An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Nutrition for sports performance: issues and opportunities
Affiliation.
- 1 School of Sport, Exercise and Health Sciences, Loughborough University, Leicestershire LE11 3TU, UK. [email protected]
- PMID: 22000743
- DOI: 10.1017/S0029665111003211
Diet can significantly influence athletic performance, but recent research developments have substantially changed our understanding of sport and exercise nutrition. Athletes adopt various nutritional strategies in training and competition in the pursuit of success. The aim of training is to promote changes in the structure and function of muscle and other tissues by selective modulation of protein synthesis and breakdown in response to the training stimulus. This process is affected by the availability of essential amino acids in the post-exercise period. Athletes have been encouraged to eat diets high in carbohydrate, but low-carbohydrate diets up-regulate the capacity of muscle for fat oxidation, potentially sparing the limited carbohydrate stores. Such diets, however, do not enhance endurance performance. It is not yet known whether the increased capacity for fat oxidation that results from training in a carbohydrate-deficient state can promote loss of body fat. Preventing excessive fluid deficits will maintain exercise capacity, and ensuring adequate hydration status can also reduce subjective perception of effort. This latter effect may be important in encouraging exercise participation and promoting adherence to exercise programmes. Dietary supplement use is popular in sport, and a few supplements may improve performance in specific exercise tasks. Athletes must be cautious, however, not to contravene the doping regulations. There is an increasing recognition of the role of the brain in determining exercise performance: various nutritional strategies have been proposed, but with limited success. Nutrition strategies developed for use by athletes can also be used to achieve functional benefits in other populations.
PubMed Disclaimer
Similar articles
- Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. [No authors listed] [No authors listed] J Am Diet Assoc. 2000 Dec;100(12):1543-56. doi: 10.1016/S0002-8223(00)00428-4. J Am Diet Assoc. 2000. PMID: 11145214
- Nutrition for power sports: middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. Stellingwerff T, Maughan RJ, Burke LM. Stellingwerff T, et al. J Sports Sci. 2011;29 Suppl 1:S79-89. doi: 10.1080/02640414.2011.589469. Epub 2011 Jul 28. J Sports Sci. 2011. PMID: 21793766 Review.
- Sport-specific nutrition: practical strategies for team sports. Holway FE, Spriet LL. Holway FE, et al. J Sports Sci. 2011;29 Suppl 1:S115-25. doi: 10.1080/02640414.2011.605459. Epub 2011 Aug 11. J Sports Sci. 2011. PMID: 21831001
- Position of Dietitians of Canada, the American Dietetic Association, and the American College of Sports Medicine: Nutrition and Athletic Performance. [No authors listed] [No authors listed] Can J Diet Pract Res. 2000 Winter;61(4):176-192. Can J Diet Pract Res. 2000. PMID: 11551367
- Nutrition for endurance sports: marathon, triathlon, and road cycling. Jeukendrup AE. Jeukendrup AE. J Sports Sci. 2011;29 Suppl 1:S91-9. doi: 10.1080/02640414.2011.610348. Epub 2011 Sep 15. J Sports Sci. 2011. PMID: 21916794 Review.
- Nutritional knowledge, attitude, and practice of professional athletes in an Iranian population (a cross-sectional study). Hasanpouri A, Rahmani B, Gharakhanlou BJ, Solaimanian S, Shahsavari S, Rasouli A, Abbasi S, Ebrahimi-Kalan A, Rouzitalab T, Hoseinabadi Z, Shiri-Shahsavar MR. Hasanpouri A, et al. BMC Sports Sci Med Rehabil. 2023 Dec 4;15(1):164. doi: 10.1186/s13102-023-00776-3. BMC Sports Sci Med Rehabil. 2023. PMID: 38049912 Free PMC article.
- Coenzyme Q10 Supplementation in Athletes: A Systematic Review. Fernandes MSS, Fidelis DEDS, Aidar FJ, Badicu G, Greco G, Cataldi S, Santos GCJ, de Souza RF, Ardigò LP. Fernandes MSS, et al. Nutrients. 2023 Sep 15;15(18):3990. doi: 10.3390/nu15183990. Nutrients. 2023. PMID: 37764774 Free PMC article. Review.
- Assessment of the Dietary Intake and Nutritional Status of Polish Professional Futsal Players: A Descriptive Study-Do Futsal Players Require Nutritional Education? Gogojewicz A, Straburzyńska-Lupa A, Podgórski T, Frajtag P, Bibrowicz K, Śliwicka E. Gogojewicz A, et al. Nutrients. 2023 Aug 25;15(17):3720. doi: 10.3390/nu15173720. Nutrients. 2023. PMID: 37686752 Free PMC article.
- Metabolic and Body Composition Changes in Ice Hockey Players Using an Ergogenic Drug (Cytoflavin). Zaborova V, Kurshev V, Kryuchkova K, Anokhina V, Malakhovskiy V, Morozova V, Sysoeva V, Zimatore G, Bonavolontà V, Guidetti L, Dronina Y, Kravtsova E, Shestakov D, Gurevich K, Heinrich KM. Zaborova V, et al. Biology (Basel). 2023 Jan 29;12(2):214. doi: 10.3390/biology12020214. Biology (Basel). 2023. PMID: 36829493 Free PMC article.
- Four Weeks of Hericium erinaceus Supplementation Does Not Impact Markers of Metabolic Flexibility or Cognition. Grozier CD, Alves VA, Killen LG, Simpson JD, O'Neal EK, Waldman HS. Grozier CD, et al. Int J Exerc Sci. 2022 Oct 1;15(2):1366-1380. eCollection 2022. Int J Exerc Sci. 2022. PMID: 36582308 Free PMC article.
- Search in MeSH
Related information
- PubChem Compound
- PubChem Substance
LinkOut - more resources
Full text sources.
- Cambridge University Press
- Ovid Technologies, Inc.
- MedlinePlus Consumer Health Information
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Sports Nutrition and Performance
- February 2019
- In book: Nutrition in Health and Disease
- Publisher: InTech Open

- Universidad Católica San Antonio de Murcia
- This person is not on ResearchGate, or hasn't claimed this research yet.

Abstract and Figures
![research paper on sports nutrition How to calculate sweat rate? [43].](https://www.researchgate.net/profile/Javier-Marhuenda/publication/331257271/figure/fig1/AS:728746269106180@1550758058622/How-to-calculate-sweat-rate-43_Q320.jpg)
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Sukendro Sukendro

- Syed Kamaruzaman

- Alfiah Rizqi Azizah

- Amiruddin Amiruddin
- Anannya Shah
- Sibel AYYILDIZ

- PSYCHOL SPORT EXERC

- Sports Nutr Rev J

- J. Porta-Manceñido
- PRIMARY CARE

- Jessica R. Stumbo
- Maurie Joe Luetkemeier
- Joseph Michael Hanisko
- Kyle Mathiew Aho
- C.A. Darley

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Advertisement
Basic Principles of Sports Nutrition
- Gastroenterology, Critical Care, and Lifestyle Medicine (SA McClave, Section Editor)
- Published: 10 August 2016
- Volume 5 , pages 213–222, ( 2016 )
Cite this article

- Anna Grout 1 ,
- Stephen A. McClave 1 , 2 ,
- Melina B. Jampolis 1 ,
- Kristine Krueger 1 ,
- Ryan T. Hurt 1 ,
- Sarah Landes 1 &
- Laszlo Kiraly 1
2523 Accesses
7 Citations
Explore all metrics
Proper nutrition is a key component in the preparation and training of the competitive athlete. The dietary recommendations for sports nutrition are surprisingly conventional, similar to that for the prevention of chronic disease (such as cancer, diabetes, stroke, hypertension, and cardiovascular disease). Few specialized supplements are required above a well-balanced diet of sufficient protein and carbohydrate. Total caloric requirements, macronutrient composition, and need for electrolyte/micronutrient repletion may vary from one sport to another (and between various positions within a single sport). The type and duration of the sporting event affect the utilization of energy systems, substrate availability, and the training adaptations required to optimize athleticism. Undernutrition, dehydration, and electrolyte abnormalities can reduce cognition, endurance, thermoregulation, overall performance, and recovery. A properly designed dietary program throughout training, competition, and the off-season should benefit and help protect both the recreational and the elite athlete.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Optimizing Nutrition for Exercise and Sports

Maximizing Nutrition and Supplements for Masters Athletes

Sports Nutrition
Papers of particular interest, published recently, have been highlighted as: •• of major importance.
Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501–28. This paper is the latest edition of joint guidelines produced by three societies and serves as a comprehensive review of the topic .
Article PubMed Google Scholar
Wolff A. The new training table. Sports Illustrated. 2011. p. 122–30.
Google Scholar
Paffenbarger Jr RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–13.
Loucks AB. Energy balance and energy availability. In: Maughan RJ, editor. Sports Nutrition, The Encyclopaedia of Sports Medicine, and IOC Medical Commission Publication. West Sussex: John Wiley & Sons, Ltd; 2013. p. 72–87.
Chapter Google Scholar
Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. 2011;21(2):97–104.
Article CAS PubMed Google Scholar
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, et al. The IOC consensus statement: beyond the female athlete triad—relative energy deficiency in sport (RED-S). Br J Sports Med. 2014;48(7):491–7. This report expands the concept of the female athlete triad to a full spectrum of eating disorders related to an energy deficiency .
Steffes GD, Megura AE, Adams J, Claytor RP, Ward RM, Horn TS, et al. Prevalence of metabolic syndrome risk factors in high school and NCAA division I football players. J Strength Cond Res. 2013;27(7):1749–57.
Stellingwerff T, Maughan RJ, Burke LM. Nutrition for power sports: middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. J Sports Sci. 2011;29 Suppl 1:S79–89.
Sundgot-Borgen J, Meyer NL, Lohman TG, Ackland TR, Maughan RJ, Stewart AD, et al. How to minimise the health risks to athletes who compete in weight-sensitive sports review and position statement on behalf of the Ad Hoc Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission. Br J Sports Med. 2013;47(16):1012–22.
Ackland TR, Lohman TG, Sundgot-Borgen J, Maughan RJ, Meyer NL, Stewart AD, et al. Current status of body composition assessment in sport: review and position statement on behalf of the ad hoc research working group on body composition health and performance, under the auspices of the I.O.C. Medical Commission. Sports Med. 2012;42(3):227–49.
http://www.ncaa.org/health-and-safety/sport-science-institute/body-composition-what-are-athletes-made.html (Accesed 6-22-16).
American Dietetic Association; Dietitians of Canada; American College of Sports Medicine, Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41(3):709–31. This report is the 2009 edition of the guidelines supported by the three separate societies (see reference #1) and is also a very good comprehensive review of the topic .
Article Google Scholar
Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab. 2014;306(8):E989–97.
van Essen M, Gibala MJ. Failure of protein to improve time trial performance when added to a sports drink. Med Sci Sports Exerc. 2006;38(8):1476–83.
Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44 Suppl 1:S87–96.
Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45 Suppl 1:5–12.
Article PubMed Central Google Scholar
Grobler LA, Collins M, Lambert MI, Sinclair-Smith C, Derman W, St Clair Gibson A, et al. Skeletal muscle pathology in endurance athletes with acquired training intolerance. Br J Sports Med. 2004;38(6):697–703.
Article CAS PubMed PubMed Central Google Scholar
Zoorob R, Parrish ME, O’Hara H, Kalliny M. Sports nutrition needs: before, during, and after exercise. Prim Care. 2013;40(2):475–86. This paper highlights the hydration and nutrition strategies through an athletic competative event .
Burke LM, Maughan RJ. The governor has a sweet tooth-mouth sensing of nutrients to enhance sports performance. Eur J Sport Sci. 2015;15(1):29–40.
Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery. J Sports Sci. 2004;22(1):15–30.
Misner B. Food alone may not provide sufficient micronutrients for preventing deficiency. J Int Soc Sports Nutr. 2006;3(1):51–5.
Article PubMed PubMed Central Google Scholar
Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20(7-8):632–44.
Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. 2006;16(5):453–84.
Larson-Meyer DE, Willis KS. Vitamin D and athletes. Curr Sports Med Rep. 2010;9(4):220–6.
Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41(5):1102–10.
Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43(2):335–43.
Shirreffs SM, Sawka MN. Fluid and electrolyte needs for training, competition, and recovery. J Sports Sci. 2011;29 Suppl 1:S39–46.
Jeukendrup A, Carter J, Maughan RJ. Competition fluid and fuel. In: Burke L, Deakin V, editors. Clinical sports nutrition. 5th ed. North Ryde: McGraw-Hill Australia Pty Ltd; 2015. p. 377–419.
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82. This provides a comprehensive discussion of the female athlete triad .
Baker LB, Dougherty KA, Chow M, Kenney WL. Progressive dehydration causes a progressive decline in basketball skill performance. Med Sci Sports Exerc. 2007;39(7):1114–23.
Lis D, Stellingwerff T, Kitic CM, Ahuja KD, Fell J. No effects of a short-term gluten-free diet on performance in nonceliac athletes. Med Sci Sports Exerc. 2015;47(12):2563–70.
Dunford M, Smith M. Dietary supplements and ergogenic aids. In: Nutrition for Health, Fitness, and Sport. 5th ed. New York: McGraw-Hill; 2006.
Braun H, Koehler K, Geyer H, Kleiner J, Mester J, Schanzer W. Dietary supplement use among elite young German athletes. Int J Sport Nutr Exerc Metab. 2009;19(1):97–109.
Maughan RJ. Riskks and rewards in dietary supplement use by athletes. In: Maughan RJ, editor. Sports Nutrition, the encyclopedia of sports medicine. West Sussex: John Wiley & Sons; 2014.
Leone JE, Sedory EJ, Gray KA. Recognition and treatment of muscle dysmorphia and related body image disorders. J Athl Train. 2005;40(4):352–9.
PubMed PubMed Central Google Scholar
Download references
Author information
Authors and affiliations.
Department of Medicine, University of Louisville School of Medicine, Louisville, KY, USA
Anna Grout, Stephen A. McClave, Melina B. Jampolis, Kristine Krueger, Ryan T. Hurt, Sarah Landes & Laszlo Kiraly
Division of Gastroenterology, Hepatology and Nutrition, University of Louisville School of Medicine, Louisville, KY, 40202, USA
Stephen A. McClave
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stephen A. McClave .
Ethics declarations
Conflict of interest.
Anna Grout declares that she has no conflict of interest.
Stephen A. McClave declares that he has no conflict of interest.
Melina B. Jampolis sits on the scientific board of directors for TerraVia and has received compensation from Prevention Pharmaceuticals for serving as a paid spokesperson.
Kristine Krueger declares that she has no conflict of interest.
Ryan T. Hurt declares that he has no conflict of interest.
Sarah Landes declares that she has no conflict of interest.
Laszlo Kiraly declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gastroenterology, Critical Care, and Lifestyle Medicine
Rights and permissions
Reprints and permissions
About this article
Grout, A., McClave, S.A., Jampolis, M.B. et al. Basic Principles of Sports Nutrition. Curr Nutr Rep 5 , 213–222 (2016). https://doi.org/10.1007/s13668-016-0177-3
Download citation
Published : 10 August 2016
Issue Date : September 2016
DOI : https://doi.org/10.1007/s13668-016-0177-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sports nutrition
- Body composition
- Energy expenditure
- Muscle glycogen
- Find a journal
- Publish with us
- Track your research
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Nutrition and Athletic Performance
This joint position statement is authored by the American Dietetic Association (ADA), Dietitians of Canada (DC), and American College of Sports Medicine (ACSM). The content appears in ADA style. This paper is being published concurrently in Medicine & Science in Sports & Exercise® and in the Journal of the American Dietetic Association, and the Canadian Journal of Dietetic Practice and Research. Individual name recognition is reflected in the acknowledgments at the end of the statement.

