatment goals set by their physicians. Regular or daily glucose monitoring is performed to ascertain the control achieved by the treatment and the diabetes progression [ 28 ]. However, this comes with some challenges including poor compliance as a result of the regular pricking of the patients and inability to monitor glucose levels at certain times of the day (e.g., sleeping and driving times). The overall impact is irregular monitoring of the glucose level which can lead to dangerous fluctuations that may worsen diabetic complications. To circumvent this challenge, continuous glucose monitoring (CGM) systems are essential. The implantation of biosensors (e.g., amperometric sensors) subcutaneously had been used to achieve CGM for 10 days; however, this has its drawbacks including instabilities and the need for a weekly change of the implantation [ 29 , 30 ].
Nanomedicine can overcome the aforementioned obstacles in CGM. The glucose-sensing device has three key components: a detector, a transducer, and a reporter. The detector measures the glucose level while the transducer converts the measurement into an output signal. The reporter finally processes the signal into an interpretable form. For an effective measure of the glucose level, the glucose sensors are usually made of nanoparticles in nanotechnology which are made from mainly three molecules: glucose oxidase, glucose-binding proteins, and glucose-binding small molecules [ 24 , 31 ]. The coupling of these nanoparticles as transducers enables the accurate detection of glucose in a patient-friendly and rapid manner [ 31 ].
Insulin shots are the mainstay in the management of T1DM and T2DM. The conventional approach of insulin delivery involves the use of needle injections. Even though some needles have been significantly improved to be painless during delivery, the thought of needles alone could be discouraging [ 32 ]. This significantly affects the compliance of patients to insulin use. Moreover, the lingering time between the time of glucose measurement and the insulin dosing in addition to the hindrance in the absorption of insulin ensuing the conventional subcutaneous injection, do not allow for a close plasma glucose control which leads to fluctuations and times of hyperglycemia [ 24 ]. An approach that is non-invasive will be well accepted by both patients and medical practitioners to improve compliance and the overall outcome of treatment.
To overcome the recent delivery challenges faced by the conventional approaches, microcomputer closed-loop or nano pumps are being developed to ensure the timely delivery of insulin while ensuring continuous glucose monitoring. In other words, this system is built to link insulin delivery to plasma glucose concentration. This will prevent the risk of plasma glucose fluctuations [ 26 , 33 ]. Other less invasive means of insulin delivery that involve the use of nanoparticles are also being explored to facilitate insulin delivery orally, transdermally, and/or via inhalation [ 26 ].
4.3. Medical Nutrition Therapy in Diabetes
Medical nutrition therapy (MNT) is a “nutrition-based treatment provided by a registered dietitian nutritionist.” It comprises nutrition diagnosis and therapeutic and professional counseling services that aid in the management of DM. MNT is a critical aspect of diabetes education and management. Recommendations on MNT by international collaborative groups for diabetes management have attempted to reform and provide courses for adverse nutritional transition [ 34 , 35 ]. For instance, MNT has been employed for the treatment of GDM because carbohydrate (CHO) is the main causative agent as a result of its impact on glycaemia. According to the Institute of Medicine, pregnant women require a minimum of 175 g CHO per day, and low-CHO diets already in use traditionally for the treatment of GDM have proven to be safe [ 36 ]. Moreover, MNT has been reported to be critical in the management of other types of DM and as such has significantly impacted patients, especially women and newborns [ 37 ]. Primarily, MNT ensures the maintenance of euglycemia via adequacy in weight gain in pregnancy and growth of fetus while avoiding ketogenesis and metabolic acidosis. Nonetheless, MNT is yet to establish the optimal diet in terms of energy content and macronutrient distribution, quality, and amount, among others, in DM [ 37 ]. Reports have shown that the nutritional requirements for GDM patients are the same for all pregnancy cases when their carbohydrate intake is taken into special cognizance. Currently, a low-glycemic index diet has been reported to be more favorable in the management of GDM than the traditional intervention of carbohydrates restriction even though the evidence is still restrained [ 37 ]. Caloric restrictions are very vital in the management of overweight or obesity.
Reports have charged MNT with the design of signature diet strategies that will be suitable medically as well as patient focused. By this, it is hoped that practicing diabetologists and registered dieticians (RDs) will partner to furnish nutritional guidelines based on evidence for use by MNT for the prevention and management of DM and related comorbidities [ 38 ]. Indications show that MNT may be a potent, easily available, and cheap therapeutic technique and could be an essential tool for DM prevention and management [ 35 ].
4.4. Gene Therapy and Diabetes Mellitus
Gene therapy is a technique that involves remedying the symptoms of an ailment orchestrated by a defective gene via the incorporation of the exogenous normal gene. Its advantage is that a single treatment can be used to cure any type of disease and currently, gene therapy is opening up novel treatment options in different branches of medicine [ 39 ]. At present, gene manipulation is not limited to the addition of a gene but also gene modulation and editing [ 40 , 41 ]. Gene therapy can also be explained as a method of introduction of a gene or gene manipulation within a cell as a curative regimen in the treatment of disease [ 42 ]. The objective of this approach is to remedy abnormal genes that have been implicated as the causative agent in any ailment and to successfully halt the beginning of the ailment or prevent its continuation. The gene therapy approach involves three major intervention methods: (i) delivery of a new gene into the body, (ii) substitution of the abnormal gene with a working gene, and (iii) disabling the malfunction genes responsible for the ailment [ 42 , 43 ]. Gene therapy can be further classified into somatic gene therapy or germline gene therapy. While the primary target in somatic gene therapy is the somatic cells often referred to as the diseased cells, the reproductive cells are the targets in germline gene therapy. Germline therapy halts the development of the disease in subsequent generations [ 43 ]. The application of gene therapies as trends in evolving therapeutics is due to its potential for the treatment of diverse ailments including DM, autoimmune disorders, heart diseases, and cancers among others that are difficult to manage using conventional therapies [ 44 ].
T1DM is an autoimmune ailment marked by T-cell-orchestrated self-damage of the islet beta cells responsible for the secretion of insulin. Its management is problematic and complex, particularly using conventional drugs. Thus, gene therapy is partly an emerging promising therapeutic alternative in its treatment [ 45 , 46 ]. The etiology of T1DM is multifactorial involving both environmental and genetic factors akin to any other autoimmune disease. In the recent past, researchers have favourably pointed out many genes accountable for the evolution of T1DM [ 47 ]. Thus, alteration or grappling with these genes employing gene therapy techniques will probably foster better comprehensible management of the ailment or even cure T1DM.
Even though gene therapy for DM majorly centres on T1DM, many genes have been evaluated as a probable treatment for T2DM as the ailment has a compelling genetic susceptibility [ 48 ]. About 75 independent genetic loci have been identified for T2DM via genetic linked studies and different novel therapeutic targets have also been determined [ 46 ]. Genetic loci might have a huge impact on drug response in contrast to the incidence and development of diseases whose effects are limited [ 49 ]. Many genetic loci exist with prospects for T2DM gene therapy. For instance, nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) is a good example. NLRP3 inhibition mitigates inflammation, guard against apoptosis of pancreatic b-cells including the prevention of development of T2DM in mice [ 50 ]. Hypothetically, all genes associated with the beginning, evolution, and deterioration of T2DM are probable targets. In Table 1 [ 51 ], the genes that modulate the homeostasis of glucose, ameliorate insulin synthesis or/and responsiveness, and improve diabetic mellitus-induced complications are abridged for simplicity.
Promising targets that can be employed for T2DM gene therapy.