It is the position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine that physical activity, athletic performance, and recovery from exercise are enhanced by optimal nutrition. These organizations recommend appropriate selection of foods and fluids, timing of intake, and supplement choices for optimal health and exercise performance. This updated position paper couples a rigorous, systematic, evidence-based analysis of nutrition and performance-specific literature with current scientific data related to energy needs, assessment of body composition, strategies for weight change, nutrient and fluid needs, special nutrient needs during training and competition, the use of supplements and ergogenic aids, nutrition recommendations for vegetarian athletes, and the roles and responsibilities of the sports dietitian. Energy and macronutrient needs, especially carbohydrate and protein, must be met during times of high physical activity to maintain body weight, replenish glycogen stores, and provide adequate protein to build and repair tissue. Fat intake should be sufficient to provide the essential fatty acids and fat-soluble vitamins and to contribute energy for weight maintenance. Although exercise performance can be affected by body weight and composition, these physical measures should not be a criterion for sports performance and daily weigh-ins are discouraged. Adequate food and fluid should be consumed before, during, and after exercise to help maintain blood glucose concentration during exercise, maximize exercise performance, and improve recovery time. Athletes should be well hydrated before exercise and drink enough fluid during and after exercise to balance fluid losses. Sports beverages containing carbohydrates and electrolytes may be consumed before, during, and after exercise to help maintain blood glucose concentration, provide fuel for muscles, and decrease risk of dehydration and hyponatremia. Vitamin and mineral supplements are not needed if adequate energy to maintain body weight is consumed from a variety of foods. However, athletes who restrict energy intake, use severe weight-loss practices, eliminate one or more food groups from their diet, or consume unbalanced diets with low micronutrient density may require supplements. Because regulations specific to nutritional ergogenic aids are poorly enforced, they should be used with caution and only after careful product evaluation for safety, efficacy, potency, and legality. A qualified sports dietitian and, in particular, the Board Certified Specialist in Sports Dietetics in the United States, should provide individualized nutrition direction and advice after a comprehensive nutrition assessment.
POSITION STATEMENT
It is the position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine that physical activity, athletic performance, and recovery from exercise are enhanced by optimal nutrition. These organizations recommend appropriate selection of food and fluids, timing of intake, and supplement choices for optimal health and exercise performance.
This ADA position paper uses ADA's Evidence Analysis Process and information from the ADA Evidence Analysis Library (EAL). Similar information is also available from DC's Practice-based Evidence in Nutrition (PEN). The use of an evidence-based approach provides important added benefits to earlier review methods. The major advantage of the approach is the more rigorous standardization of review criteria, which minimizes the likelihood of reviewer bias and increases the ease with which disparate articles may be compared. For a detailed description of the methods used in the evidence analysis process, access the ADA's Evidence Analysis Process at http://adaeal.com/eaprocess/ .
Conclusion Statements are assigned a grade by an expert work group based on the systematic analysis and evaluation of the supporting research evidence: grade I = good, grade II = fair, grade III = limited, grade IV = expert opinion only, and grade V = a grade is not assignable because there is no evidence to support or refute the conclusion.
Evidence-based information for this and other topics can be found at www.adaevidencelibrary.com and www.dieteticsatwork.com/pen and subscriptions for non-ADA members are purchasable at https://www.adaevidencelibrary.com/store.cfm . Subscriptions for DC and non-DC members are available for PEN at http://www.dieteticsatwork.com/pen_order.asp
The following key points summarize the current energy, nutrient, and fluid recommendations for active adults and competitive athletes. These general recommendations can be adjusted by sports nutrition experts to accommodate the unique concerns of individual athletes regarding health, sports, nutrient needs, food preferences, and body weight and body composition goals.
- Athletes need to consume adequate energy during periods of high-intensity and/or long-duration training to maintain body weight and health and maximize training effects. Low energy intakes can result in loss of muscle mass; menstrual dysfunction; loss of or failure to gain bone density; an increased risk of fatigue, injury, and illness; and a prolonged recovery process.
- Body weight and composition should not be used as the sole criterion for participation in sports; daily weigh-ins are discouraged. Optimal body fat levels depend on the sex, age, and heredity of the athlete and may be sport-specific. Body fat assessment techniques have inherent variability and limitations. Preferably, weight loss (fat loss) should take place during the off-season or begin before the competitive season and involve a qualified sports dietitian.
- Carbohydrate recommendations for athletes range from 6 to 10 g·kg −1 body weight·d −1 (2.7-4.5 g·lb −1 body weight·d −1 ). Carbohydrates maintain blood glucose levels during exercise and replace muscle glycogen. The amount required depends on the athlete's total daily energy expenditure, type of sport, sex, and environmental conditions.
- Protein recommendations for endurance and strength-trained athletes range from 1.2 to 1.7 g·kg −1 body weight·d −1 (0.5-0.8 g·lb −1 body weight·d −1 ). These recommended protein intakes can generally be met through diet alone, without the use of protein or amino acid supplements. Energy intake sufficient to maintain body weight is necessary for optimal protein use and performance.
- Fat intake should range from 20% to 35% of total energy intake. Consuming ≤20% of energy from fat does not benefit performance. Fat, which is a source of energy, fat-soluble vitamins, and essential fatty acids, is important in the diets of athletes. High-fat diets are not recommended for athletes.
- Athletes who restrict energy intake or use severe weight-loss practices, eliminate one or more food groups from their diet, or consume high- or low-carbohydrate diets of low micronutrient density are at greatest risk of micronutrient deficiencies. Athletes should consume diets that provide at least the recommended dietary allowance (RDA) for all micronutrients.
- Dehydration (water deficit in excess of 2-3% body mass) decreases exercise performance; thus, adequate fluid intake before, during, and after exercise is important for health and optimal performance. The goal of drinking is to prevent dehydration from occurring during exercise and individuals should not drink in excess of sweating rate. After exercise, approximately 16-24 oz (450-675 mL) of fluid for every pound (0.5 kg) of body weight lost during exercise.
- Before exercise, a meal or snack should provide sufficient fluid to maintain hydration, be relatively low in fat and fiber to facilitate gastric emptying and minimize gastrointestinal distress, be relatively high in carbohydrate to maximize maintenance of blood glucose, be moderate in protein, be composed of familiar foods, and be well tolerated by the athlete.
- During exercise, primary goals for nutrient consumption are to replace fluid losses and provide carbohydrates (approximately 30-60 g·h −1 ) for maintenance of blood glucose levels. These nutrition guidelines are especially important for endurance events lasting longer than an hour when the athlete has not consumed adequate food or fluid before exercise or when the athlete is exercising in an extreme environment (heat, cold, or high altitude).
- After exercise, dietary goals are to provide adequate fluids, electrolytes, energy, and carbohydrates to replace muscle glycogen and ensure rapid recovery. A carbohydrate intake of approximately 1.0-1.5 g·kg −1 body weight (0.5-0.7 g·lb −1 ) during the first 30 min and again every 2 h for 4-6 h will be adequate to replace glycogen stores. Protein consumed after exercise will provide amino acids for building and repair of muscle tissue.
- In general, no vitamin and mineral supplements are required if an athlete is consuming adequate energy from a variety of foods to maintain body weight. Supplementation recommendations unrelated to exercise, such as folic acid for women of childbearing potential, should be followed. A multivitamin/mineral supplement may be appropriate if an athlete is dieting, habitually eliminating foods or food groups, is ill or recovering from injury, or has a specific micronutrient deficiency. Single-nutrient supplements may be appropriate for a specific medical or nutritional reason (e.g., iron supplements to correct iron deficiency anemia).
- Athletes should be counseled regarding the appropriate use of ergogenic aids. Such products should only be used after careful evaluation for safety, efficacy, potency, and legality.
- Vegetarian athletes may be at risk for low intakes of energy, protein, fat, and key micronutrients such as iron, calcium, vitamin D, riboflavin, zinc, and vitamin B 12 . Consultation with a sports dietitian is recommended to avoid these nutrition problems.
EVIDENCE-BASED ANALYSIS
Studies used in the development of this position paper were identified from the PubMed database maintained by the National Library of Medicine and CENTRAL database, as well as through research articles and literature reviews. Five topic-specific questions were identified for evidence-based analysis ( Fig. 1 ) and incorporated into this position, updating the prior position on nutrition and performance ( 1 ). Search terms used were athlete, performance, power, strength, endurance, or competition and macronutrient, meal, carbohydrate, fat, protein, or energy. For the purpose of this analysis, inclusion criteria were adults aged 18-40 yr; all sport settings; and trained athletes, athletes in training, or individuals regularly exercising. Because the grading system used provides allowances for consideration of study design, the evidence-based analysis was not limited to randomized controlled trials. Study design preferences were randomized controlled trials or clinical controlled studies; large nonrandomized observational studies; and cohort, case-control studies. All sample sizes were included and study dropout rate could not exceed 20%. The publication range for the evidence-based analysis spanned 1995-2006. If an author was included in more than one review article or primary research articles that were similar in content, the most recent paper was accepted, and earlier versions were rejected. However, when an author was included in more than one review article or primary research article for which content differed, then both reviews could be accepted for analysis.

The following exclusion criteria were applied to all identified studies:
- Adults older than 40 yr, adults younger than 18 yr, infants, children, and adolescents
- Settings not related to sports
- Nonathletes
- Critical illness and other diseases and conditions
- Drop out rates >20%
- Publication before 1995
- Studies by same author, which were similar in content
- Articles not in English
Conclusion statements were formulated summarizing the strength of evidence with respect to each question ( Fig. 1 ). The strength of the evidence was graded using the following elements: quality, consistency across studies, quantity, and generalizability. A more detailed description of the methodology used for this evidence-based analysis may be found on the American Dietetic Association's Web site at www.eatright.org/cps/rde/xchg/ada/hs.xsl/8099_ENU_HTML.htm .
ENERGY METABOLISM
Energy expenditure must equal energy intake to achieve energy balance. The energy systems used during exercise for muscular work include the phosphagen and glycolytic (both anaerobic) and the oxidative (aerobic) pathways. The phosphagen system is used for events lasting no longer than a few seconds and of high intensity. Adenosine triphosphate (ATP) and creatine phosphate provide the readily available energy present within the muscle. The amount of ATP present in the skeletal muscles (∼5 mmol·kg −1 wet weight) is not sufficient to provide a continuous supply of energy, especially at high exercise intensities. Creatine phosphate is an ATP reserve in muscle that can be readily converted to sustain activity for ∼3-5 min ( 2 ). The amount of creatine phosphate available in skeletal muscle is approximately four times greater than ATP and, therefore, is the primary fuel used for high-intensity, short-duration activities such as the clean and jerk in weight lifting or the fast break in basketball.
The anaerobic glycolytic pathway uses muscle glycogen and glucose that are rapidly metabolized anaerobically through the glycolytic cascade. This pathway supports events lasting 60-180 s. Approximately 25%-35% of total muscle glycogen stores are used during a single 30-s sprint or resistance exercise bout. Neither the phosphagen nor the glycolytic pathway can sustain the rapid provision of energy to allow muscles to contract at a very high rate for events lasting greater than ∼2-3 min.
The oxidative pathway fuels events lasting longer than 2-3 min. The major substrates include muscle and liver glycogen, intramuscular, blood, and adipose tissue triglycerides and negligible amounts of amino acids from muscle, blood, liver, and the gut. Examples of events for which the major fuel pathway is the oxidative pathway include a 1500-m run, marathon, half-marathon, and endurance cycling or ≥1500-m swimming events. As oxygen becomes more available to the working muscle, the body uses more of the aerobic (oxidative) pathways and less of the anaerobic (phosphagen and glycolytic) pathways. Only the aerobic pathway can produce much ATP over time via the Krebs cycle and the electron transport system. The greater dependence on aerobic pathways does not occur abruptly, nor is one pathway ever relied on exclusively. The intensity, duration, frequency, type of activity, sex, and fitness level of the individual, as well as prior nutrient intake and energy stores, determine when the crossover from primarily aerobic to anaerobic pathways occurs ( 2 ).
Conversion of energy sources over time.
Approximately 50%-60% of energy during 1-4 h of continuous exercise at 70% of maximal oxygen capacity is derived from carbohydrates and the rest from free fatty acid oxidation ( 3 ). A greater proportion of energy comes from oxidation of free fatty acids, primarily those from muscle triglycerides as the intensity of the exercise decreases ( 3 ). Training does not alter the total amount of energy expended but rather the proportion of energy derived from carbohydrates and fat ( 3 ). As a result of aerobic training, the energy derived from fat increases and from carbohydrates decreases. A trained individual uses a greater percentage of fat than an untrained person does at the same workload( 2 ). Long-chain fatty aids derived from stored muscle triglycerides are the preferred fuel for aerobic exercise for individuals involved in mild- to moderate-intensity exercise ( 4 ).
ENERGY REQUIREMENTS
Meeting energy needs is a nutrition priority for athletes. Optimum athletic performance is promoted by adequate energy intake. This section will provide information necessary to determine energy balance for an individual. Energy balance occurs when energy intake (the sum of energy from foods, fluids, and supplement products) equals energy expenditure or the sum of energy expended as basal metabolic rate (BMR), the thermic effect of food, the thermic effect of activity (TEA), which is the energy expended in planned physical activity, and nonexercise activity thermogenesis ( 5 ). Spontaneous physical activity is also included in the TEA.
Athletes need to consume enough energy to maintain appropriate weight and body composition while training for a sport ( 6 ). Although usual energy intakes for many intensely training female athletes might match those of male athletes per kilogram body weight, some female athletes may consume less energy than they expend. Low energy intake (e.g., <1800-2000 kcal·d −1 ) for female athletes is a major nutritional concern because a persistent state of negative energy balance can lead to weight loss and disruption of endocrine function ( 7-10 ).
Inadequate energy intake relative to energy expenditure compromises performance and negates the benefits of training. With limited energy intake, fat and lean tissue will be used for fuel by the body. Loss of lean tissue mass results in the loss of strength and endurance, as well as compromised immune, endocrine, and musculoskeletal function ( 11 ). In addition, long-term low energy intake results in poor nutrient intake, particularly of the micronutrients, and may result in metabolic dysfunctions associated with nutrient deficiencies as well as lowered resting metabolic rate (RMR). The newer concept of energy availability, defined as dietary intake minus exercise energy expenditure normalized to fat-free mass (FFM), is the amount of energy available to the body to perform all other functions after exercise training expenditure is subtracted. Many researchers have suggested that 30 kcal·kg −1 FFM·d −1 might be the lower threshold of energy availability for females ( 12-15 ).
Estimation of energy needs of athletes and active individuals can be done using a variety of methods. The Dietary Reference Intakes (DRI) ( 15,17 ) and the Dietary Guidelines 2005 ( 16 ) ( http://www.health.gov/dietaryguidelines/dga2005/report/HTML/D3_Disccalories.htm ) provide energy recommendations for men and women who are slightly to very active, which are based on predictive equations developed using the doubly labeled water technique thatcan also be used to estimate energy needs of athletes ( Fig. 2 ).

Energy expenditure for different types of exercise is dependent on the duration, frequency, and intensity of the exercise, the sex of the athlete, and prior nutritional status. Heredity, age, body size, and FFM also influence energy expenditure. The more energy used in activity, the more calories needed to achieve energy balance.
Typical laboratory facilities are usually not equipped to determine total energy expenditure. Therefore, predictive equations are often used to estimate BMR or RMR. The two prediction equations considered to most closely estimate energy expenditure are the Cunningham equation (1980) ( 18 ) and the Harris-Benedict equation ( 19 ). Because the Cunningham equation requires that lean body mass be known, sports dietitians typically use the Harris-Benedict equation. To estimate total energy expenditure, BMR or RMR is then multiplied by the appropriate activity factor of 1.8-2.3 (representing moderate to very heavy physical activity levels, respectively). Numeric guidelines such as these ( 8 ) only provide an approximation of the average energy needs of an individual athlete. An alternative method for estimating exercise energy expenditure is to use metabolic equivalents (METs) recorded during a 24-h period ( 20 ). Any of these methods can be used to estimate energy expenditure for the determination of energy intake requirements and provide the sports dietitian with a basis to guide the athletes or active individuals in meeting their energy needs.
BODY COMPOSITION
Body composition and body weight are two of the many factors that contribute to optimal exercise performance. Taken together, these two factors may affect an athlete's potential for success for a given sport. Body weight can influence an athlete's speed, endurance, and power, whereas body composition can affect an athlete's strength, agility, and appearance. A lean body, i.e., one with greater muscle/fat ratio, is often advantageous in sports where speed is involved.
Athletic performance cannot be accurately predicted based solely on body weight and composition given that many factors affect body composition ( 21 ). Some sports dictate that athletes make changes in body weight and composition that may not be best for the individual athlete. Athletes who participate in weight-class sports-such as wrestling or lightweight rowing-may be required to lose or gain weight to qualify for a specific weight category. Athletes who participate in body-conscious sports, such as dance, gymnastics, figure skating, or diving, may be pressured to lose weight and body fat to have a lean physique, although their current weight for health and performance is appropriate. With extreme energy restrictions, losses of both muscle and fat mass may adversely influence an athlete's performance.
Individualized assessment of an athlete's body composition and body weight or body image may be advantageous for the improvement of athletic performance. Age, sex, genetics, and the requirements of the sport are factors that impact the individual athlete's body composition. An optimal competitive body weight and relative body fatness should be determined when an athlete is healthy and performing at his or her best.
Methodology and equipment to perform body composition assessments must be accessible and cost-effective. Not all of the following methods meet these criteria for the practitioner. In addition, athletes and coaches should know that there are errors associated with all body composition techniques and that it is not appropriate to set a specific body fat percentage goal for an individual athlete. Rather, a range of target percentages of body fat values should be recommended.
Assessment methodology.
Three levels of assessment techniques are used to assess body composition ( 22 ). Direct assessment based on analysis of cadavers, although not used in clinical practice, is designated as a Level 1 technique. The other two technique levels are indirect assessments (Level II) and doubly indirect assessments (Level III). Hydrodensitometry, or underwater weighing, dual-energy x-ray absorptiometry (DXA), and air displacement plethysmography are Level II techniques, and skinfold measurements and bioelectrical impedance analysis (BIA) are Level III techniques. Levels II and III techniques are used in practice by sports dietitians.
Underwater weighing, once considered the criterion standard, is no longer common. DXA, originally developed to assess bone mineral, can be used for body composition analysis ( 21 ). Although DXA is fairly accurate, quick, and noninvasive, the cost of and access to the instrument limits its use in practice. Air displacement plethysmography (BodPod; Life Measurement, Inc, Concord, CA) is also used to determine body composition by body density ( 22 ), and body fat percentage is calculated using the equation of either Siri ( 23 ) or Brozek ( 24 ). Although this method provides valid and reliable assessment of body composition, it may underestimate body fat in adults and children by 2%-3% ( 25 ).
Two of the most commonly used Level III methods are skinfold measurements and BIA. In addition, measures of body weight, height, wrist and girth circumferences, and skinfold measurements are routinely used by sports dietitians to assess body composition. Usually, seven skinfold sites are used including abdominal, biceps, front thigh, medial calf, subscapular, supraspinale, and triceps. The standard techniques and definitions of each of these sites are provided by Heymsfield et al. ( 22 ) and Marfell-Jones et al. ( 26 ). Prediction equations using skinfold measurements to determine body fat content are numerous ( 22 ). Approximately 50%-70% of the variance in body density is accounted for by this measurement. In addition, population differences limit the ability to interchange the prediction equations and standardization of skinfold sites and skinfold measurement techniques vary from investigator to investigator. Even the skinfold caliper is a source of variability ( 22 ). Despite the inherent problems of skinfold measurement, this technique remains a method of choice because it is convenient and inexpensive. The US Olympic Committee (USOC) is using the International Society for Advances in Kinanthropometry (ISAK) techniques ( 26 ) as efforts are underway to standardize measures worldwide. The USOC advocates using the sum of seven skinfolds (mm) based on ISAK landmarks, marking skinfold sites on the body, reporting duplicate measures, and communicating the results as a range, rather than percentage of body fat.
BIA is based on the principle that an electrical signal is more easily conducted through lean tissue than fat or bone ( 22 ). Fat mass is estimated by subtracting the BIA-determined estimate of FFM from total body mass. Whole body resistance to the flow of an electrical current conducted through the body by electrodes placed on wrists and ankles can provide fairly accurate estimates of total body water and FFM ( 22 ). Bioelectrical impedance analysis is dependent on several factors that can cause error in the measurement and must be taken into account to obtain a fairly accurate estimate. Hydration status is the most important factor that may alter the estimated percentage body fat. The prediction accuracy of BIA is similar to skinfold assessments, but BIA may be preferable because it does not require the technical skill associated with skinfold measurements ( 27 ). Currently, upper and lower body impedance devices have been developed but have not been evaluated in an athletic population.
Body composition and sports performance.
Body fat percentage of athletes varies depending on the sex of the athlete and the sport. The estimated minimal level of body fat compatible with health is 5% for males and 12% for females ( 22 ); however, optimal body fat percentages for an individual athlete may be much higher than these minimums and should be determined on an individual basis. The ISAK sum of seven skinfolds indicates that the range of values for the athletic population is 30-60 mm for males and 40-90 mm for females ( 26 ). Body composition analysis should not be used as a criterion for selection of athletes for athletic teams. Weight management interventions should be thoughtfully designed to avoid detrimental outcomes with specific regard for performance, as well as body composition (i.e., loss of lean body mass). See Figure 3 for practical guidelines for weight management of athletes.