| Class | Genes | Main Function |
|---|---|---|
| Genes modulating homeostasis of glucose | GLUTs | Involved in the re-absorption of filtered glucose from the kidney into the bloodstream |
| SGLTs | Partake profoundly in muscle and hepatic glucose fluxes | |
| FGFs | Functions significantly in the homeostasis of glucose | |
| SIRT6 | Connected with an expression of GLUTs and increased glycolysis | |
| Genes enhancing the secretion of insulin and/or sensitivity | GLP-1 and its analogs/agonists | Boost the survival of beta-cell, provoke the expression of the insulin gene, and synthesis |
| GPGRs and their agonists | Enhances the secretion of insulin and GLP-1 | |
| CTB-APSL | Enhances secretion of insulin and insulin resistance | |
| IKK E, TBK1 | Linked with diminution in weight, insulin resistance, fatty liver as well as inflammation | |
| Genes attenuating diabetic induced complications | IL-1b | Linked with inflammation and b-cell failure |
| ADPN | Attenuates diabetic nephropathy | |
| TGF-a | Has a function in DKD linked with nephron reduction | |
| NLRP3 | Attenuates diabetic cardiomyopathy | |
| CDKN2A/2B | connected with modulation of T-cell phenotype and chronic inflammation | |
| HSP70 | Connected with bioenergetics of mitochondrion and diabetic sensory neuropathy | |
| MicroRNAs | Implicated in the modulation of diabetic microvasculature |
Legend: HSP70 = heat shock protein 70; NLRP3 = nucleotide-binding oligomerization domain-like receptor protein 3; SGLTs = sodium-glucose co-transporters; GLUTs, glucose transporters; SIRT6=Sirtuin 6; FGFs = fibroblast growth factors; GPGRs = G protein–coupled receptors; GLP-1= glycogen-like peptide 1; ADPN = adiponectin; CTB APSL = cholera toxin B subunit and active peptide from shark liver; TGF-a = transforming growth factor-alpha; DKD = diabetic kidney disease [ 51 ].
4.5. Stem Cell Therapy in Diabetes
The conventional approaches in the management of DM do not resolve the causes of the ailment and are laden with adverse effects. Hence, there is a quest for a desirable different therapeutic regimen. The cellular-based therapeutic technique currently in use in DM management is based on the pancreas or islet-cell transplantation to revive the beta cells for insulin secretion. This approach is restricted due to a lack of donor organs. These problems lead to the exploration of the possibility of constructing beta cells using stem cells. The peculiar rebuilding potential of stem cells might be an important tool that could be used in the management of DM. Development of replenishable islets source using stem cells might avert the recent supply/demand problems in the transplantation of islet and furnish DM subjects with a prolonged source of beta cells for insulin secretion. Hence, in the management of DM, stem cell investigation has become a promising approach [ 52 ].
The stem cell DM therapy is aimed at the replacement of malfunctioning or damaged pancreatic cells by employing pluripotent or multipotent stem cells. This technique has exploited the ability of various kinds of stem cells including induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), and adult stem cells using diverse methods to produce surrogate beta cells or to bring back the physiologic role of the beta cell [ 53 ].
Advancement in technology has facilitated the development of stem cells using different kinds of tissue sources such as adipose tissue, skin, bone marrow, umbilical cord blood, periosteum, and dental pulp. In searching for promising stem cells, the first organ of choice is usually the pancreas. Studies with animal models have indicated that a small number of pancreatic tissue when made available could bring back the optimum pancreatic beta-cell mass [ 54 ]. This is sequel to the differentiated beta cells from the pancreatic duct undergoing replication and dedifferentiation culminating in the formation of pluripotent cells which in turn synthesize more beta cells. Additional study suggested that these ductal cells populations could be produced in vitro and directed to produce insulin synthesizing clusters [ 55 , 56 ].
Moreover, the haemopoietic adult stem cells such as HSCs and mesenchymal stem cells (MSCs) have the potential to transdifferentiate into so many cell lineages such as the brain, liver, and lung as well as gastrointestinal tract cells [ 57 , 58 , 59 ]. A different group of researchers experimented on the multipotent differentiation of haemapoietic progenitors to replenish the beta cell number in T1DM. It was reported that the bone marrow of mouse was differentiated ex vivo into functional beta cells [ 60 ]. Relatedly, studies using the mice model indicated that cells of the bone marrow could be amenable to the pancreas as a target and that elevated blood glucose could be normalized [ 61 ]. An experiment with autologous HSCs demonstrated an improvement in T1DM and T2DM [ 62 , 63 ]. These studies furnish potential outcomes for the usage of autologous HSCs in the management of DM.
4.6. Latest Inventions in Diabetes Management
In addition to the aforementioned innovations in the management of diabetes, several drugs are still at different stages of clinical trial for eventual use. Others are ready and have been recently introduced into the market.
4.6.1. Drugs Recently Introduced
Tirzepatide: The drug was recently approved by the FDA under the trade name mounjaro for the treatment of T2DM [ 64 ]. Tirzepatide is an injectable given under the skin once in a week which targets the receptors of hormones which play central role in the metabolism of glucose. These hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). While the GLP-1 reduces blood glucose by several mechanisms, including stimulating insulin secretion and suppressing glucagon release during hyperglycemia, GIP stimulates insulin release during hyperglycemia, but it also stimulates glucagon release during hypoglycemia.
Tirzepatide acts as agonist to their receptors [ 65 ], hence elongating their functions which results in blood glucose control. The efficacy of tirzepatide was established against a placebo, a GLP-1 receptor agonist (semaglutide) and two long-acting insulin analogs either as monotherapy or in combination with other antidiabetic agents [ 64 ]. In comparison to the placebo, it lowered the HbA1c by 11.6% and 1.5% as monotherapy and combination therapy, respectively. In comparison to other antidiabetic drugs, at the highest dose of 15 mg, it lowered the HbA1c 0.5% more than semaglutide, 0.9% more than insulin degludec and 1.0% more than insulin glargine [ 64 ]. Because of the efficacy therein and the once in a week dosing, tirzepatide provides a desirable paradigm shift in the management of T2DM.
4.6.2. Drugs in the Pipeline
Several drug candidates are at different phases of development for the management of DM. These are listed below.
LY3502970: LY3502970 is a partial agonist, biased toward G-protein activation over β-arrestin recruitment at the (GLP-1 receptor (GLP-1R). The molecule is highly potent and selective against other class B G-protein-coupled receptors (GPCRs) with a pharmacokinetic profile favorable for oral administration [ 66 ]. It is a product that is currently being developed by Eli lilly.
SCO-094: SCO-094 is a drug candidate identified by SCOHIA company which has a dual target of the receptors of GIP and GLP-1 [ 67 ]
Ladarixin (LDX): Ladarixin is an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset T1DM [ 68 ]. It is a drug candidate developed by Dompe Farmaceutici. Short term LDX treatment of newly diagnosed patients with T1DM had no appreciable effect on preserving residual beta cell function [ 68 ].
5. Discussion of Major Findings
DM is a complex, progressive, and multifactorial metabolic disorder needing more complex treatments over time. Globally, researchers have worked assiduously in the discovery and development of novel drugs for the treatment of diabetes. There is significant progress in research into the cause and management of T1DM [ 69 ]. Mounting evidence indicates that modern insulin therapy in combination with glucose self-monitoring including blood pressure and lipid monitoring has profoundly improved the long-term prognosis of T1DM [ 70 ]. The literature indicates that regular exercise and improved diet may enhance the quality of life for diabetic subjects but in the absence of adequate exercise and diet, medications may help diabetic persons regulate their blood glucose level. Moreover, implantation of insulin producing cells could furnish the basal glucose level essential for maintaining glucose homeostasis in vivo and thus hinder long-term injury from occurring in different tissues regardless of hormone administration [ 71 ].
The attainment of the full potential of gene therapy technique could be obtained via the design of gene delivery vectors that are safe, efficient, and specific and/ or the development of a technique for engineering of cell, in which the stem cell seems to be of great importance. Thus, the establishment of a reliable, sensitive, and acutely monitored feedback system is needed for the generation of a safe and efficient vector to facilitate diabetes gene therapy for clinical trial. Probably, the curtailment of islet transplantation rejection is the first clinical technique to DM gene therapy approach. On the other hand, insulin gene therapy is carried out in concert with conventional insulin treatment culminating in tight glycemic regulation in the absence of fasting hypoglycemia in T1DM subjects, as reported in T1DM rats [ 72 ].
Physical activity and nutrition therapy could help individuals with DM achieve metabolic goals. Employing diverse lifestyle approaches might help. Regulation of metabolic parameters such blood pressure, glucose, glycated hemoglobin, lipids, and body weight including the assessment of life quality are critical in determining the level of treatment goals by lifestyle changes [ 73 ]. However, different countries have focused on DM management and its complications on the normalization of glycemic control as assessed by hemoglobin A1 or fasting blood glucose which only addresses the need of subjects who were already diabetic. Thus, it is imperative to design programs for the early detection of altered glucose metabolism and to carry out robust approaches for the normalization of this changed state. Furthermore, through robust prevention strategies, better diagnostic tests, early risk detection, and management of the risks will help mitigate the incidence of DM and reduce or prevent events associated with end-organ failure [ 73 ].