Conclusion statement.
Four studies have reported inconclusive findings related to the effects of energy and protein restriction on athletic performance, but carbohydrate restriction has been shown to be detrimental. For weight-class athletes, two studies show that weight loss preceding athletic competition may have no significant effect on measures of performance, depending on refeeding protocol. (Evidence Grade III = Limited) . ( www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=250448 ).
MACRONUTRIENT REQUIREMENTS FOR EXERCISE
Athletes do not need a diet substantially different from that recommended in the Dietary Guidelines for Americans ( 16 ) and Eating Well with Canada's Food Guide ( 28 ). Although high-carbohydrate diets (more than 60% of energy intake) have been advocated in the past, caution is recommended in using specific proportions as a basis for meal plans for athletes. For example, when energy intake is 4000-5000 kcal·d −1 , even a diet containing 50% of the energy from carbohydrate will provide 500-600 g of carbohydrate (or approximately 7-8 g·kg −1 (3.2-3.6 g·lb −1 ) for a 70-kg (154 lb) athlete), an amount sufficient to maintain muscle glycogen stores from day to day ( 29 ). Similarly, if protein intake for this plan was 10% of energy intake, absolute protein intake (100-125 g·d −1 ) could exceed the recommended protein intake for athletes (1.2-1.7 g·kg −1 ·d −1 or 84-119 g in a 70-kg athlete). Conversely, when energy intake is less than 2000 kcal·d −1 , a diet providing 60% of the energy from carbohydrate may not be sufficient to maintain optimal carbohydrate stores (4-5 g·kg −1 or 1.8-2.3 g·lb −1 ) in a 60-kg (132 lb) athlete.
Protein metabolism during and after exercise is affected by sex, age, intensity, duration, and type of exercise, energy intake, and carbohydrate availability. More detailed reviews of these factors and their relationship to protein metabolism and needs of active individuals can be found elsewhere ( 30,31 ). The current recommended dietary allowance (RDA) is 0.8 g·kg −1 body weight and the acceptable macronutrient distribution range (AMDR) for protein intake for adults older than 18 yr is 10%-35% of total calories ( 15 ). Because there is not a strong body of evidence documenting that additional dietary protein is needed by healthy adults who undertake endurance or resistance exercise, the current DRI for protein and amino acids does not specifically recognize the unique needs of routinely active individuals and competitive athletes. However, recommending protein intakes in excess of the RDA to maintain optimum physical performance is commonly done in practice.
Endurance athletes.
An increase in protein oxidation during endurance exercise, coupled with nitrogen balance studies, provides the basis for recommending increased protein intakes for recovery from intense endurance training ( 32 ). Nitrogen balance studies suggest that dietary protein intake necessary to support nitrogen balance in endurance athletes ranges from 1.2 to 1.4 g·kg −1 ·d −1 ( 29-31 ). These recommendations remain unchanged, although recent studies have shown that protein turnover may become more efficient in response to endurance exercise training ( 29,32 ). Ultra-endurance athletes who engage in continuous activity for several hours or consecutive days of intermittent exercise should also consume protein at or slightly above 1.2-1.4 g·kg −1 ·d −1 ( 32 ). Energy balance, or the consumption of adequate calories, particularly carbohydrates, to meet those expended, is important to protein metabolism so that amino acids are spared for protein synthesis and not oxidized to assist in meeting energy needs ( 33,34 ). In addition, discussion continues as to whether sex differences in protein-related metabolic responses to exercise exist ( 35,36 ).
Strength athletes.
Resistance exercise may necessitate protein intake in excess of the RDA, as well as that needed for endurance exercise, because additional protein, essential amino acids in particular, is needed along with sufficient energy to support muscle growth ( 30,31 ). This is particularly true in the early phase of strength training when the most significant gains in muscle size occurs. The amount of protein needed to maintain muscle mass may be lower for individuals who routinely resistance train because of more efficient protein use ( 30,31 ). Recommended protein intakes for strength-trained athletes range from approximately 1.2 to 1.7 g·kg −1 ·d −1 ( 30,32 ).
Protein and amino acid supplements.
High-protein diets have been popular throughout history. Although earlier investigations in this area involved supplementation with individual amino acids ( 37,38 ), more recent work has shown that intact high-quality proteins such as whey, casein, or soy are effectively used for the maintenance, repair, and synthesis of skeletal muscle proteins in response to training ( 39 ). Protein or amino acids consumed near strength and endurance exercise can enhance maintenance of, and net gains in, skeletal muscle ( 39,40 ). Because protein or amino acid supplementation has not been shown to positively impact athletic performance ( 41,42 ), recommendations regarding protein supplementation are conservative and directed primarily at optimizing the training response to and the recovery period after exercise. From a practical perspective, it is important to conduct a thorough nutrition assessment specific to the athlete's goals before recommending protein powders and amino acid supplements to athletes.
Fat is a necessary component of a normal diet, providing energy and essential elements of cell membranes and associated nutrients such as vitamins A, D, and E. The acceptable macronutrient distribution range (AMDR) for fat is 20%-35% of energy intake ( 17 ). The Dietary Guidelines for Americans ( 16 ) and Eating Well with Canada's Food Guide ( 28 ) make recommendations that the proportion of energy from fatty acids be 10% saturated, 10% polyunsaturated, 10% monounsaturated, and include sources of essential fatty acids. Athletes should follow these general recommendations. Careful evaluation of studies suggesting a positive effect of consuming diets for which fat provides ≥70% of energy intake on athletic performance ( 43,44 ) does not support this concept ( 45 ).
VITAMINS AND MINERALS
Micronutrients play an important role in energy production, hemoglobin synthesis, maintenance of bone health, adequate immune function, and protection of body against oxidative damage. They assist with synthesis and repair of muscle tissue during recovery from exercise and injury. Exercise stresses many of the metabolic pathways where micronutrients are required, and exercise training may result in muscle biochemical adaptations that increase micronutrient needs. Routine exercise may also increase the turnover and loss of these micronutrients from the body. As a result, greater intakes of micronutrients may be required to cover increased needs for building, repair, and maintenance of lean body mass in athletes ( 46 ).
The most common vitamins and minerals found to be of concern in athletes' diets are calcium and vitamin D, the B vitamins, iron, zinc, magnesium, as well as some antioxidants such as vitamins C and E, β-carotene, and selenium ( 46-50 ). Athletes at greatest risk for poor micronutrient status are those who restrict energy intake or have severe weight-loss practices, who eliminate one or more of the food groups from their diet, or who consume unbalanced and low micronutrient-dense diets. These athletes may benefit from a daily multivitamin-and-mineral supplement. Use of vitamin and mineral supplements does not improve performance in individuals consuming nutritionally adequate diets ( 46-48,50 ).
B Vitamins:Thiamin, Riboflavin, Niacin, Vitamin B 6 , Pantothenic Acid, Biotin, Folate, Vitamin B 12
Adequate intake of B vitamins is important to ensure optimum energy production and the building and repair of muscle tissue ( 48,51 ). The B-complex vitamins have two major functions directly related to exercise. Thiamin, riboflavin, niacin, pyridoxine (B 6 ), pantothenic acid, and biotin are involved in energy production during exercise ( 46,51 ), whereas folate and vitamin B 12 are required for the production of red blood cells, for protein synthesis, and in tissue repair and maintenance including the CNS. Of the B vitamins, riboflavin, pyridoxine, folate, and vitamin B 12 are frequently low in female athletes' diets, especially those who are vegetarian or have disordered eating patterns ( 47,48 ).
Limited research has been conducted to examine whether exercise increases the need for the B-complex vitamins ( 46,48 ). Some data suggest that exercise may slightly increase the need for these vitamins as much as twice the current recommended amount ( 48 ); however, these increased needs can generally be met with higher energy intakes. Although short-term marginal deficiencies of B vitamins have not been observed to impact performance, severe deficiency of vitamin B 12 , folate, or both may result in anemia and reduced endurance performance ( 46,47,52 ). Therefore, it is important that athletes consume adequate amounts of these micronutrients to support their efforts for optimal performance and health.
Vitamin D is required for adequate calcium absorption, regulation of serum calcium and phosphorus levels, and promotion of bone health. Vitamin D also regulates the development and homeostasis of the nervous system and skeletal muscle ( 53-55 ). Athletes who live at northern latitudes or who train primarily indoors throughout the year, such as gymnasts and figure skaters, are at risk for poor vitamin D status, especially if they do not consume foods fortified with vitamin D ( 50,56,57 ). These athletes would benefit from supplementation with vitamin D at the DRI level (5 μg·d −1 or 200 IU for ages 19-49 yr) ( 54,56,58-61 ). A growing number of experts advocate that the RDA for vitamin D is not adequate ( 53,62,63 ).
Antioxidants: Vitamins C and E, β-Carotene, and Selenium
The antioxidant nutrients, vitamins C and E, β-carotene, and selenium, play important roles in protecting cell membranes from oxidative damage. Because exercise can increase oxygen consumption by 10- to 15-fold, it has been hypothesized that long-term exercise produces a constant "oxidative stress" on the muscles and other cells ( 49 ) leading to lipid peroxidation of membranes. Although short-term exercise may increase levels of lipid peroxide by-products ( 64 ), habitual exercise has been shown to result in an augmented antioxidant system and reduced lipid peroxidation ( 50,65 ). Thus, a well-trained athlete may have a more developed endogenous antioxidant system than a sedentary person. Whether exercise increases the need for antioxidant nutrients remains controversial. There is little evidence that antioxidant supplements enhance physical performance ( 49,50,64,66 ). Athletes at greatest risk for poor antioxidant intakes are those following a low-fat diet, restricting energy intakes, or limiting dietary intakes of fruits, vegetables, and whole grains ( 29,66 ).
The evidence that a combination of antioxidants or single antioxidants such as vitamin E may be helpful in reducing inflammation and muscle soreness during recovery from intense exercise remains unclear ( 42,67 ). Although the ergogenic potential of vitamin E concerning physical performance has not been clearly documented, endurance athletes may have a higher need for this vitamin. Indeed, vitamin E supplementation has been shown to reduce lipidperoxidation during aerobic/endurance exercise and have a limited effect with strength training ( 66 ). There is some evidence that vitamin E may attenuate exercise-induced DNA damage and enhance recovery in certain active individuals; however, more research is needed ( 66 ). Athletes should be advised not to exceed the tolerable upper intake levels (UL) for antioxidants because higher doses could be pro-oxidative with potential negative effects ( 46,64,68 ).
Vitamin C supplements do not seem to have an ergogenic effect if the diet provides adequate amounts of this nutrient. Because strenuous and prolonged exercise has been shown to increase the need for vitamin C, physical performance can be compromised with marginal vitamin C status or deficiency. Athletes who participate in habitual prolonged, strenuous exercise should consume 100-1000 mg of vitamin C daily ( 47,69,70 ).
Minerals: Calcium, Iron, Zinc, and Magnesium
The primary minerals low in the diets of athletes, especially female athletes, are calcium, iron, zinc, and magnesium ( 47 ). Low intakes of these minerals are often due to energy restriction or avoidance of animal products ( 70 ).
Calcium is especially important for growth, maintenance and repair of bone tissue, maintenance of blood calcium levels, regulation of muscle contraction, nerve conduction, and normal blood clotting. Inadequate dietary calcium and vitamin D increase the risk of low bone mineral density and stress fractures. Female athletes are at greatest risk for low bone mineral density if energy intakes are low, dairy products and other calcium-rich foods are inadequate or eliminated from the diet, and menstrual dysfunction is present ( 47,52,55,71-73 ).
Supplementation with calcium and vitamin D should be determined after nutrition assessment. Current recommendations for athletes with disordered eating, amenorrhea, and risk for early osteoporosis are 1500 mg of elemental calcium and 400-800 IU of vitamin D per day ( 50,72,73 ).
Iron is required for the formation of oxygen-carrying proteins, hemoglobin and myoglobin, and for enzymes involved in energy production ( 50,74 ). Oxygen-carrying capacity is essential for endurance exercise as well as normal function of the nervous, behavioral, and immune systems ( 64,74 ). Iron depletion (low iron stores) is one of the most prevalent nutrient deficiencies observed among athletes, especially females ( 75 ). Iron deficiency, with or without anemia, can impair muscle function and limit work capacity ( 47,58,75,76 ). Iron requirements for endurance athletes, especially distance runners, are increased by approximately 70% ( 58,74 ). Athletes who are vegetarian or regular blood donors should aim for an iron intake greater than their respective RDA (i.e., >18 mg and >8 mg, for men and women respectively).
The high incidence of iron depletion among athletes is usually attributed to inadequate energy intake. Other factors that can impact iron status include vegetarian diets that have poor iron availability, periods of rapid growth, training at high altitudes, increased iron losses in sweat, feces, urine, menstrual blood, intravascular hemolysis, foot-strike hemolysis, regular blood donation, or injury ( 50,75,77 ). Athletes, especially women, long-distance runners, adolescents, and vegetarians should be screened periodically to assess and monitor iron status ( 75,77,78 ).
Because reversing iron deficiency anemia can require 3-6 months, it is advantageous to begin nutrition intervention before iron deficiency anemia develops ( 47,75 ). Although depleted iron stores (low serum ferritin) are more prevalent in female athletes, the incidence of iron deficiency anemia in athletes is similar to that of the nonathlete female population ( 50,75,77 ). Chronic iron deficiency, with or without anemia, that results from consistently poor iron intake can negatively impact health, physical, and mental performance and warrants prompt medical intervention and monitoring ( 76,78 ).
Some athletes may experience a transient decrease in serum ferritin and hemoglobin at the initiation of training due to hemodilution after an increase in plasma volume known as "dilutional" or "sports anemia" and may not respond to nutrition intervention. These changes seem to be a beneficial adaptation to aerobic training, which do not negatively impact performance ( 50 ).
In athletes who are iron-deficient, iron supplementation not only improves blood biochemical measures and iron status but also increases work capacity as evidenced by increasing oxygen uptake, reducing heart rate, and decreasing lactate concentration during exercise ( 47 ). There is some evidence that athletes who are iron-deficient but do not have anemia may benefit from iron supplementation ( 50,75 ). Recent findings provide additional support for improved performance (i.e., less skeletal muscle fatigue) when iron supplementation was prescribed as 100-mg ferrous sulfate for 4-6 wk ( 76 ). Improving work capacity and endurance, increasing oxygen uptake, reducing lactate concentrations, and reducing muscle fatigue are benefits of improved iron status ( 50 ).
Zinc plays a role in growth, building and repair of muscle tissue, energy production, and immune status. Diets low in animal protein, high in fiber and vegetarian diets, in particular, are associated with decreased zinc intake ( 50,52 ). Zinc status has been shown to directly affect thyroid hormone levels, BMR, and protein use, which in turn can negatively affect health and physical performance ( 50 ).
Survey data indicate that a large number of North Americans have zinc intakes below recommended levels ( 74,75,79 ). Athletes, particularly females, are also at risk for zinc deficiency ( 79 ). The impact of low zinc intakes on zinc status is difficult to measure because clear assessment criteria have not been established and plasma zinc concentrations may not reflect changes in whole-body zinc status ( 47,79 ). Decreases in cardiorespiratory function, muscle strength, and endurance have been noted with poor zinc status ( 47 ). The UL for zinc is 40 mg ( 74 ). Athletes should be cautioned against single-dose zinc supplements because they often exceed this amount, and unnecessary zinc supplementation may lead to low HDL cholesterol and nutrient imbalances by interfering with absorption of other nutrients such as iron and copper ( 47 ). Further, the benefits of zinc supplementation to physical performance have not been established.
Magnesium plays a variety of roles in cellular metabolism (glycolysis, fat, and protein metabolism) and regulates membrane stability and neuromuscular, cardiovascular, immune, and hormonal functions ( 47,55 ). Magnesium deficiency impairs endurance performance by increasing oxygen requirements to complete submaximal exercise. Athletes in weight-class and body-conscious sports, such as wrestling, ballet, gymnastics, and tennis, have been reported to consume inadequate dietary magnesium. Athletes should be educated about good food sources of magnesium. In athletes with low magnesium status, supplementation might be beneficial ( 47 ).
Sodium, Chloride, and Potassium
Sodium is a critical electrolyte, particularly for athletes with high sweat losses ( 80-83 ). Many endurance athletes will require much more than the UL for sodium (2.3 g·d −1 ) and chloride (3.6 g·d −1 ). Sports drinks containing sodium (0.5-0.7 g·L −1 ) and potassium (0.8-2.0 g·L −1 ), as well as carbohydrate, are recommended for athletes especially in endurance events (>2 h) ( 50,80,82,83 ).
Potassium is important for fluid and electrolyte balance, nerve transmission, and active transport mechanisms. During intense exercise, plasma potassium concentrations tend to decline to a lesser degree than sodium. A diet rich in a variety of fresh vegetables, fruits, nuts / seeds, dairy foods, lean meats, and whole grains is usually considered adequate for maintaining normal potassium status among athletes ( 32,83 ).
Being well hydrated is an important consideration for optimal exercise performance. Because dehydration increases the risk of potentially life-threatening heat injury such as heat stroke, athletes should strive for euhydration before, during, and after exercise. Dehydration (loss of >2% body weight) can compromise aerobic exercise performance, particularly in hot weather, and may impair mental/cognitive performance ( 83 ).
The American College of Sports Medicine's (ACSM) Position Stand on exercise and fluid replacement ( 83 ) provides a comprehensive review of the research and recommendations for maintaining hydration before, during, and after exercise. In addition, ACSM has published position stands specific to special environmental conditions ( 84,85 ). The major points from these position stands are the basis for the following recommendations.
Fluid and Electrolyte Recommendations
- Before exercise
At least 4 h before exercise, individuals should drink approximately 5-7 mL·kg −1 body weight (∼2-3 mL·lb −1 ) of water or a sport beverage. This would allow enough time to optimize hydration status and for excretion of any excess fluid as urine. Hyperhydration with fluids that expand the extra- and intracellular spaces (e.g., water and glycerol solutions) will greatly increase the risk of having to void during competition ( 83 ) and provides no clear physiologic or performance advantage over euhydration. This practice should be discouraged ( 83 ).
- During exercise
Athletes dissipate heat produced during physical activity by radiation, conduction, convection, and vaporization of water. In hot, dry environments, evaporation accounts for more than 80% of metabolic heat loss. Sweat rates for any given activity will vary according to ambient temperature, humidity, body weight, genetics, heat acclimatization state, and metabolic efficiency. Depending on the sport and condition, sweat rates can range from as little as 0.3 to as much as 2.4 L·h −1 ( 83 ). In addition to water, sweat also contains substantial but variable amounts of sodium. The average concentration of sodium in sweat approximates 50 mmol·L −1 or approximately 1 g·L −1 (although concentrations vary widely). There are modest amounts of potassium and small amounts of minerals such as magnesium and chloride lost in sweat.
The intent of drinking during exercise is to avert a water deficit in excess of 2% of body weight. The amount and rate of fluid replacement is dependent on the individual athlete's sweat rate, exercise duration, and opportunities to drink ( 83 ). Readers are referred to the ACSM position stand for specific recommendations related to body size, sweat rates, types of work, etc., and are encouraged to individualize hydration protocols when possible ( 83 ).
Consumption of beverages containing electrolytes and carbohydrates can help sustain fluid and electrolyte balance and endurance exercise performance ( 83 ). The type, intensity, and duration of exercise and environmental conditions will alter the need for fluids and electrolytes. Fluids containing sodium and potassium help replace sweat electrolyte losses, whereas sodium stimulates thirst and fluid retention and carbohydrates provides energy. Beverages containing 6%-8% carbohydrate are recommended for exercise events lasting longer than 1 h ( 83 ).
Fluid balance during exercise is not always possible because maximal sweat rates exceed maximal gastric emptying rates that in turn limit fluid absorption, and most often, rates of fluid ingestion by athletes during exercise fall short of amounts that can be emptied from the stomach and absorbed by the gut. Gastric emptying is maximized when the amount of fluid in the stomach is high and reduced with hypertonic fluids or when carbohydrate concentration is greater than 8%.
Disturbances of fluid and electrolyte balance that can occur in athletes include dehydration, hypohydration, and hyponatremia ( 83 ). Exercise-induced dehydration develops because of fluid losses that exceed fluid intake. Although some individuals begin exercise euhydrated and dehydrate over an extended duration, athletes in some sports might start training or competing in a dehydrated state because the interval between exercise sessions is inadequate for full rehydration ( 82 ). Another factor that may predispose an athlete to dehydration is "making weight" as a prerequisite for a specific sport or event. Hypohydration, a practice of some athletes competing in weight-class sports (i.e., wrestling, boxing, lightweight crew, martial arts, etc.), can occur when athletes dehydrate themselves before beginning a competitive event. Hypohydration can develop by fluid restriction, certain exercise practices, diuretic use, or sauna exposure before an event. In addition, fluid deficits may span workouts for athletes who participate in multiple or prolonged daily sessions of exercise in the heat ( 84 ).
Hyponatremia (serum sodium concentration less than 130 mmol·L −1 ) can result from prolonged, heavy sweating with failure to replace sodium, or excessive water intake. Hyponatremia is more likely to develop in novice marathoners who are not lean, who run slowly, who sweat less, or who consume excess water before, during, or after an event ( 83 ).
Skeletal muscle cramps are associated with dehydration, electrolyte deficits, and muscle fatigue. Non-heat-acclimatized American football players commonly experience dehydration and muscle cramping particularly during formal preseason practice sessions in late summer. Athletes participating in tennis matches, long-cycling races, late-season triathlons, soccer, and beach volleyball are also susceptible to dehydration and muscle cramping. Muscle cramps also occur in winter-sport athletes such as cross-country skiers and ice hockey players. Muscle cramps are more common in profuse sweaters who experience large sweat sodium losses ( 83 ).
- After exercise
Because many athletes do not consume enough fluids during exercise to balance fluid losses, they complete their exercise session dehydrated to some extent. Given adequate time, intake of normal meals and beverages will restore hydration status by replacing fluids and electrolytes lost during exercise. Rapid and complete recovery from excessive dehydration can be accomplished by drinking at least 16-24 oz (450-675 mL) of fluid for every pound (0.5 kg) of body weight lost during exercise. Consuming rehydration beverages and salty foods at meals/snacks will help replace fluid and electrolyte losses ( 83 ).
Special Environmental Conditions
Hot and humid environments..
The risk for dehydration and heat injury increases dramatically in hot, humid environments ( 84 ). When the ambient temperature exceeds body temperature, heat cannot be dissipated by radiation. Moreover, the potential to dissipate heat by evaporation of sweat is substantially reduced when the relative humidity is high. There is a very high risk of heat illness when temperature and humidity are both high. If competitive events occur under these conditions, it is necessary to take every precaution to ensure that athletes are well hydrated, have ample access to fluids, and are monitored for heat-related illness.