Besides glycemic control, multifactorial interventions using different treatment regimen, including nanotechnology, gene therapy, stem cell, medical nutrition therapy, and lifestyle modification have yielded significant results in ameliorating the impact of DM but not without some challenges. Regardless of the promising nature of nanotechnology and its projected ability to turn around the fortunes in diabetes management, it is still faced with some challenges. One of the major limitations is the cost. Most of the gadgets required for CGM, and insulin delivery are very expensive. This limits their use to the rich class even when diabetes cuts across different economic classes. More so, there is an increased risk of infection via the implantation of sensors and cannulas which increases inflammation and could be frightening sometimes [ 24 ].
Notwithstanding the merits linked with the gene therapy approach, there could equally be problems. For example, genes introduced employing a viral vector might provoke an immune response and aggravate the disease condition [ 74 ]. Additionally, gene therapy studies are still mostly carried out using animal models and their safety is yet to be validated in humans [ 46 ].
Currently, it is established that gene delivery technology is the primary hurdle for successful gene therapy. The prime factors for an effective gene delivery technique include efficiency, stability, specificity, safety, and convenience. Thus, the greatest obstacle in gene therapy is the method of delivery of the corrective gene to the target site safely and efficiently. There is, therefore, a requirement of desirable gene delivery technology or vector to furnish the therapeutic potential where required. The two main vectors currently employed are viral and non-viral vectors. The merit of the non-viral vector is that it has low immunity, a low financial burden, and its preparation is convenient but the major obstacles for its extensive use emanate from the inefficiency of delivery method and expression of gene transiently [ 75 ]. Contrastingly, reports show that viral vectors are more efficient in gene delivery as several of them use a distinct mechanism for DNA delivery to the cells. Viral vectors are arranged as viral particles having precisely the important modulated sequences of the virus and from which all the genes of the virus have been excised. These viruses, when prepared very well, are defective that after target cell infection, there is no probable replication or infection theoretically [ 76 ]. Viral DNA is integrated with the genome of the host cell, thereby bestowing the capability for sturdy therapeutic gene expression.
Despite the fact that viral vectors are more efficient in comparison to non-viral vectors as gene delivery systems, there are still challenges associated with them, including inflammation, cytotoxicity, and immunogenicity which are needed to be looked into during the construction of viral vector system [ 46 ].
Notwithstanding the huge and novel impacts recorded in the applicable areas of stem cell biology in the management of DM, it is still in its primitive stage. A lot of hurdles still hinder the progression of stem cell research technologically and ethically, including:
The use of ESCs is confronted with the formation of teratomas and the danger of malignancy [ 77 ], thus raising safety concerns. This makes it imperative for a thorough investigation and screening of the probable adverse effects prior to its deployment in clinical trials and human treatment.
The primary hurdle associated with transplantation is autoimmune rejection. This makes it necessary for a stable and appropriate regimen for immunosuppression. There is a need for the stabilization of current transplantation protocols with the standard testing module. The transplantation of stem cells needs a few experimental works to appraise the problems linked with the stability, durability, and the survival of the transplanted cell with appropriate vascular and neural support in the new microenvironment.
The challenges of scale-up problems arise after the optimization of the appropriate developmental procedures. The number of cells must be enough to cope with the requisite request for future research including clinical investigations. Hence, an efficient method is required for the maximization of the yield via an adjustment in the culture requirements. The stem cells’ scale-up ability is needed for future exploration for the provision of surplus transplanted cellular reserves in order to strike equilibrium between demand and usage.
As a result of where it is obtained from, the ESCs are the potential targets for the ethicists. Normally, ESCs are obtained from embryos not fertilized or used during ex vivo fertilization in hospitals. Informed consent is usually required in the procurement of these ESCs from the donor prior to the usage in clinical research. Sadly, though, in the majority of instances, there is the destruction of the embryo during the process of obtaining the cells from the embryo, and this questions the source of life and the ethical license to terminate the fetus. Adult stem cells are preferable to embryonic ones as the controversy about their usage is limited. The current advancement in technology in induced pluripotent stem cell research is to allow the use of ones’ stem cells for diverse uses [ 78 ]. The adult cells are reprogrammed in such cases to pluripotent conditions and thereafter transformed into working beta cells. This approach might eventually resolve the impasse linked with ESCs and contribute to further safety issues likely to be tackled later in the future.
6. Conclusions
DM has become a public clinical challenge that requires urgent attention and the increasing trend in its cases is suggested to continue for more decades. Currently, there is no permanent cure for DM. Many treatment regimens have shown promising results in DM management. Yet, notwithstanding the potential of these giant treatment plans, DM remains a serious challenge that may continue to threaten public health. Thus, the problems encountered in each of these approaches need to be addressed to achieve a robust, efficient, and safe clinical management plan. There is a need for optimal metabolic regulation of glucose, blood pressure, and body weight which requires proper education and support for the improvement of diet, physical activity, and reduction in body weight. To effectively and successfully manage the control of this disease, an emphasis on public policies to reinforce health care access and resources, the promotion of a patient-centred care approach, and health-promoting infrastructures at environmental level are required.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Conception and Design: C.A., C.O.E. and I.A.; Data Collection: C.A., C.O.E., P.N.O. and N.A.O.; Data Analysis and Table Creation: C.A., P.M.A., J.C. and B.O.A. Writing the Manuscript: C.A., C.O.E., P.M.A., N.A.O., J.C., B.O.A., P.N.O. and I.A.; Vetting the manuscript for intellectual content: I.A.; Approval of the manuscript for submission: All the authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare that they have no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
COMMENTS
The US Food and Drug Administration (FDA) has cleared the Omnipod 5 for use by people aged 18 and older with type 2 diabetes, a first for any automated insulin delivery (AID) system. The Omnipod 5 ...
TO THE EDITOR. We are fortunate to have read the article, which was written by Feng et al[] and published in the World Journal of Diabetes.We extend our felicitations to the authors for completing the aforementioned retrospective study and providing new insights into treating type 2 diabetes mellitus (T2DM) patients with Hashimoto's thyroiditis (HT).
Diabetes mellitus (DM) is a chronic disorder that affects carbohydrate, protein, and fat metabolism, leading to abnormal blood glucose levels. 1 It is classified into two main types: type 1 and type 2 diabetes (T2D). 2 Type 1 diabetes typically occurs in children but can manifest in adults, particularly in their late 30s and early 40s. Patients with type 1 diabetes are usually not obese and ...
Diabetes mellitus is a chronic metabolic disorder characterized by high blood sugar levels, which can lead to severe health issues if not managed effectively. Recent statistics indicate a significant global impact, with 463 million adults diagnosed worldwide and this projected to rise to 700 million by 2045. Type 1 diabetes is an autoimmune disorder where the immune system attacks pancreatic ...
Research output: Contribution to journal › Article › peer-review. Overview; Original language: English: Pages (from-to) 234-238: Number of pages: 5: ... Bedtime dosing of glyburide and the treatment of type II diabetes mellitus. American Journal of the Medical Sciences, 308(4), 234-238.
Several studies have demonstrated that older adults with type 2 diabetes mellitus (T2DM) have a higher risk of falls compared to those without T2DM, which may lead to disability and a lower quality of life. While, limited prospective studies have quantified the associations in southern China. We conducted a longitudinal cohort study to quantify the associations between T2DM and falls and ...
Diabetes mellitus type 2; General fitness training; ... 2020 — Losing a few kilograms in weight almost halves people's risk of developing Type 2 diabetes, according to a large scale research ...
Annals of Sports Medicine and Research . ISSN: 2379-0571 Annals of Sports Medicine and Research . Downolad Brochure. Journal Menu . Home; ... Weijers RNM (2024) Type 2 Diabetes Mellitus: Insulin Resistance Loses Relevance. Ann Sports Med Res 11(2): 1227. Show Citation. Hide Citation. Received : 28 Jun 2024 Accepted : 31 Jul 2024 ...
Diabetes mellitus (DM) is commonest endocrine disorder that affects more than 100 million people. worldwide (6% po pulation). It is caused b y deficiency or ineffective production of insulin by ...
Different classes of diabetes mellitus, type 1, type 2, gestational diabetes and other types of diabetes mellitus are compared in terms of diagnostic criteria, etiology and genetics. The molecular genetics of diabetes received extensive attention in recent years by many prominent investigators and research groups in the biomedical field.