Cold environments.
It is possible for dehydration to occur in cool or cold weather ( 85 ). Factors contributing to dehydration in cold environments include respiratory fluid losses and sweat losses that occur when insulated clothing is worn during intense exercise. Dehydration can also occur because of low rates of fluid ingestion. If an athlete is chilled and available fluids are cold, the incentive to drink may be reduced. Finally, removal of multiple layers of clothing to urinate may be inconvenient and difficult for some athletes, especially women, and they may voluntarily limit fluid intake ( 86 ).
Fluid losses beyond those associated with any exercise performed may occur at altitudes >2500 m (8200 ft) consequent to mandatory diuresis and high respiratory water losses, accompanied by decreased appetite. Respiratory water losses may be as high as 1900 mL·d −1 (1.9 L·d −1 ) in men and 850 mL·d −1 (0.85 L·d −1 ) in women ( 87,88 ). Total fluid intake at high altitude approaches 3-4 L·d −1 to promote optimal kidney function and maintain urine output of ∼1.4 L in adults ( 87 ).
THE TRAINING DIET
The fundamental differences between an athlete's diet and that of the general population are that athletes require additional fluid to cover sweat losses and additional energy to fuel physical activity. As discussed earlier, it is appropriate for much of the additional energy to be supplied as carbohydrate. The proportional increase in energy requirements seems to exceed the proportional increase in needs for most other nutrients. Accordingly, as energy requirements increase, athletes should first aim to consume the maximum number of servings appropriate for their needs from carbohydrate-based food groups (bread, cereals and grains, legumes, milk/alternatives, vegetables, and fruits). Energy needs for many athletes will exceed the amount of energy (kcal·d −1 ) in the upper range of servings for these food groups. Conversely, athletes who are small and/or have lower energy needs will need to pay greater attention to making nutrient-dense food choices to obtain adequate carbohydrate, protein, essential fats, and micronutrients.
With regard for the timing of meals and snacks, common sense dictates that food and fluid intake around workouts be determined on an individual basis with consideration for an athlete's gastrointestinal characteristics as well as the duration and intensity of the workout. For example, an athlete might tolerate a snack consisting of milk and a sandwich 1 h before a low-intensity workout but would be uncomfortable if the same meal was consumed before a very hard effort. Athletes in heavy training or doing multiple daily workouts may need to eat more than three meals and three snacks per day and should consider every possible eating occasion. These athletes should consider eating near the end of a workout, having more than one afternoon snack, or eating a substantial snack before bed.
Twenty-three studies investigating consumption of a range of macronutrient composition during the training period on athletic performance were evaluated. Nine studies have reported that the consumption of a high-carbohydrate diet (>60% of energy) during the training period and the week before competition results in improved muscle glycogen concentrations and/or significant improvements in athletic performance. Two studies reported no additional performance benefits when consuming level above 6 g carbohydrates·kg −1 body weight. Two studies report sex differences; women may have less ability to increase muscle glycogen concentrations through increased carbohydrate consumption, especially when energy intake is insufficient. One study based on the consumption of a high-fat diet (>65% of energy) for10 d followed by a high-carbohydrate diet (>65% of energy) for 3 d reported a significant improvement in athletic performance. Nine studies report no significant effects of macronutrient composition on athletic performance during the training period and week before competition. (Evidence Grade II = Fair) . ( www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=250447 ).
Pre-Exercise Meal
Eating before exercise, as opposed to exercising in the fasting state, has been shown to improve performance ( 89,90 ). The meal or snack consumed before competition or an intense workout should prepare athletes for the upcoming activity and leave the individual neither hungry nor with undigested food in the stomach. Accordingly, the following general guidelines for meals and snacks should be used: sufficient fluid should be ingested to maintain hydration, foods should be relatively low in fat and fiber to facilitate gastric emptying and minimize gastrointestinal distress, high in carbohydrate to maintain blood glucose and maximize glycogen stores, moderate in protein, and familiar to the athlete.
The size and timing of the pre-exercise meal are interrelated. Because most athletes do not like to compete on a full stomach, smaller meals should be consumed near the event to allow for gastric emptying, whereas larger meals can be consumed when more time is available before exercise or competition. Amounts of carbohydrate shown to enhance performance have ranged from approximately 200 to 300 g of carbohydrate for meals consumed 3-4 h before exercise. Studies report either no effect or beneficial effects of pre-event feeding on performance ( 91-98 ). Data are equivocal concerning whether the glycemic index of carbohydrate in the pre-exercise meal affects performance ( 92,99-102 ).
Although the above guidelines are sound and effective, the athlete's individual needs must be emphasized. Some athletes consume and enjoy a substantial meal (e.g., pancakes, juice, and scrambled eggs) 2-4 h before exercise or competition; however, others may experience severe gastrointestinal distress after such a meal and need to rely on liquid meals. Athletes should always ensure that they know what works best for themselves by experimenting with new foods and beverages during practice sessions and planning ahead to ensure they will have access to these foods at the appropriate time.
Nineteen studies investigating the consumption of a range of macronutrient composition during the 24 h before competition on athletic performance were evaluated. Of eight studies, six reported no significant effect of meal consumption 90 min to 4 h before trials on athletic performance. Six studies that focused on the consumption of food or beverage within the hour before competition reported no significant effects on athletic performance, despite hyperglycemia, hyperinsulinemia, increased carbohydrate oxidation, and reduced free fatty acid availability. Variations in research methodology on glycemic index of meals consumed before competition have led to inconclusive findings. (Evidence Grade II = Fair) . ( www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=250452 ).
During Exercise
Current research supports the benefit of carbohydrate consumption in amounts typically provided in sport drinks (6%-8%) to endurance performance in events lasting 1 h or less ( 103-105 ), especially in athletes who exercise in the morning after an overnight fast when liver glycogen levels are decreased. Providing exogenous carbohydrate during exercise helps maintain blood glucose levels and improve performance ( 106 ).
For longer events, consuming 0.7 g carbohydrates·kg −1 body weight·h −1 (approximately 30-60 g·h −1 ) has been shown unequivocally to extend endurance performance ( 107,108 ). Consuming carbohydrates during exercise is even more important in situations when athletes have not carbohydrate-loaded, not consumed pre-exercise meals, or restricted energy intake for weight loss. Carbohydrate intake should begin shortly after the onset of activity; consuming a given amount of carbohydrate as a bolus after 2 h of exercise is not as effective as consuming the same amount at 15- to 20-min intervals throughout the 2 h of activity ( 109 ). The carbohydrate consumed should yield primarily glucose; fructose alone is not as effective and may cause diarrhea, although mixtures of glucose and fructose, other simple sugars and maltodextrins, seem effective ( 107 ). If the same total amount of carbohydrate and fluid is ingested, the form of carbohydrate does not seem to matter. Some athletes may prefer to use a sport drink, whereas others may prefer to consume a carbohydrate snack or sports gel and consume water. As described elsewhere in this document, adequate fluid intake is also essential for maintaining endurance performance.
Thirty-six studies investigating the consumption of a range of macronutrient composition during competition on athletic performance were evaluated. Seven studies based on carbohydrate consumption during exercise lasting less than 60 min show conflicting results on athletic performance. However, of 17 studies based on carbohydrate consumption during exercise lasting greater than 60 min, 5 reported improved metabolic response, and 7 of 12 studies reported improvements in athletic performance. Evidence is inconclusive regarding the addition of protein to carbohydrate during exercise on athletic performance. Seven studies based on consumption of pre-exercise meals in addition to carbohydrate consumption during exercise suggest enhanced athletic performance. (Evidence Grade II = Fair) . ( www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=250453 ).
The timing and composition of the postcompetition or postexercise meal or snack depend on the length and intensity of the exercise session (i.e., whether glycogen depletion occurred) and on when the next intense workout will occur. For example, most athletes will finish a marathon with depleted glycogen stores, whereas glycogen depletion would be less marked after a 90-min training run. Because athletes competing in a marathon are not likely to perform another race or hard workout the same day, the timing and composition of the postexercise meal is less critical for these athletes. Conversely, a triathlete participating in a 90-min run in the morning and a 3-h cycling workout in the afternoon needs to maximize recovery between training sessions. The postworkout meal assumes considerable importance in meeting this goal.
Timing of postexercise carbohydrate intake affects glycogen synthesis over the short term ( 110 ). Consumption of carbohydrates within 30 min after exercise (1.0-1.5 g carbohydrate·kg −1 at 2-h intervals up to 6 h is often recommended) results in higher glycogen levels after exercise than when ingestion is delayed for 2 h ( 111 ). It is unnecessary for athletes who rest one or more days between intense training sessions to practice nutrient timing about glycogen replenishment provided sufficient carbohydrates are consumed during the 24-h period after the exercise bout ( 112 ). Nevertheless, consuming a meal or snack near the end of exercise may be important for athletes to meet daily carbohydrate and energy goals.
The type of carbohydrate consumed also affects postexercise glycogen synthesis. When comparing simple sugars, glucose and sucrose seem equally effective when consumed at a rate of 1.0-1.5 g·kg −1 body weight for 2 h; fructose alone is less effective ( 113 ). With regard to whole foods, consumption of carbohydrate with a high glycemic index results in higher muscle glycogen levels 24 h after a glycogen-depleting exercise as compared with the same amount of carbohydrates provided as foods with a low glycemic index ( 114 ). Application of these findings, however, must be considered in conjunction with the athlete's overall diet. When isocaloric amounts of carbohydrates or carbohydrates plus protein and fat are provided after endurance ( 115 ) or resistance exercise ( 116 ), glycogen synthesis rates are similar. Including protein in a postexercise meal, however, may provide needed amino acids for muscle protein repair and promote a more anabolic hormonal profile ( 33 ).
Twenty-five studies investigating the consumption of a range of macronutrient composition during the recovery period were evaluated. Nine studies report that consumption of diets higher in carbohydrate (>65% carbohydrate or 0.8-1.0 g carbohydrates·kg −1 body weight·h −1 ) during the recovery period increases plasma glucose and insulin concentrations and increases muscle glycogen resynthesis. Provided that carbohydrate intake is sufficient, four studies show no significant benefit of additional protein intake and two studies show no significant effect of meal timing on muscle glycogen resynthesis during the recovery period. Studies focusing on carbohydrate consumption during recovery periods of 4 h or more suggest improvements in athletic performance. (Evidence Grade II = Fair) . ( www.adaevidencelibrary.com/conclusion.cfm?conclusion_statement_id=250451 ).
DIETARY SUPPLEMENTS AND ERGOGENIC AIDS
The overwhelming number and increased availability of sports supplements presents an ongoing challenge for the practitioner and the athlete to keep up-to-date about the validity of the claims and scientific evidence. Although dietary supplements and nutritional ergogenic aids, such as nutritional products that enhance performance, are highly prevalent, the fact remains that very few improve performance ( 117-119 ) and some may cause concern.
In the United States, the Dietary Supplements and Health Education Act of 1994 allows supplement manufacturers to make health claims regarding the effect of products on body structure or function but not therapeutic claims to "diagnose, mitigate, treat, cure, or prevent" a specific disease or medical condition ( 117,120 ). As long as a special supplement label indicates the active ingredients and the entire ingredients list is provided, claims for enhanced performance can be made, valid or not. The Act, however, made the FDA responsible for evaluating and enforcing safety. In 2003, the US/FDA Task Force on Consumer Health Information for Better Nutrition proposed a new system for evaluating health claims that uses an evidence-based model and is intended to help consumers determine effectiveness of ergogenic aids and dietary supplements more reliably ( 117 ). Although all manufacturers are required by the FDA to analyze the identity, purity, and strength of all of their products' ingredients, they are not required to demonstrate the safety and efficacy of their products.
Canada regulates supplements as medicine or as natural health products (NHP). Products regulated in Canada as NHP must comply with Natural Health Products Regulations (2003) and manufacturers are allowed to make a full range of claims (structure/function, risk reduction, treatment, prevention) as supported by scientific evidence ( 117 ). In Canada, sports supplements such as sport drinks, protein powders, energy bars, and meal replacement products/beverages are regulated by Health Canada's Canadian Food Inspection Agency, whereas energy drinks, vitamin/mineral and herbal supplements, vitamin-enhanced water, and amino acid supplements fall under the NHP Regulations. Anabolic steroids are considered drugs and are tightly regulated under the Controlled Drugs & Substances Act.
Sports dietitians should consider the following factors in evaluating nutrition-related ergogenic aids: validity of the claims relative to the science of nutrition and exercise, quality of the supportive evidence provided (double-blinded, placebo-controlled scientific studies vs testimonials), and health and legal consequences of the claim ( 121,122 ). The safety of ergogenic aids remains in question. Possible contamination of dietary supplements and ergogenic aids with banned or nonpermissable substances remains an issue of concern. Therefore, sports dietitians and athletes must proceed with caution when considering the use of these types of products. Ultimately, athletes are responsible for the product they ingest and any subsequent consequences. Dietary supplements or ergogenic aids will never substitute for genetic makeup, years of training, and optimum nutrition.
Both national [National Collegiate Athletic Association (NCAA; www.ncaa.org ), United States Anti-Doping Agency ( www.usantidoping.org ) ] and international sports organizations [World Anti-Doping Agency (WADA; ( www.wada-ama.org )] limit the use of certain ergogenic aids and require random urine testing of athletes to ensure that certain products are not consumed. In Canada, the Canadian Centre for Ethics in Sport ( www.cces.ca ) is the organization which checks for banned substances.
The ethical use of performance-enhancing substances is a personal choice and remains controversial ( 117 ). Therefore, it is important that the qualified sports nutrition professional keep an open mind when working with elite athletes to effectively assess, recommend, educate, and monitor athletes who contemplate using or actively take dietary supplements and/or ergogenic aids ( 117 ). Credible and responsible information regarding the use of these products should be made available by qualified health professionals such as Board Certified Specialists in Sports Dietetics (CSSD) who carefully evaluate the risk-benefit ratio, including a complete dietary assessment.
It is beyond the scope of this article to address the multitude of ergogenic aids used by athletes in North America. From a practical perspective, however, most ergogenic aids can be classified into one of four categories: 1. those that perform as claimed; 2. those that may perform as claimed but for which there is insufficient evidence of efficacy at this time; 3. those that do not perform as claimed; and 4. those that are dangerous, banned, or illegal and, therefore, should not be used ( 122 ).
- 1. Ergogenic aids that perform as claimed
Creatine is currently the most widely used ergogenic aid among athletes wanting to build muscle and enhance recovery ( 118,123-125 ). Creatine has been shown to be effective in repeated short bursts of high-intensity activity in sports that derive energy primarily from the ATP-CP energy system such as sprinting and weight lifting but not for endurance sports such as distance running ( 32,117,126-128 ). Most of the researches on creatine have been conducted in a laboratory setting with male athletes.
The most common adverse effects of creatine supplementation are weight (fluid) gain, cramping, nausea, and diarrhea ( 32,117,129 ). Although widely debated, creatine is generally considered safe for healthy adults, despite anecdotal reports of dehydration, muscle strains/tears, and kidney damage ( 130-132 ). Although the effects of long-term use of creatine remain unknown, studies to date do not show any adverse effects in healthy adults from creatine supplementation ( 133 ). Nevertheless, health care professionals should carefully screen athletes using creatine for any risk of liver or kidney dysfunction or, in rare instances, anterior compartment syndrome.
The potential ergogenic effects of caffeine may be more closely related to its role as a CNS stimulant and the associated decreased perception of effort as opposed to its role in mobilizing of free fatty acids and sparing of muscle glycogen ( 117,134 ). In 2004, WADA moved caffeine from the restricted list to its Monitoring Programme. However, caffeine is still a restricted substance by the NCAA, where a positive doping test would be a caffeine level >15 μg·mL −1 of urine. New evidence shows that caffeine, when used in moderation, does not cause dehydration or electrolyte imbalance ( 135-138 ). However, when rapid hydration is necessary, athletes should rely on noncaffeinated and nonalcoholic beverages.
The use of high-energy drinks containing caffeine can be ergolytic and potentially dangerous when used in excess or in combination with stimulants or alcohol or other unregulated herbals and should be discouraged ( 32,117,139-141 ). Adverse effects of caffeine are anxiety, jitteriness, rapid heartbeat, gastrointestinal distress, and insomnia, and it could be ergolytic for novice users ( 134,142 ). There is little evidence to promote use of caffeine alone as a weight-loss aid ( 118 ).
Sports drinks, gels, and bars.
Sports drinks, gels, and bars are commonly used as convenient dietary supplements or ergogenic aids for busy athletes and active people. It is important that qualified nutrition professionals educate consumers about label reading, product composition, and appropriate use of these products (before, during, and after training and competition).
Sodium bicarbonate.
Sodium bicarbonate may be an effective ergogenic aid as a blood buffer (role in acid-base balance and prevention of fatigue), but its use is not without unpleasant adverse effects such as diarrhea ( 117,143 ).
Current evidence indicates that protein and amino acid supplements are no more or no less effective than food when energy is adequate for gaining lean body mass ( 30,31,117 ). Although widely used, protein powders and amino acid supplements are a potential source for illegal substances such as nandrolone, which may not be listed on the ingredient label ( 144,145 ).
- 2. Ergogenic aids that may perform as claimed but for which there is insufficient evidence
The ergogenic aids that have claims as health and performance enhancers include glutamine, β-hydroxymethylbutyrate, colostrum, and ribose ( 117 ). Preliminary studies concerning these ergogenic aids are inconclusive as performance enhancers. These substances are not banned from use by athletes ( www.wada-ama.org/en/prohibitedlist.ch2 ).
- 3. Ergogenic aids that do not perform as claimed
The majority of ergogenic aids currently on the market are in this category ( 122 ). These include amino acids, bee pollen, branched chain amino acids, carnitine, chromium picolinate, cordyceps, coenzyme Q10, conjugated linoleic acid, cytochrome C , dihydroxyacetone, γ-oryzanol, ginseng, inosine, medium-chain triglycerides, pyruvate, oxygenated water, and vanadium. This list is by no means exhaustive, and it is likely that other substances would be best placed in this category. Similarly, it is possible for any of these compounds to eventually move from this to another category after appropriate scientific inquiry and evaluation. To date, however, none of these products has been shown to enhance performance and many have had adverse effects ( 122 ).
- 4. Ergogenic aids that are dangerous, banned, or illegal
The ergogenic aids in this category should not be used and are banned by WADA. Examples are androstenedione, dehydroepiandrosterone, 19-norandrostenedione, 19-norandrostenediol, and other anabolic, androgenic steroids, Tribulus terrestris , ephedra, strychnine, and human growth hormone. Because this is an evolving field, sports dietitians need to consistently consider the status of various nutritional ergogenic aids.
The Vegetarian Athlete
The Position Statement of the American Dietetic Association and Dietitians of Canada on vegetarian diets (2003) provides appropriate dietary guidance for vegetarian athletes. This article provides additional considerations for vegetarians who participate in exercise. Well-planned vegetarian diets seem to effectively support parameters that influence athletic performance, although studies on this population are limited ( 31,146 ). Plant-based high-fiber diets may reduce energy availability. Monitoring body weight and body composition is the preferred means of determining whether energy needs are met. Some individuals, especially women, may switch to vegetarianism as a means of avoiding red meat and/or restricting energy intake to attain a lean body composition favored in some sports. Occasionally, this may be a red flag for disordered eating and increase the risk for the female athlete triad ( 72,73 ). Because of this association, coaches, trainers, and other health professionals should be alert when an athlete becomes a vegetarian and should ensure that appropriate weight is maintained.
Although most vegetarian athletes meet or exceed recommendations for total protein intake, their diets often provide less protein than those of nonvegetarians ( 31 ). Thus, some individuals may need more protein to meet training and competition needs ( 31 ). Protein quality of plant-based diets should be sufficient provided a variety of foods that supply adequate energy is consumed ( 31 ). Protein quality is a potential concern for individuals who avoid all animal proteins such as milk and meat (i.e., vegans). Their diets may be limited in lysine, threonine, tryptophan, or methionine ( 39 ).
Because plant proteins are less well digested than animal proteins, an increase in intake of approximately 10% protein is advised ( 15 ). Therefore, protein recommendations for vegetarian athletes approximate 1.3-1.8 g·kg −1 ·d −1 ( 52 ). Vegetarians with relatively low energy intakes should choose foods wisely to ensure protein intakes are consistent with these recommendations.
Vegetarian athletes may be at risk for low intakes of energy, fat, vitamins B 12 , riboflavin, and D, calcium, iron, and zinc, which are readily available from animal proteins. Iron is of particular concern because of the low bioavailability of nonheme plant sources. Iron stores of vegetarians are generally lower than omnivores ( 52 ). Vegetarian athletes, especially women, may be at greater risk for developing iron deficiency or anemia. Routine monitoring of iron status is recommended for vegetarian athletes, especially during periods of rapid growth (i.e., adolescence and pregnancy). Very low fat diets or avoidance of all animal protein may lead to a deficiency of essential fatty acids. Sport dietitians should educate novice vegetarian athletes on resources for menu planning, cooking, and shopping-especially high-quality plant protein combinations and acceptable animal sources (i.e., dairy and eggs) as well as foods rich in or fortified with key nutrients (calcium, vitamins D, B 12 , and riboflavin, iron, and zinc) ( 52 ).
Roles and Responsibilities of the Sports Dietitian
As nutrition information advances in quantity and complexity, athletes and active individuals are presented with a myriad of choices and decisions about appropriate and effective nutrition for activity and performance. Increasingly, athletes and active individuals seek professionals to guide them in making optimal food and fluid choices. Although many athletes and active individuals view winning or placing in an event to be the ultimate evidence of the effectiveness of their dietary regimens, sports dietitians should address the combined goals of health and fitness, enhanced capacity to train, and optimal athletic performance. Therefore, sports dietitians should be competent in the following areas:
- Conduct comprehensive nutrition assessment and consultation
- Educate in food selection, purchasing, and preparation
- Provide medical nutrition therapy in private practice, health care, and sports settings
- Identify and treat nutritional issues that impact health and performance
- Address energy balance and weight management issues
- Address nutritional challenges to performance (gastrointestinal disturbances, iron depletion, eating disorders, female athlete triad, food allergies, and supplement use)
- Track and document measurable outcomes of nutrition services
- Promote wound and injury healing
- Oversee menu planning and design, including pre- and postevent and travel
- Develop and oversee nutrition polices and procedures
- Evaluate the scientific literature and provide evidence-based assessment and application
Responsibilities
- Apply sports nutrition science to fueling fitness and performance
- Develop personalized nutrition and hydration strategies
- Advise on dietary supplements, ergogenic aids, meal and fluid replacement products, sports drinks, bars, and gels
- Evaluate dietary supplements and sports foods for legality, safety, and efficacy
- Provide nutrition strategies to delay fatigue during exercise and speed recovery from training
- Help enhance athletic training capacity and performance
- Participate in identifying and treating disordered eating patterns
- Provide nutrition strategies to reduce risk of illness/injury and facilitate recovery
- Promote career longevity for collegiate and professional athlete and all active individuals
- Recruit and retain clients and athletes in practice
- Provide sports nutrition as member of multidisciplinary/medical/health care teams
- Provide reimbursable services (diabetes medical nutrition therapy)
- Design and conduct sports team education
- Serve as a mentor for developing sports dietetics professionals
- Maintain credential(s) by actively engaging in profession-specific continuing education activities
The aforementioned responsibilities should be routine expectations of sporting and sports medicine organizations that employ qualified sports dietitians and of clients and athletes seeking valid sports nutrition information and advice.
In 2005, the Commission on Dietetic Registration (CDR; the credentialing agency of the American Dietetic Association) created a specialty credential for food and nutrition professionals who specialize in sports dietetic practice. The Board Certification Specialist in Sports Dietetics (CSSD) credential is designed as the premier professional sports nutrition credential in the United States. Specialists in Sports Dietetics provide safe, effective, evidence-based nutrition assessment, guidance, and counseling for health and performance for athletes, sport organizations, and physically active individuals and groups. The credential requires current Registered Dietitian (RD) status, maintenance of RD status for a minimum of 2 yr, and documentation of 1500 sports specialty practice hours as an RD within the past 5 yr. For more information, readers are referred to www.cdrnet.org/whatsnew/Sports.htm .
ADA/DC/ACSM position adopted by the ADA House of Delegates Leadership Team on July 12, 2000 and reaffirmed on May 25, 2004; approved by Dietitians of Canada on July 12, 2000 and approved by the American College of Sports Medicine Board of Trustees on October 17, 2000. The Coaching Association of Canada endorses this position paper. This position is in effect until December 31, 2012. ADA/DC/ACSM authorizes republication of the position, in its entirety, provided full and proper credit is given. Readers may copy and distribute this article, providing such distribution is not used to indicate an endorsement of product or service. Commercial distribution is not permitted without the permission of ADA. Requests to use portions of the position must be directed to ADA headquarters at 800/877-1600, ext 4835, or [email protected] .
- American College of Sports Medicine: Nancy R. Rodriguez, PhD, RD, CSSD, FACSM (University of Connecticut, Storrs, CT)
- American Dietetic Association: Nancy M. DiMarco, PhD, RD, CSSD, FACSM (Texas Woman's University, Denton, TX)
- Dietitians of Canada: Susie Langley, MS, RD, CSSD (69 McGill Street, Toronto, ON, Canada)
- American Dietetic Association:
Sharon Denny, MS, RD (ADA Knowledge Center, Chicago, IL);
Mary H. Hager, PhD, RD, FADA (ADA Government Relations, Washington, DC)
Melinda M. Manore, PhD, RD, CSSD (Oregon State University, Corvallis, OR)
Esther Myers, PhD, RD, FADA (ADA Scientific Affairs, Chicago, IL);
Nanna Meyer, PhD, RD, CSSD (University of Colorado, Colorado Springs, CO)
James Stevens, MS, RD (Metropolitan State College of Denver, Denver, CO)
Jennifer A. Weber, MPH, RD (ADA Government Relations, Washington, DC)
- Dietitians of Canada:
Rennie Benedict, MSc, RD (Department of Kinesiology & Applied Health, University of Winnipeg, Winnipeg, MB)
Marilyn Booth, MSc, RD (Registered Dietitian and Exercise Consultant, Ottawa, ON)
Patricia Chuey, MSc, RD (Manager Nutrition Affairs, Overwaitea Food Group, Vancouver, BC)
Kelly Anne Erdman, MSc, RD (University of Calgary Sport Medicine Centre, Calgary AB)
Marielle Ledoux, PhD, PDt (Department of Nutrition, Faculty of Medicine, Université de Montréal, QC)
Heather Petrie, MSc, PDt (Nutrition Cosultant, Halifax, NS)
Pamela Lynch, MHE, PDt (Nutrition Counseling Services & Associates; Mount Saint Vincent University, Department of Applied Human Nutrition, Halifax, NS)
Elizabeth (Beth) Mansfield, MSc, RD, PhD Candidate (McGill University, Montreal, QC)
- American College of Sports Medicine:
Susan Barr, PhD, RDN (University of British Columbia, Vancouver, BC)
Dan Benardot, PhD, DHC, RD (Georgia State University, Atlanta, GA)
Jacqueline Berning, PhD, RD (University of Colorado Springs, Colorado Springs, CO)
Andrew Coggan, PhD (Washington University School of Medicine, St. Louis, MO)
Melinda Manore, PhD, RD (Oregon State University, Corvallis, OR)
Brian Roy, PhD (Brock University, St. Catharines, ON)
Assistance from Lisa M. Vislocky, PhD, University of Connecticut, Storrs, CT, in preparing the references is acknowledged.
APC WORKGROUP
Christine M. Palumbo, MBA, RD (chair); Pat M. Schaaf, MS, RD; Doug Kalman, PhD, RD, FACN (content advisor); Roberta Anding, MS, RD, LD, CDE, CSSD (content advisor).
The authors thank the reviewers for their many constructive comments and suggestions. The reviewers were not asked to endorse this position or the supporting paper.
ADA NUTRITION AND ATHLETIC PERFORMANCE POSITION STAND REFERENCES
Eal conclusion statement-training diet(23 references).
Achten J, Halson SL, Moseley L, Rayson MP, Casey A, Jeukendrup AE. Higher dietary carbohydrate content during intensified running training results in better maintenance of performance and mood state. J Appl Physiol . 2004;96:1331-40.
Burke LM, Hawley JA, Schabort EJ, St Clair Gibson A, Mujika I, Noakes TD. Carbohydrate loading failed to improve 100-km cycling performance in a placebo-controlled trial. J Appl Physiol . 2000;88:1284-90.
Burke LM, Hawley JA, Angus DJ, et al. Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med Sci Sports Exerc . 2002;34:83-91.
Bussau VA, Fairchild TJ, Rao A, Steele P, Fournier PA. Carbohydrate loading in human muscle: an improved 1 day protocol. Eur J Appl Physiol . 2002;87:290-5.
Carey AL, Staudacher HM, Cummings NK, et al. Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. J Appl Physiol . 2001;91:115-22.
Casey A, Short AH, Curtis S, Greenhaff PL. The effect of glycogen availability on power output and the metabolic response to repeated bouts of maximal, isokinetic exercise in man. Eur J Appl Physiol Occup Physiol . 1996;72:249-55.
Erlenbusch M, Haub M, Munoz K, MacConnie S, Stillwell B. Effect of high-fat or high-carbohydrate diets on endurance exercise: a meta-analysis. Int J Sport Nutr Exerc Metab . 2005;15:1-14.
Fairchild TJ, Fletcher S, Steele P, Goodman C, Dawson B, Fournier PA. Rapid carbohydrate loading after a short bout of near maximal-intensity exercise. Med Sci Sports Exerc . 2002;34:980-6.
Fleming J, Sharman MJ, Avery NG, et al. Endurance capacity and high-intensity exercise performance responses to a high fat diet. Int J Sport Nutr Exerc Metab . 2003;13:466-78.
Goedecke JH, Christie C, Wilson G, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999;48:1509-17.
Hawley JA, Palmer GS, Noakes TD. Effects of 3 days of carbohydrate supplementation on muscle glycogen content and utilisation during a 1-h cycling performance. Eur J Appl Physiol Occup Physiol . 1997;75:407-12.
Horvath PJ, Eagen CK, Fisher NM, Leddy JJ, Pendergast DR. The effects of varying dietary fat on performance and metabolism in trained male and female runners. J Am Coll Nutr . 2000;19:52-60.
Lambert EV, Goedecke JH, Zyle C, et al. High-fat diet versus habitual diet prior to carbohydrate loading: effects of exercise metabolism and cycling performance. Int J Sport Nutr Exerc Metab . 2001;11:209-25.
Pitsiladis YP, Duignan C, Maughan RJ. Effects of alterations in dietary carbohydrate intake on running performance during a 10 km treadmill time trial. Br J Sports Med . 1996;30:226-31.
Pizza FX, Flynn MG, Duscha BD, Holden J, Kubitz ER. A carbohydrate loading regimen improves high intensity, short duration exercise performance. Int J Sport Nutr . 1995;5:110-6.
Reznik Dolins K, Boozer CN, Stoler F, Bartels M, DeMeersmane R, Contento I. Effect of variable carbohydrate intake on exercise performance in female endurance cyclists. Int J Sport Nutr Exerc Metab . 2003;13:422-35.
Rockwell MS, Rankin JW, Dixon H. Effects of muscle glycogen on performance of repeated sprints and mechanisms of fatigue. Int J Sport Nutr Exerc Metab . 2003;13:1-14.
Roltsch MH, Flohr JA, Brevard PB. The effect of diet manipulations on aerobic performance. Int J Sport Nutr Exerc Metab . 2002;12:480-9.
Rowlands DS, Hopkins WG. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism . 2002;51:678-90.
Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM, Hawley JA. Effect of short-term fat adaptation on high-intensity training. Med Sci Sports Exerc . 2002;34:449-55.
Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol . 1995;78:1360-8.
Tarnopolsky MA, Zawada C, Richmond LB, et al. Gender differences in carbohydrate loading are related to energy intake. J Appl Physiol . 2001;91:225-30.
Van Zant RS, Conway JM, Seale JL. A moderate carbohydrate and fat diet does not impair strength performance in moderately trained males. J Sports Med Phys Fitness . 2002;42:31-7.
EAL Conclusion Statement-During Exercise (36 References)
Anantaraman R, Carmines AA, Gaesser GA, Weltman A. Effects of carbohydrate supplementation on performance during 1 hour of high-intensity exercise. Int J Sports Med . 1995;16:461-5.
Anastasiou CA, Kavouras SA, Koutsari C, et al. Effect of maltose-containing sports drinks on exercise performance. Int J Sport Nutr Exerc Metab . 2004;14:609-25.
Andrews JL, Sedlock DA, Flynn MG, Navalta JW, Ji H. Carbohydrate loading and supplementation in endurance-trained women runners. J Appl Physiol . 2003;95:584-90.
Ball TC, Headley SA, Vanderburgh PM, Smith JC. Periodic carbohydrate replacement during 50 min of high-intensity cycling improves subsequent sprint performance. Int J Sport Nutr . 1995;5:151-8.
Below PR, Mora-Rodriguez R, Gonzalez-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc . 1995;27:200-10.
Brundle S, Thayer R, Taylor AW. Comparison of fructose and glucose ingestion before and during endurance cycling to exhaustion. J Sports Med Phys Fitness . 2000;40:343-9.
Burke LM, Claassen A, Hawley JA, Noakes TD. Carbohydrate intake during prolonged cycling minimizes effect of glycemic index of preexercise meal. J Appl Physiol . 1998;85:2220-6.
Carter JM, Jeukendrup AE, Mann CH, Jones DA. The effect of glucose infusion on glucose kinetics during a 1-h time trial. Med Sci Sports Exerc . 2004;36:1543-50.
Chryssanthopoulos C, Williams C. Pre-exercise carbohydrate meal and endurance running capacity when carbohydrates are ingested during exercise. Int J Sports Med . 1997;18:543-8.
Chryssanthopoulos C, Williams C, Nowitz A, Kotsiopoulou C, Vleck V. The effect of a high carbohydrate meal on endurance running capacity. Int J Sport Nutr Exerc Metab . 2002;12:157-71.
Chryssanthopoulos C, Williams C, Nowitz A. Influence of a carbohydrate-electrolyte solution ingested during running on muscle glycogen utilisation in fed humans. Int J Sports Med . 2002;23:279-84.
Claassen A, Lambert EV, Bosch AN, Rodger M, St Clair Gibson A, Noakes TD. Variability in exercise capacity and metabolic response during endurance exercise after a low carbohydrate diet. Int J Sport Nutr Exerc Metab . 2005;15:97-116.
Clark VR, Hopkins WG, Hawley JA, Burke LM. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med Sci Sports Exerc . 2000;32:1642-7.
Davis JM, Welsh RS, De Volve KL, Alderson NA. Effects of branched-chain amino acids and carbohydrate on fatigue during intermittent, high-intensity running. Int J Sports Med . 1999;20:309-14.
De Bock K, Richter EA, Russell AP, et al. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol . 2005;564:649-60.
Desbrow B, Anderson S, Barrett J, Rao E, Hargreaves M. Carbohydrate-electrolyte feedings and 1 h time trial cycling performance. Int J Sport Nutr Exerc Metab . 2004;14:541-9.
Earnest CP, Lancaster SL, Rasmussen CJ, et al. Low vs. high glycemic index carbohydrate gel ingestion during simulated 64-km cycling time trial performance. J Strength Cond Res . 2004;18:466-72.
el Sayed MS, Rattu AJ, Lin X, Reilly T. Effects of active warm-down and carbohydrate feeding on free fatty acid concentrations after prolonged submaximal exercise. Int J Sport Nutr . 1996;6:337-47.
Febbraio MA, Chiu A, Angus DJ, Arkinstall MJ, Hawley JA. Effects of carbohydrate ingestion before and during exercise on glucose kinetics and performance. J Appl Physiol . 2000;89:2220-6.
Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Substrate metabolism when subjects are fed carbohydrate during exercise. Am J Physiol . 1999;276:E828-35.
Ivy JL, Res PT, Sprague RC, Widzer MO. Effect of a carbohydrate-protein supplement on endurance performance during exercise of varying intensity. Int J Sport Nutr Exerc Metab . 2003;13:382-95.
Jeukendrup A, Brouns F, Wagenmakers AJ, Saris WH. Carbohydrate-electrolyte feedings improve 1 h time trial cycling performance. Int J Sports Med . 1997;18:125-9.
Jeukendrup AE, Wagenmakers AJ, Stegen JH, Gijsen AP, Brouns F, Saris WH. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Am J Physiol . 1999;276:E672-83.
Kang J, Robertson RJ, Denys BG, et al. Effect of carbohydrate ingestion subsequent to carbohydrate supercompensation on endurance performance. Int J Sport Nutr . 1995;5:329-43.
Kimber NE, Ross JJ, Mason SL, Speedy DB. Energy balance during an Ironman Triathlon in male and female triathletes. Int J Sport Nutr Exerc Metab . 2002;12:47-62.
McConell G, Kloot K, Hargreaves M. Effect of timing of carbohydrate ingestion on endurance exercise performance. Med Sci Sports Exerc . 1996;28:1300-4.
Meyer T, Gabriel HH, Auracher M, Scharhag J, Kindermann W. Metabolic profile of 4 h cycling in the field with varying amounts of carbohydrate supply. Eur J Appl Physiol . 2003;88:431-7.
Millard-Stafford ML, Sparling PB, Rosskopf LB, Snow TK. Should carbohydrate concentration of a sports drink be less than 8% during exercise in the heat? Int J Sport Nutr Exerc Metab . 2005;15:117-30.
Nassis GP, Williams C, Chisnall P. Effect of a carbohydrate-electrolyte drink on endurance capacity during prolonged intermittent high intensity running. Br J Sports Med . 1998;32:248-52.
Nicholas CW, Tsintzas K, Boobis L, Williams C. Carbohydrate-electrolyte ingestion during intermittent high-intensity running. Med Sci Sports Exerc . 1999;31:1280-6.
Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci . 1995;13:283-90.
Riddell MC, Partington SL, Stupka N, Armstrong D, Rennie C, Tarnopolsky MA. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance-trained athletes. Int J Sport Nutr Exer Metab . 2003:13:407-21.
Rowlands DS, Hopkins WG. Effect of high-fat, high-carbohydrate, and high-protein meals on metabolism and performance during endurance cycling. Int J Sport Nutr Exerc Metab . 2002;12:318-35.
Saunders MJ, Kane MD, Todd MK. Effects of a carbohydrate-protein beverage on cycling endurance and muscle damage. Med Sci Sports Exerc . 2004;36:1233-8.
Sugiura K, Kobayashi K. Effect of carbohydrate ingestion on sprint performance following continuous and intermittent exercise. Med Sci Sports Exerc . 1998;30:1624-30.
Tsintzas OK, Williams C, Wilson W, Burrin J. Influence of carbohydrate supplementation early in exercise on endurance running capacity. Med Sci Sports Exerc . 1996;28:1373-9.
EAL Conclusion Statement-Pre-exercise Meal (19 References)
Achten J, Jeukendrup AE. Effects of pre-exercise ingestion of carbohydrate on glycaemic and insulinaemic responses during subsequent exercise at differing intensities. Eur J Appl Physiol . 2003;88:466-71.
Cramp T, Broad E, Martin D, Meyer BJ. Effects of preexercise carbohydrate ingestion on mountain bike performance. Med Sci Sports Exerc . 2004;36:1602-9.
DeMarco HM, Sucher KP, Cisar CJ, Butterfield GE. Pre-exercise carbohydrate meals: application of glycemic index. Med Sci Sports Exerc . 1999;31:164-70.
Diboll DC, Boone WT, Lindsey LR. Cardiovascular and metabolic responses during 30 minutes of treadmill exercise shortly after consuming a small, high-carbohydrate meal. Int J Sports Med . 1999;20:384-9.
Febbraio MA, Stewart KL. CHO feeding before prolonged exercise: effect of glycemic index on muscle glycogenolysis and exercise performance. J Appl Physiol . 1996;81:1115-20.
Febbraio MA, Keenan J, Angus DJ, Campbell SE, Garnham AP. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J Appl Physiol . 2000;89:1845-51.
Jentjens RL, Cale C, Gutch C, Jeukendrup AE. Effects of pre-exercise ingestion of differing amounts of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol . 2003;88:444-52.
Kirwan JP, Cyr-Campbell D, Campbell WW, Scheiber J, Evans WJ. Effects of moderate and high glycemic index meals on metabolism and exercise performance. Metabolism . 2001;50:849-55.
Kirwan JP, O'Gorman DJ, Cyr-Campbell D, Campbell WW, Yarasheski KE, Evans WJ. Effects of a moderate glycemic meal on exercise duration and substrate utilization. Med Sci Sports Exerc . 2001;33:1517-23.
Moseley L, Lancaster GI, Jeukendrup AE. Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol . 2003;88:453-8.
Okano G, Sato Y, Takumi Y, Sugawara M. Effect of 4h preexercise high carbohydrate and high fat meal ingestion on endurance performance and metabolism. Int J Sports Med . 1996;17:530-4.
Okano G, Sato Y, Murata Y. Effect of elevated blood FFA levels on endurance performance after a single fat meal ingestion. Med Sci Sports Exerc . 1998;30:763-8.
Palmer GS, Clancy MC, Hawley JA, Rodger IM, Burke LM, Noakes TD. Carbohydrate ingestion immediately before exercise does not improve 20 km time trial performance in well trained cyclists. Int J Sports Med . 1998;19:415-8.
Paul D, Jacobs KA, Geor RJ, Hinchcliff KW. No effect of pre-exercise meal on substrate metabolism and time trial performance during intense endurance exercise. Int J Sport Nutr Exerc Metab . 2003;13:489-503.
Schabort EJ, Bosch AN, Weltan SM, Noakes TD. The effect of a preexercise meal on time to fatigue during prolonged cycling exercise. Med Sci Sports Exerc . 1999;31:464-71.
Sparks MJ, Selig SS, Febbraio MA. Pre-exercise carbohydrate ingestion: effect of the glycemic index on endurance exercise performance. Med Sci Sports Exerc . 1998;30:844-9.
Wee SL, Williams C, Gray S, Horabin J. Influence of high and low glycemic index meals on endurance running capacity. Med Sci Sports Exerc . 1999;31:393-9.
Wee SL, Williams C, Tsintzas K, Boobis L. Ingestion of a high-glycemic index meal increases muscle glycogen storage at rest but augments its utilization during subsequent exercise. J Appl Physiol . 2005;99:707-14.
Whitley HA, Humphreys SM, Campbell IT, et al. Metabolic and performance responses during endurance exercise after high-fat and high-carbohydrate meals. J Appl Physiol . 1998;85:418-24.
EAL Conclusion Statement-Recovery (25 References)
Abt G, Zhou S, Weatherby R. The effect of a high-carbohydrate diet on the skill performance of midfield soccer players after intermittent treadmill exercise. J Sci Med Sport . 1998;1:203-12.
Bloomer RJ, Sforzo GA, Keller BA. Effects of meal form and composition on plasma testosterone, cortisol, and insulin following resistance exercise. Int J Sport Nutr Exerc Metab . 2000;10:415-24.
Bosher KJ, Potteiger JA, Gennings C, Luebbers PE, Shannon KA, Shannon RM. Effects of different macronutrient consumption following a resistance-training session on fat and carbohydrate metabolism. J Strength Cond Res . 2004;18:212-9.
Burke LM, Collier GR, Beasley SK, et al. Effect of coingestion of fat and protein with carbohydrate feedings on muscle glycogen storage. J Appl Physiol . 1995;78:2187-92.
Burke LM, Collier GR, Davis PG, Fricker PA, Sanigorski AJ, Hargreaves M. Muscle glycogen storage after prolonged exercise: effect of the frequency of carbohydrate feedings. Am J Clin Nutr . 1996;64:115-9.
Burke LM, Collier GR, Broad EM, et al. Effect of alcohol intake on muscle glycogen storage after prolonged exercise. J Appl Physiol . 2003;95:983-90.
Carrithers JA, Williamson DL, Gallagher PM, Godard MP, Schulze KE, Trappe SW. Effects of postexercise carbohydrate-protein feedings on muscle glycogen restoration. J Appl Physiol . 2000;88:1976-82.
Haub MD, Haff GG, Potteiger JA. The effect of liquid carbohydrate ingestion on repeated maximal effort exercise in competitive cyclists. J Strength Cond Res . 2003;17:20-5.
Haub MD, Potteiger JA, Jacobsen DJ, Nau KL, Magee LA, Comeau MJ. Glycogen replenishment and repeated maximal effort exercise: effect of liquid carbohydrate. Int J Sport Nutr . 1999;9:406-15.
Ivy JL, Goforth HW Jr, Damon BM, McCauley TR, Parsons EC, Price TB. Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J Appl Physiol . 2002;93:1337-44.
Jentjens RL, van Loon LJ, Mann CH, Wagenmakers AJ, Jeukendrup AE. Addition of protein and amino acids to carbohydrates does not enhance postexercise muscle glycogen synthesis. J Appl Physiol . 2001;91:839-46.
Kimber NE, Heigenhauser GJ, Spriet LL, Dyck DJ. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J Physiol . 2003;548:919-27.
Nicholas CW, Green PA, Hawkins RD, Williams C. Carbohydrate intake and recovery of intermittent running capacity. Int J Sport Nutr . 1997;7:251-60.
Parkin JA, Carey MF, Martin IK, Stojanovska L, Febbraio MA. Muscle glycogen storage following prolonged exercise: effect of timing of ingestion of high glycemic index food. Med Sci Sports Exerc . 1997;29:220-4.
Roy BD, Tarnopolsky MA. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J Appl Physiol . 1998;84 890-6.
Roy BD, Tarnopolsky MA, MacDougall JD, Fowles J, Yarasheski KE. Effect of glucose supplement timing on protein metabolism after resistance training. J Appl Physiol . 1997;82:1882-8.
Siu PM, Wong SH, Morris JG, Lam CW, Chung PK, Chung S. Effect of frequency of carbohydrate feedings on recovery and subsequent endurance run. Med Sci Sports Exerc . 2004;36:315-23.
Stevenson E, Williams C, Biscoe H. The metabolic responses to high carbohydrate meals with different glycemic indices consumed during recovery from prolonged strenuous exercise. Int J Sport Nutr Exerc Metab . 2005;15:291-307.
Tarnopolsky MA, Bosman M, Macdonald JR, Vandeputte D, Martin J, Roy BD. Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. J Appl Physiol . 1997;83:1877-83.
van Hall G, Shirreffs SM, Calbet JA. Muscle glycogen resynthesis during recovery from cycle exercise: no effect of additional protein ingestion. J Appl Physiol . 2000;88:1631-6.
van Loon LJ, Saris WH, Kruijshoop M, Wagenmakers AJ. Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr . 2000;72:106-11.
van Loon LJ, Schrauwen-Hinderling VB, Koopman R, et al. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am J Physiol Endocrinol Metab . 2003;285:E804-11.
Williams MB, Raven PB, Fogt DL, Ivy JL. Effects of recovery beverages on glycogen restoration and endurance exercise performance. J Strength Cond Res . 2003;17:12-9.
Wong SH, Williams C. Influence of different amounts of carbohydrate on endurance running capacity following short term recovery. Int J Sports Med . 2000;21:444-52.
Wong SH, Williams C, Adams N. Effects of ingesting a large volume of carbohydrate-electrolyte solution on rehydration during recovery and subsequent exercise capacity. Int J Sport Nutr Exerc Metab . 2000;10:375-93.
EAL Conclusion Statement-Energy Balance and Sports Performance (8 References)
Can F, Yilmaz I, Erden Z. Morphological characteristics and performance variables of women soccer players. J Strength Cond Res . 2004;18:480-5.
Filaire E, Maso F, Degoutte F, Jouanel P, Lac G. Food restriction, performance, psychological state and lipid values in judo athletes. Int J Sports Med . 2001;22:454-9.
Finn KJ, Dolgener FA, Williams RB. Effects of carbohydrate refeeding on physiological responses and psychological and physical performance following acute weight reduction in collegiate wrestlers. J Strength Cond Res . 2004;18:328-33.
Jarvis M, McNaughton L, Seddon A, Thompson D. The acute 1-week effects of the Zone diet on body composition, blood lipid levels, and performance in recreational endurance athletes. J Strength Cond Res . 2002;16:50-7.
Mourier A, Bigard AX, de Kerviler E, Roger B, Legrand H, Guezennec CY. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med . 1997;18:47-55.
Noel MB, VanHeest JL, Zaneteas P, Rodgers CD. Body composition in Division I football players. J Strength Cond Res . 2003;17:228-37.
Tarnopolsky MA, Cipriano N, Woodcroft C, et al. Effects of rapid weight loss and wrestling on muscle glycogen concentration. Clin J Sport Med . 1996;6:78-84.
Zachwieja JJ, Ezell DM, Cline AD, et al. Short-term dietary energy restriction reduces lean body mass but not performance in physically active men and women. Int J Sports Med . 2001;22:310-6.
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Exercise and fluid replacement, progression models in resistance training for healthy adults, appropriate physical activity intervention strategies for weight loss and..., the female athlete triad.
MINI REVIEW article
New opportunities to advance the field of sports nutrition.

- 1 Department of Physical Performance, Norwegian School of Sport Sciences, Oslo, Norway
- 2 Gatorade Sports Science Institute, PepsiCo Life Sciences, Barrington, IL, United States
- 3 Gatorade Sports Science Institute, PepsiCo Life Sciences, Global R&D, Leicestershire, United Kingdom
- 4 Canadian Sport Institute-Pacific, Victoria, BC, Canada
- 5 Exercise Science, Physical and Health Education, University of Victoria, Victoria, BC, Canada
- 6 School of Sport and Health Sciences, University of Brighton, Eastbourne, United Kingdom
Sports nutrition is a relatively new discipline; with ~100 published papers/year in the 1990s to ~3,500+ papers/year today. Historically, sports nutrition research was primarily initiated by university-based exercise physiologists who developed new methodologies that could be impacted by nutrition interventions (e.g., carbohydrate/fat oxidation by whole body calorimetry and muscle glycogen by muscle biopsies). Application of these methods in seminal studies helped develop current sports nutrition guidelines as compiled in several expert consensus statements. Despite this wealth of knowledge, a limitation of the current evidence is the lack of appropriate intervention studies (e.g., randomized controlled clinical trials) in elite athlete populations that are ecologically valid (e.g., in real-life training and competition settings). Over the last decade, there has been an explosion of sports science technologies, methodologies, and innovations. Some of these recent advances are field-based, thus, providing the opportunity to accelerate the application of ecologically valid personalized sports nutrition interventions. Conversely, the acceleration of novel technologies and commercial solutions, especially in the field of biotechnology and software/app development, has far outstripped the scientific communities' ability to validate the effectiveness and utility of the vast majority of these new commercial technologies. This mini-review will highlight historical and present innovations with particular focus on technological innovations in sports nutrition that are expected to advance the field into the future. Indeed, the development and sharing of more “big data,” integrating field-based measurements, resulting in more ecologically valid evidence for efficacy and personalized prescriptions, are all future key opportunities to further advance the field of sports nutrition.
Introduction
Innovation has always been at the forefront of sport. Recent examples include drafting in cycling, clap skates in speed skating and more recently, carbon plate shoes in running. Sports nutrition is a relatively young discipline with <100 scientific papers published per year in the early 1990s, to about 3,500 per year today and a myriad of books ( Figure 1 ). Much of this progress was brought about by exercise physiologists who developed new methods and technologies within their laboratories (e.g., treadmills and ergometers) at universities around the world to study trained athletes (e.g., distance runners and cyclists) ( Hawley et al., 2015 ). Next to sports science, these developments promoted the emergence of another new discipline, that of sports nutrition. Some of the major innovations and corresponding knowledge milestones for sports nutrition research, combined with sports science research, are summarized in Figure 2 .
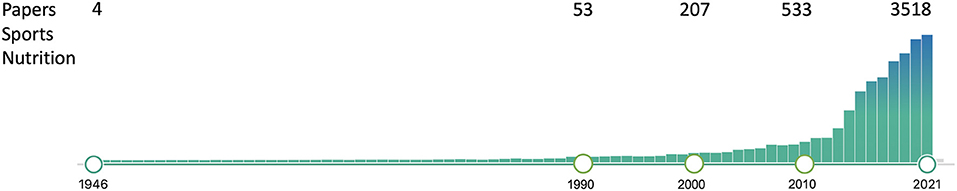
Figure 1 . Publications in Pubmed using the search term “Sports Nutrition” as of December, 2021 1 .
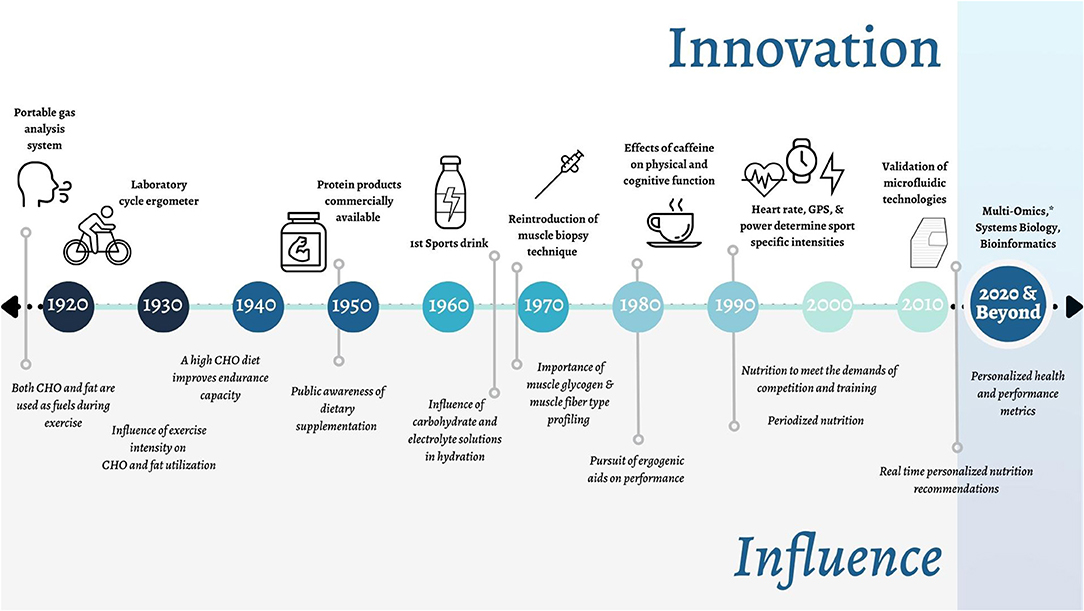
Figure 2 . Timeline of key innovations in Sports Nutrition and their respective influence on the field. *Including: genomics, transcriptomics, metabolomics, proteomics, phenomics, and other related omics (e.g., epigenomics).
In 2003, the International Olympic Committee (IOC) working group on sports nutrition concluded “The amount, composition, and timing of food intake can profoundly affect sports performance. Good nutritional practice will help athletes train hard, recover quickly and adapt more effectively with less risk of illness and injury” ( IOC Consensus Statement on Sports Nutrition, 2004 ). Nearing two decades later and these recommendations remain as pertinent. Despite all this progress, excitement and scientific endeavor, the ability to determine the impact of sports nutrition for different groups of athletes (e.g., different sports, ethnic groups, and sex) is still elusive. For example, there is substantial evidence for carbohydrate (CHO) ingestion before, during and after exercise ( Burke et al., 2011 ; Stellingwerff and Cox, 2014 ). However, it is difficult to separate the performance benefits of CHO ingestion per se vs. all other variables during competition (e.g., environment, competition, technology, equipment, and psychology).
Failure to establish the impact of any sports and exercise science intervention may result in a decline in recognition of the disciplines role in supporting the health and performance of athletes. This applies not only to the elite performers but also the young athlete, the exercising public, and the elderly. Therefore, this mini-review will focus on the opportunities to accelerate knowledge and practice of sports nutrition via the integration of technological innovations. We first highlight the present knowledge then propose ways of integrating technical advances and personalized prescription. Particular reference will be given to personalized prescriptions that are transforming and modernizing other life sciences.
The Present
Although, we primarily think of innovation in sports nutrition as directed at athlete performance outcomes, we also need to be innovative in our methods of synthesizing and dispersing research. The sports nutrition research data generated to date, have been compiled in several consensus documents on general sports ( Thomas D. T. et al., 2016 ), team sports ( Collins et al., 2021 ), and dietary supplements ( Maughan et al., 2018 ). However, a limitation of the current evidence base informing all sport science and sports medicine consensus guidelines is the lack of appropriate intervention studies (e.g., randomized controlled clinical trials) in elite populations ( McKay et al., 2022 ) that are ecologically valid (e.g., in real-life training and competition settings). Furthermore, often in practice, sports nutrition recommendations are retrospective fitted to sports in which the original research was not completed. For example much of the research to inform “stop-and-go” type sports has primarily been completed in soccer ( Williams and Rollo, 2015 ). There is also a primary focus on male subjects in the sports nutrition literature in general; yet most guidelines are “assumed” to be ideal for females as well. Furthermore, in the last 5 years, the focus has shifted from original research to reviews in the area of sports nutrition. Of the published articles, between 17.6 and 20.2% have been reviews (512–570), of which 4.4–6.0 meta-analyses (128–223). Some of this focus on reviews of all types rather than original research is enforced due to restrictions imposed due to Covid-19. However, there is a continuing need for original research in order to advance the discipline.
Consensus meetings and subsequent statements are fundamental in the generation of expert driven guidelines. Over the past 5 years, consensus statements in Sports Nutrition ranged between 0.3 and 0.5% ( Williams and Rollo, 2015 ; Jeukendrup, 2017 ; Pitsiladis et al., 2017 ; Sutehall et al., 2018 ; Burke et al., 2019 ; Stellingwerff et al., 2019 ; Baker et al., 2020 ; Muniz-Pardos et al., 2021 ) of the published articles. Historically, consensus statements, such as the International Olympic Committee (IOC) consensus on sports supplements ( Maughan et al., 2018 ) are drafted following in-person meetings of leading medical and scientific content experts. However, it is appreciated that such meetings can be costly in terms of budget, time and the carbon footprint of travel. More recently, major consensus statements have implemented remote online approaches, including the entire 2019 World Athletics (formerly IAAF) Nutrition for Athletics Consensus Update (featuring over 40 authors across 17 papers, e.g., 12). A remote consensus approach provides the opportunity to involve a wider contribution on topic guidelines rather than fewer selected opinion leaders. Establishing online working communication platforms as well as documents may also allow consensus documents to be updated with greater frequently or at pace with current literature. In summary, consensus statements informed by original research and meta-analyses, will require a greater reliance on new digital based approaches, while also respecting the need for in person meetings amongst experts.
Integrating Technical Advances to Field-Based Metrics
Most paradigms in sports nutrition have been established using laboratory experimentation, while neglecting evidence from in situ or field experimentation. This results in studies with limited ecological validity. In order to establish the efficacy of nutrition parameters for performance enhancement for all relevant populations, we need to better understand the competition demands of sport ( Stellingwerff et al., 2019 ). Recent advances in wearable technologies and real-time monitoring have accelerated the shift in research from the laboratory to the field in order to enhance ecological validity. This trend poses a real opportunity for all sport science disciplines, including sports nutrition, to embrace these technological developments. One such recent example was the implementation of live performance feedback of athletes (during 10,000 m, marathon, and race-walk events) competing in the heat at Tokyo 2020 ( Muniz-Pardos et al., 2021 ). Briefly, the aim of implementing this wireless technology during Tokyo Olympic Games was to help characterize the physiological and thermal strain experienced by athletes, as well as determine future management of athletes during a medical emergency as a result of a more timely and accurate diagnosis. The real-time monitoring comprised a smartwatch application, designed to collect, process and transmit a wide range of physiological, biomechanical, bioenergetics, and environmental data. This project was a success in terms of technological innovation but also general acceptance by athletes and sport's governing bodies. Such projects provide the opportunity for other new and valid sensors to assess performance- and health-related parameters particularly relevant to sports nutrition. One example is microfluidic technologies integrated into wearable patches to provide athletes instant feedback on sweat rate and sweat composition ( Baker et al., 2020 ). Wider adoption of such technologies will create more symbiotic relationships between sport, health and technology by harnessing the unique demands of elite sport (e.g., the need for unobtrusive devices that provide real-time feedback).
Given their symbiotic relationship, the evolution of sports nutrition, and sports science requires more holistic approaches with input from all major disciplines (e.g., coaching science, environmental physiology, and sports biomechanics), stakeholders, sponsors, and interested industry ( Pitsiladis et al., 2017 ). In recent years, physiology, nutrition, and technical advances have become increasingly integrated as part of new sport performance innovation strategies. A pertinent example is the Sub2 marathon project which was a novel proof-of-concept idea motivated by the need to focus on a holistic approach whilst promoting clean sport (i.e., high performance marathon running without doping) ( Pitsiladis et al., 2017 ). This was the first dedicated international research initiative launched in 2014 made up of multidisciplinary scientists from academia, elite athletes and strategic industry partners across many sport science and medicine domains. An exciting Sub2 innovation with particular sports nutrition focus was the carbohydrate “hydrogel” development. This innovative concept in sports drinks was tested in elite athletes training in Ethiopia and Kenya. The novel aspect of the gel was that it allowed runners to ingest and tolerate CHO concentrations much higher than would normally be possible to ingest while running (e.g., 30% CHO) ( Sutehall et al., 2018 ). This was important because a common challenge for runners is to meet CHO ingestion guidelines without experiencing gastrointestinal complaints ( Jeukendrup, 2017 ). This sports drink was subsequently trialed and tested in the field with positive response by elite runners during marathons and cyclists in the tour de France ( Sutehall et al., 2020 ). One laboratory-based study has confirmed improved running performance, greater carbohydrate oxidation and lower GI symptoms following hydrogel ingestion compared with a standard CHO solution ( Rowe et al., 2022 ). However, other laboratory-based studies have not reported any of these advantages following hydrogel ingestion compared to the ingestion of carbohydrate-electrolyte sport beverages ( Baur et al., 2019 ; King et al., 2020 ; McCubbin et al., 2020 ). Nevertheless, it is a great example of sports nutrition innovation specific to the needs of the sport in the field. The hydrogel innovation was adopted by both the breaking 2 , 3 . and INEOS 159 4 . projects to break the 2-h marathon barrier, a reflection of the perceived value of this putative innovation.
Combining emerging technologies are ideal to better our understanding of performance and to objectively test the impact of nutritional strategies in laboratory or real performance environments ( Table 1 ). Such innovations will also allow other sports, beyond the mainly studied endurance sports cycling and running, to be evaluated in terms of sports nutrition impact. The utilization of these technologies, and co-ordinated research, may allow for the rapid generation of large data sets across many other types of sport that have yet to be included in sports nutrition research. As such, this approach will (i) speed our knowledge of sports that are difficult to study, (ii) gain data from regional populations under-represented in the literature, and (iii) inform the advice of how specific nutrition guidelines maybe transferred to the field. Accordingly, Table 1 highlights examples of existing and emerging technologies and methodologies that are “field-based” and relatively non-invasive that may continue to drive and refine sports nutrition research, interventions and recommendations.
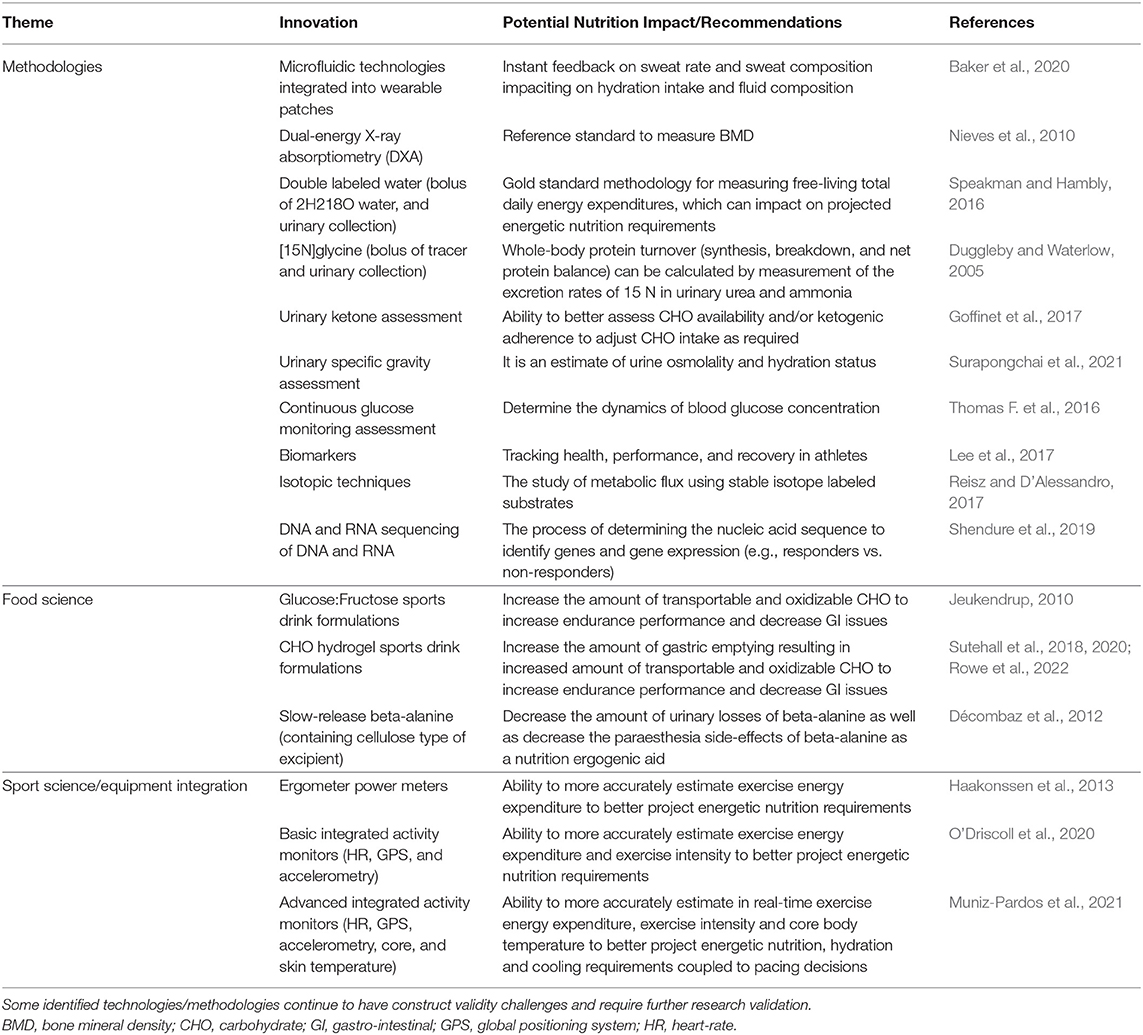
Table 1 . Examples of existing or potential “in field” non-invasive technologies or methodologies that may drive current or future nutrition studies, interventions, and/or recommendations.
The wearable/technological revolution promises in the near future to improve the ability to monitor a whole range of physiological parameters in the field. For example, apps, devices, and entire ecosystems are being developed and destined to improve the quality of dietary intake methods and therefore the accurately of athletes' daily energy intake (EI) ( Ferrara et al., 2019 ). These technological developments may enable the energy availability (EA) of individual athletes (i.e., calculated as EI–EEE/fat free mass) to be more accurately monitored. Correspondingly, a more comprehensively study of the athlete in situ would be possible. Thus, this approach represents an unprecedented opportunity to mitigate many unresolved issues in the field of sports nutrition such as relative energy deficiency in sport (RED-S) ( Mountjoy et al., 2018 ). Importantly, the recent explosion of wearable technology/apps/devices with often unsubstantiated claims require quality assurance standards for wearable devices. Such concerns have prompted the International Federation of Sports Medicine (FIMS) to create a global standard for wearable devices in sport and fitness ( Ash et al., 2020 , 2021 ). Organizations involved in sports nutrition also have the opportunity to engage in quality assurance processes to safeguard the credibility of the innovations in sports nutrition.
Personalized Prescriptions
There is no such thing as an “average” athlete. However, a key question is if there is an added value of personalized nutrition vs. general guidelines? Importantly, technology innovations will allow the individual response to a sports nutrition intervention to be determined. For instance, to find the individual recommendation of carbohydrate and fluid during exercise, we need knowledge about the energy demands of the sport, sweat losses, gastrointestinal limits, personal taste preferences and every element of the event. This needs to include research on different sport categories and target groups. This also presents the opportunity to follow athletes over a longer period of time, without associated human labor or time costs. For instance, to establish the extent to which an individual responds to different nutritional interventions, we need to conduct repeated testing in the same individual on several occasions. And the more complex the sport and its environment, the more test repetitions may be needed to establish the magnitude of impact of an intervention. It is also imperative that we determine athlete compliance with prescribed nutritional interventions. Such data will allow the evaluation of education and behavior change strategies, which may also provide opportunity for personalization.
The research on personalized sports nutrition will undoubtedly be the focus in the near future due to the technological advances in genomics technologies such as genetic sequencing. For instance, is has been suggested that the impact of DNA sequencing will become on a par with that of the microscope ( Shendure et al., 2019 ). Sports nutrition and sports science are encouraged to use these powerful technologies and to keep up with rapid developments to increase the chances of finding the best solutions possible. Such technologies are routinely used in biomedical research and precision medicine applications, such as for cancer, stroke and Alzheimer's disease, thus, vital lessons can be learned and transferred to sports nutrition. However, it is essential that these technological developments are not “oversold” and that their application in the field is founded on evidence-based research and not driven by commercial interests. At present, the use of genetic testing in both sports nutrition and sports science is at a very early stage. The consensus in the scientific literature being that genetic testing in sport science has very low clinical utility and should not be sold ( Guasch-Ferré et al., 2018 ; Tanisawa et al., 2020 ). This is in contrast to the ever-increasing number of companies selling genetic testing, supported by unfounded claims ( Webborn et al., 2015 ; Vlahovich et al., 2017 ; Tanisawa et al., 2020 ). The market value of genetic testing; USD 10.80 Billion in 2020, is forecast to reach USD 23.14 Billion by 2027 5 .
A more precision-based sports nutrition will also need to consider the other components of the “omics” cascade in addition to genomics (e.g., transcriptomics, metabolomics, proteomics, and single cell sequencing). Furthermore, such approaches may utilize powerful bioinformatics methods, such as machine learning and artificial intelligence to integrate the different layers of biological data required for understanding the functional consequences with the real time assessment of the “phenome” using 5G and 6G, sensors, devices and applications ( Mancin et al., 2021 ). The identification of relevant non-invasive biomarkers are attractive to athletes and practitioners, due to the speed and increased frequency of collection vs. traditional blood draws or questionnaires. However, these technologies should be adopted in accordance with ethical principles and within national/international regulatory frameworks, which require further development.
New Approaches to Fulfill Knowledge Gaps