Recent Advances. ADA-funded researchers use the money from their awards to conduct critical diabetes research. In time, they publish their findings in order to inform fellow scientists of their results, which ensures that others will build upon their work. Ultimately, this cycle drives advances to prevent diabetes and to help people burdened by it.
I. Introduction. Diabetes mellitus, a metabolic disease defined by elevated fasting blood glucose levels due to insufficient insulin production, has reached epidemic proportions worldwide (World Health Organization, 2020).Type 1 and type 2 diabetes (T1D and T2D, respectively) make up the majority of diabetes cases with T1D characterized by autoimmune destruction of the insulin-producing ...
Among the U.S. population overall, crude estimates for 2021 were: 29.7 million people of all ages—or 8.9% of the U.S. population—had diagnosed diabetes. 352,000 children and adolescents younger than age 20 years—or 35 per 10,000 U.S. youths—had diagnosed diabetes. This includes 304,000 with type 1 diabetes.
Gestational diabetes mellitus (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation) ... maternal-fetal medicine, pediatrics, diabetes research, biostatistics, and other related fields. The panel recommended a two-step approach to screening that used a 1-h 50-g glucose load test ...
Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-1585. Crossref. ... Chronicle of Diabetes Research and Practice, 3, 1, ...
Correlation between thyroid hormone sensitivity and diabetic peripheral neuropathy in euthyroid patients with type 2 diabetes mellitus. ... Research Open Access 21 Aug 2024 Communications Medicine.
Physical activity recommendations and precautions may vary by diabetes type. The primary types of diabetes are type 1 and type 2. Type 1 diabetes (5%-10% of cases) results from cellular-mediated autoimmune destruction of the pancreatic β-cells, producing insulin deficiency ().Although it can occur at any age, β-cell destruction rates vary, typically occurring more rapidly in youth than in ...
No significant association was noted between the presence of MS and prostate cancer development. On multivariate analysis, diabetes mellitus continued to protect against the development of prostate cancer, this was more pronounced in the absence of MS (HR=0.43, P=0.01 for diabetes in the absence of MS; HR=0.64, P=0.08 in the presence of MS).
The purpose of this study is to evaluate the impact of a digital storytelling intervention derived through a community-based participatory research (CBPR) approach on type 2 diabetes mellitus (T2D) outcomes among Hispanic adults with poorly controlled type 2 diabetes mellitus (T2D) in primary care settings through a randomized clinical trial.
With the development of these new monitoring and treatment modalities along with quantitative methods of measuring complications, the stage was set to test the glucose hypothesis ().In 1974, the U.S. Congress passed the National Diabetes Research and Education Act (PL 93-3540), which required the National Institutes of Health (NIH) to establish a National Commission on Diabetes.
Diabetes Mellitus. Diabetes mellitus is defined as a metabolic disorder of multiple etiologies characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both [1]. ... Ling Xiao, Guoyu Pan, in Clinics and Research in Hepatology and ...
This study employed bibliometric analysis to investigate recent advancements in research about the relationship between vitamin D and type 2 diabetes. Methods: We searched for articles on the topic of vitamin D and type 2 diabetes published between January 1, 2004, and December 31, 2023. The search was performed on February 20, 2024, using the ...
with type 1 diabetes mellitus in the US Authors: Orighomisan Agboghoroma, Kory Heier, Meredith Duncan, Anna Kucharska-Newton, Mary E Lacy BACKGROUND RESEARCH QUESTION METHODS RESULTS CONCLUSIONS ACKNOWLEDGEMENT & REFERENCES Cardiovascular disease (CVD) is a major cause of morbidity and mortality for people with type 1 diabetes (T1D).
The incidence of diabetes mellitus is rapidly increasing, and this condition often results in significant metabolic disease and severe complications. Nurses have a crucial role in monitoring, educating and supporting people with diabetes, as well as their families and significant others. This article provides an overview of the main types and ...
Methods. In this trial involving participants with type 2 diabetes of less than 10 years' duration who were receiving metformin and had glycated hemoglobin levels of 6.8 to 8.5%, we compared the ...
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR ...
Diabetes mellitus (DM) is a long-standing, complicated, and non-transmissible endocrine ailment that is growing rapidly and has posed clinical challenges globally, often linked with threats related to complicated metabolic development in patients. ... Nanotechnology in diabetes research has played several roles in improving the outcome of ...