Given recent technological breakthroughs, there are exciting opportunities for sports nutrition research to take gigantic leaps in the near future. Until now, most sports nutrition and sports physiology studies are performed in controlled laboratory environments and often study the effect of single nutrients. There is opportunity for sports nutrition research to embrace real world settings using real solutions and a more holistic approaches, such as performance benefits of whole foods, whole-body effects of low EA and “targeted nutritional periodization.” One example is a study using tracer technologies to compare the effect of whole eggs vs. egg whites on post exercise muscle protein synthesis ( van Vliet et al., 2017 ). New study designs should focus on real life settings that are strictly monitored with use of new technological advances, apps, and systems. As such, with a clear overview of nutritional demands of the sport and individual factors of impact, the extent of real-life effects of sports nutrition elements can be established.
Beyond the physiological impact of nutrients, there is also opportunity for sports nutrition research to study of cognitive and mental performance ( Habay et al., 2021 ). This shift will require sports nutrition researchers and nutritionists to adopt and further develop technological methods to allow the psychobiological determinants of performance to better defined. New research paradigms and technologies could revolutionize sports nutrition research from small landmark studies of the 1960s with mainly the authors as subjects taking muscle biopsies on themselves ( Bergström and Hultman, 1966 ), to the use of big data and collaboration between large groups of researchers. Examples of the latter are studies identifying genes implicated in hand grip strength involving over 195,000 subjects ( Willems et al., 2017 ) or investigating the effects of age, body composition, and sex on total expenditure by the doubly labeled water method in 6,421 participants from 29 countries ( Pontzer et al., 2021 ). The field of sports nutrition has the opportunity to adopt such collaborative practices combined with the application of the new and established technologies (see Table 1 ). It is reasonable to suggest that this approach will inevitably become the mainstay of personalized medicine, where treating the individual will be the norm rather than the average. If sports nutrition can embrace these challenges, it will thrive as an essential discipline and its relevance recognized in other fields ( Oikawa et al., 2021 ).
Limitations/Perspectives
While innovations are necessary and appealing, there needs to be a considered approach to implementation. Soon almost any parameter will be able to be measured or inferred, yet the use of such data especially during live performances remains to be explored. There also seems to be a trend toward 24/7 observations (e.g., Apple watch, Oura ring, WHOOP, and Biostrap). Caution is encouraged when moving from too little or no assessment to over monitored and scheduled, as a result of too much feedback and reliance on devices. For instance, the athlete should be focussing on racing/competition, not on heart rate or temperature or non-validated feedback directly from a device. Tracking may also be potentially stressful ( Andersen et al., 2020 ), albeit this remains to be determined in athlete populations. When evolving sports nutrition research with new technological advances, it is important to continuously question the application to practice as well as the reliability and reliance of devices.
The integration of new technologies in elite populations will also require closer collaborations between research and practitioners, and then directly to the athlete and coach ( Bartlett and Drust, 2021 ). However, multidisciplinary sport science and medicine teams do not come without challenges and clear communication, roles and responsibilities are essential ( Dijkstra et al., 2014 ) with the athlete and coach at the centre of accountability.
Finally, impactful implementation of these innovations and technological developments especially in elite athletic populations is going to require the continued and better integration of behavioral change psychology in sports nutrition. A recent systematic review highlighted some of the most effective behavioral strategies used in sports nutrition ( Bentley et al., 2020 ).
Conclusions
Innovation is at the core of sports nutrition research and has pushed the field forward even before sports nutrition was recognized as a separate discipline. We are at a critical juncture in the evolution of this discipline primed to utilize new technologies to support the success of specific sports and individual athletes. Sharing data in new and more efficient ways, integrating field based physiological measures, and personalized prescriptions are key opportunities to advance sports nutrition. However, technological advances should not be used in haste and must first be evaluated to determine their functionality and value to the athletes health and performance. In summary, nutrition is but one of many complex and integrated sport performance determinants, and the impact of any new intervention should be assessed along a risk-reward continuum.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Author Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
Conflict of Interest
IR and MK are employees of the Gatorade Sports Science Institute, a division of PepsiCo, Inc. KJ, TS, and YP, received speaking honoraria, for the GSSI ECSS 2021 pre-conference symposium which inspired this article. YP is the founding member of the Sub2 project ( www.sub2hrs.com ). The Sub2 project is affiliated to a non-trading company (Athlome Limited, UK) that is minor (<1.1%) shareholder of Maurten AB, Gothenburg, Sweden.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ https://pubmed.ncbi.nlm.nih.gov/?term=sports+nutrition&filter=years.1946-2021&timeline=expanded&sort=date&sort_order=asc .
2. ^ www.runnersworld.com/uk/news/a29100149/breaking2 .
3. ^ www.nike.com/gb/running/breaking2 .
4. ^ http://www.ineos159challenge.com/ .
5. ^ https://brandessenceresearch.com/requestSample/PostId/1362 .
Andersen, T. O., Langstrup, H., and Lomborg, S. (2020). Experiences with wearable activity data during self-care by chronic heart patients: qualitative study. J. Med. Internet Res. 22:e15873. doi: 10.2196/15873
PubMed Abstract | CrossRef Full Text | Google Scholar
Ash, G. I., Stults-Kolehmainen, M., Busa, M. A., Gaffey, A. E., Angeloudis, K., Muniz-Pardos, B., et al. (2021). Establishing a global standard for wearable devices in sport and exercise medicine: perspectives from academic and industry stakeholders. Sports Med . 51, 2237–2250. doi: 10.1007/s40279-021-01543-5
Ash, G. I., Stults-Kolehmainen, M., Busa, M. A., Gregory, R., Garber, C. E., Liu, J., et al. (2020). Establishing a global standard for wearable devices in sport and fitness: perspectives from the New England Chapter of the American College of Sports Medicine Members. Curr. Sports Med. Rep . 19, 45–49. doi: 10.1249/JSR.0000000000000680
Baker, L. B., Model, J. B., Barnes, K. A., Anderson, M. L., Lee, S. P., Lee, K. A., et al. (2020). Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv . 6, eabe3929. doi: 10.1126/sciadv.abe3929
Bartlett, J. D., and Drust, B. (2021). A framework for effective knowledge translation and performance delivery of Sport Scientists in professional sport. Eur. J. Sport Sci . 21, 1579–1587. doi: 10.1080/17461391.2020.1842511
Baur, D. A., Toney, H. R., Saunders, M. J., Baur, K. G., Luden, N. D., and Womack, C. J. (2019). Carbohydrate hydrogel beverage provides no additional cycling performance benefit versus carbohydrate alone. Eur. J. Appl. Physiol . 119, 2599–2608. doi: 10.1007/s00421-019-04240-4
Bentley, M. R. N., Mitchell, N., and Backhouse, S. H. (2020). Sports nutrition interventions: a systematic review of behavioural strategies used to promote dietary behaviour change in athletes. Appetite 150, 104645. doi: 10.1016/j.appet.2020.104645
Bergström, J., and Hultman, E. (1966). Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210, 309–310. doi: 10.1038/210309a0
Burke, L. M., Castell, L. M., Casa, D. J., Close, G. L., Costa, R. J. S., Desbrow, B., et al. (2019). International Association of Athletics Federations Consensus Statement 2019: nutrition for athletics. Int. J. Sport Nutr. Exerc. Metab . 29, 73–84. doi: 10.1123/ijsnem.2019-0065
Burke, L. M., Hawley, J. A., Wong, S. H. S., and Jeukendrup, A. E. (2011). Carbohydrates for training and competition. J. Sports Sci . 29(Suppl. 1), 17–S27. doi: 10.1080/02640414.2011.585473
Collins, J., Maughan, R. J., Gleeson, M., Bilsborough, J., Jeukendrup, A., Morton, J. P., et al. (2021). UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. Br. J. Sports Med . 55, 416. doi: 10.1136/bjsports-2019-101961
Décombaz, J., Beaumont, M., Vuichoud, J., Bouisset, F., and Stellingwerff, T. (2012). Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids 43, 67–76. doi: 10.1007/s00726-011-1169-7
Dijkstra, H. P., Pollock, N., Chakraverty, R., and Alonso, J. M. (2014). Managing the health of the elite athlete: a new integrated performance health management and coaching model. Br. J. Sports Med . 48, 523–531. doi: 10.1136/bjsports-2013-093222
Duggleby, S. L., and Waterlow, J. C. (2005). The end-product method of measuring whole-body protein turnover: a review of published results and a comparison with those obtained by leucine infusion. Br. J. Nutr . 94, 141–153. doi: 10.1079/BJN20051460
Ferrara, G., Kim, J., Lin, S., Hua, J., and Seto, E. (2019). A focused review of smartphone diet-tracking apps: usability, functionality, coherence with behavior change theory, and comparative validity of nutrient intake and energy estimates. JMIR Mhealth Uhealth 7, e9232. doi: 10.2196/mhealth.9232
Goffinet, L., Barrea, T., Beauloye, V., and Lysy, P. A. (2017). Blood versus urine ketone monitoring in a pediatric cohort of patients with type 1 diabetes: a crossover study. Ther. Adv. Endocrinol. Metab . 8, 3–13. doi: 10.1177/2042018816681706
Guasch-Ferré, M, Dashti, H. S., and Merino, J. (2018). Nutritional genomics and direct-to-consumer genetic testing: an overview. Adv. Nutr . 9, 128–135. doi: 10.1093/advances/nmy001
Haakonssen, E. C., Martin, D. T., Burke, L. M., and Jenkins, D. G. (2013). Energy expenditure of constant- and variable-intensity cycling: power meter estimates. Med. Sci. Sports Exerc . 45, 1833–1840. doi: 10.1249/MSS.0b013e31828e18e6
Habay, J., Van Cutsem, J., Verschueren, J., De Bock, S., Proost, M., De Wachter, J., et al. (2021). Mental fatigue and sport-specific psychomotor performance: a systematic review. Sports Med . 51, 1527–1548. doi: 10.1007/s40279-021-01429-6
Hawley, J. A., Maughan, R. J., and Hargreaves, M. (2015). Exercise metabolism: historical perspective. Cell Metab . 22, 12–17. doi: 10.1016/j.cmet.2015.06.016
IOC Consensus Statement on Sports Nutrition (2004). Available online at: https://stillmed.olympic.org/Documents/Commissions_PDFfiles/Medical_commission/IOC_CONSENSUS_STATEMENT_ON_SPORTS_NUTRITION_2003.pdf (accessed December 14, 2021).
Jeukendrup, A. E.. (2010). Carbohydrate and exercise performance: the role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 13, 452–457. doi: 10.1097/MCO.0b013e328339de9f
Jeukendrup, A. E.. (2017). Training the gut for athletes. Sports Med. 47(Suppl. 1), 101–110. doi: 10.1007/s40279-017-0690-6
PubMed Abstract | CrossRef Full Text
King, A. J., Rowe, J. T., and Burke, L. M. (2020). Carbohydrate hydrogel products do not improve performance or gastrointestinal distress during moderate-intensity endurance exercise. Int. J. Sport Nutr. Exerc. Metab . 30, 305–314. doi: 10.1123/ijsnem.2020-0102
Lee, E. C., Fragala, M. S., Kavouras, S. A., Queen, R. M., Pryor, J. L., and Casa, D. J. (2017). Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. J. Strength Cond. Res . 31, 2920–2937. doi: 10.1519/JSC.0000000000002122
Mancin, L., Rollo, I., Mota, J. F., Piccini, F., Carletti, M., Susto, G. A., et al. (2021). Optimizing microbiota profiles for athletes. Exerc. Sport Sci. Rev . 49, 42–49. doi: 10.1249/JES.0000000000000236
Maughan, R. J., Burke, L. M., Dvorak, J., Larson-Meyer, D. E., Peeling, P., Phillips, S. M., et al. (2018). IOC consensus statement: dietary supplements and the high-performance athlete. Br. J. Sports Med . 52, 439–455. doi: 10.1136/bjsports-2018-099027
McCubbin, A. J., Zhu, A., Gaskell, S. K., and Costa, R. J. S. (2020). Hydrogel carbohydrate-electrolyte beverage does not improve glucose availability, substrate oxidation, gastrointestinal symptoms or exercise performance, compared with a concentration and nutrient-matched placebo. Int. J. Sport Nutr. Exerc. Metab . 30, 25. doi: 10.1123/ijsnem.2019-0090
McKay, A. K. A., Stellingwerff, T., Smith, E. S., Martin, D. T., Mujika, I., Goosey-Tolfrey, V. L., et al. (2022). Defining training and performance calibre: a participant classification framework. Int. J. Sports Physiol. Perform . 1–15. doi: 10.1123/ijspp.2021-0451
Mountjoy, M., Sundgot-Borgen, J. K., Burke, L. M., Ackerman, K. E., Blauwet, C., Constantini, N., et al. (2018). IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med . 52, 687–697. doi: 10.1136/bjsports-2018-099193
Muniz-Pardos, B., Angeloudis, K., Guppy, F. M., Keramitsoglou, I., Sutehall, S., Bosch, A., et al. (2021). Wearable and telemedicine innovations for Olympic events and elite sport. J. Sports Med. Phys. Fitness 61, 1061–1072. doi: 10.23736/S0022-4707.21.12752-5
Nieves, J. W., Melsop, K., Curtis, M., Kelsey, J. L., Bachrach, L. K., Greendale, G., et al. (2010). Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R 2, 740–750; quiz 794. doi: 10.1016/j.pmrj.2010.04.020
O'Driscoll, R., Turicchi, J., Beaulieu, K., Scott, S., Matu, J., Deighton, K., et al. (2020). How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br. J. Sports Med . 54, 332–340. doi: 10.1136/bjsports-2018-099643
Oikawa, S. Y., Brisbois, T. D., van Loon, L. J. C., and Rollo, I. (2021). Eat like an athlete: insights of sports nutrition science to support active aging in healthy older adults. Geroscience 43, 2485–2495. doi: 10.1007/s11357-021-00419-w
Pitsiladis, Y., Ferriani, I., Geistlinger, M., de Hon, O., Bosch, A., and Pigozzi, F. (2017). A holistic antidoping approach for a fairer future for sport. Curr. Sports Med. Rep . 16, 222–224. doi: 10.1249/JSR.0000000000000384
Pontzer, H., Yamada, Y., Sagayama, H., Ainslie, P. N., Andersen, L. F., Anderson, L. J., et al. (2021). Daily energy expenditure through the human life course. Science 373, 808–812. doi: 10.1126/science.abe5017
Reisz, J. A., and D'Alessandro, A. (2017). Measurement of metabolic fluxes using stable isotope tracers in whole animals and human patients. Curr. Opin. Clin. Nutr. Metab. Care 20, 366–374. doi: 10.1097/MCO.0000000000000393
Rowe, J. T., King, R. F. G. J., King, A. J., Morrison, D. J., Preston, T., Wilson, O. J., et al. (2022). Glucose and fructose hydrogel enhances running performance, exogenous carbohydrate oxidation, and gastrointestinal tolerance. Med. Sci. Sports Exerc . 54, 129–140. doi: 10.1249/MSS.0000000000002764
Shendure, J., Balasubramanian, S., Church, G. M., Gilbert, W., Rogers, J., Schloss, J. A., et al. (2019). Publisher correction: DNA sequencing at 40: past, present and future. Nature 568:E11. doi: 10.1038/s41586-019-1120-8
Speakman, J. R., and Hambly, C. (2016). Using doubly-labelled water to measure free-living energy expenditure: some old things to remember and some new things to consider. Comp. Biochem. Physiol. A Mol. Integr. Physiol . 202, 3–9. doi: 10.1016/j.cbpa.2016.03.017
Stellingwerff, T., and Cox, G. R. (2014). Systematic review: carbohydrate supplementation on exercise performance or capacity of varying durations. Appl. Physiol. Nutr. Metab . 39, 998–1011. doi: 10.1139/apnm-2014-0027
Stellingwerff, T., Morton, J. P., and Burke, L. M. (2019). A framework for periodized nutrition for athletics. Int. J. Sport Nutr. Exerc. Metab . 29, 141–151. doi: 10.1123/ijsnem.2018-0305
Surapongchai, J., Saengsirisuwan, V., Rollo, I., Rendell, R. K., Nithitsuttibuta, K., Sainiyom, P., et al. (2021). Hydration status, fluid intake, sweat rate, and sweat sodium concentration in recreational tropical native runners. Nutrients 13:1374. doi: 10.3390/nu13041374
Sutehall, S., Galloway, S. D. R., Bosch, A., and Pitsiladis, Y. (2020). Addition of an alginate hydrogel to a carbohydrate beverage enhances gastric emptying. Med. Sci. Sports Exerc . 8, 1785–1792. doi: 10.1249/MSS.0000000000002301
Sutehall, S., Muniz-Pardos, B., Bosch, A., Di Gianfrancesco, A., and Pitsiladis, Y. (2018). Sports drinks on the edge of a new era. Curr. Sports Med. Rep . 17, 112–116. doi: 10.1249/JSR.0000000000000475
Tanisawa, K., Wang, G., Seto, J., Verdouka, I., Twycross-Lewis, R., Karanikolou, A., et al. (2020). Sport and exercise genomics: the FIMS 2019 consensus statement update. Br. J. Sports Med . 54, 969–975. doi: 10.1136/bjsports-2019-101532
Thomas, D. T., Erdman, K. A., and Burke, L. M. (2016). American College of Sports Medicine Joint Position Statement. Nutrition and athletic performance. Med. Sci. Sports Exerc . 48, 543–568. doi: 10.1249/MSS.0000000000000852
Thomas, F., Pretty, C. G., Desaive, T., and Chase, J. G. (2016). Blood glucose levels of subelite athletes during 6 days of free living. J. Diabetes Sci. Technol . 10, 1335–1343. doi: 10.1177/1932296816648344
van Vliet, S., Shy, E. L., Sawan, S. A., Beals, J. W., West, D. W., Skinner, S. K., et al. (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr . 106, 1401–1412. doi: 10.3945/ajcn.117.159855
Vlahovich, N., Hughes, D., Griffiths, L. R., Wang, G., Pitsiladis, Y. P., and Eynon, N. (2017). Genetic testing for exercise prescription and injury prevention: AIS-Athlome consortium joint statement. BMC Genomics 18(Suppl 8), 818. doi: 10.1186/s12864-017-4185-5
Webborn, N., Williams, A., McNamee, M., Bouchard, C., Pitsiladis, Y., Ahmetov, I., et al. (2015). Direct-to-consumer genetic testing for predicting sports performance and talent identification: Consensus statement. Br. J. Sports Med . 49, 1486–1491. doi: 10.1136/bjsports-2015-095343
Willems, S. M., Wright, D. J., Day, F. R., Trajanoska, K., Joshi, P. K., Morris, J. A., et al. (2017). Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat. Commun . 8, 16015. doi: 10.1038/ncomms16015
Williams, C., and Rollo, I. (2015). Carbohydrate nutrition and team sport performance. Sports Med . 45(Suppl 1), S13–S22. doi: 10.1007/s40279-015-0399-3
Keywords: innovation, wearables, technology, performance, health, diet, wellness, athletes
Citation: Jonvik KL, King M, Rollo I, Stellingwerff T and Pitsiladis Y (2022) New Opportunities to Advance the Field of Sports Nutrition. Front. Sports Act. Living 4:852230. doi: 10.3389/fspor.2022.852230
Received: 10 January 2022; Accepted: 18 January 2022; Published: 17 February 2022.
Reviewed by:
Copyright © 2022 Jonvik, King, Rollo, Stellingwerff and Pitsiladis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yannis Pitsiladis, y.pitsiladis@brighton.ac.uk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Open access
- Published: 02 February 2010
ISSN exercise & sport nutrition review: research & recommendations
- Richard B Kreider 1 ,
- Colin D Wilborn 2 ,
- Lem Taylor 2 ,
- Bill Campbell 3 ,
- Anthony L Almada 4 ,
- Rick Collins 5 ,
- Mathew Cooke 6 ,
- Conrad P Earnest 7 ,
- Mike Greenwood 8 ,
- Douglas S Kalman 9 ,
- Chad M Kerksick 10 ,
- Susan M Kleiner 11 ,
- Brian Leutholtz 8 ,
- Hector Lopez 12 ,
- Lonnie M Lowery 13 ,
- Ron Mendel 14 ,
- Abbie Smith 10 ,
- Marie Spano 15 ,
- Robert Wildman 16 ,
- Darryn S Willoughby 8 ,
- Tim N Ziegenfuss 17 &
- Jose Antonio 18
Journal of the International Society of Sports Nutrition volume 7 , Article number: 7 ( 2010 ) Cite this article
535k Accesses
268 Citations
251 Altmetric
Metrics details
Sports nutrition is a constantly evolving field with hundreds of research papers published annually. For this reason, keeping up to date with the literature is often difficult. This paper is a five year update of the sports nutrition review article published as the lead paper to launch the JISSN in 2004 and presents a well-referenced overview of the current state of the science related to how to optimize training and athletic performance through nutrition. More specifically, this paper provides an overview of: 1.) The definitional category of ergogenic aids and dietary supplements; 2.) How dietary supplements are legally regulated; 3.) How to evaluate the scientific merit of nutritional supplements; 4.) General nutritional strategies to optimize performance and enhance recovery; and, 5.) An overview of our current understanding of the ergogenic value of nutrition and dietary supplementation in regards to weight gain, weight loss, and performance enhancement. Our hope is that ISSN members and individuals interested in sports nutrition find this review useful in their daily practice and consultation with their clients.

Introduction
Sports nutrition professionals need to know how to evaluate the scientific merit of articles and advertisements about exercise and nutrition products so they can separate marketing hype from scientifically-based training and nutritional practices. In order to help ISSN members keep informed about the latest in sports nutrition, we have updated the ISSN Exercise & Sports Nutrition Review that was used to help launch the JISSN (originally called the Sports Nutrition Review Journal). This paper provides an overview of: 1.) The definitional category of ergogenic aids and dietary supplements; 2.) How dietary supplements are legally regulated; 3.) How to evaluate the scientific merit of nutritional supplements; 4.) General nutritional strategies to optimize performance and enhance recovery; and, 5.) An overview of our current understanding of the ergogenic value in regards to weight gain, weight loss, and performance enhancement supplements. We have also categorized nutritional supplements into 'apparently effective', 'possibly effective', 'too early to tell', and 'apparently ineffective' as well a description of our general approach into educating athletes about sports nutrition. Over the last five years there have been many changes to our original categorization of supplements. In addition, a number of new supplements have been introduced to the market are reviewed in this article. While some may not agree with all of our interpretations of the literature and/or categorization of a particular supplement, and some classifications may change over time as more research is forthcoming, these interpretations are based on current available scientific evidence and have been well received within the broader scientific community. Our hope is that ISSN members find this information useful in their daily practice and consultation with their clients.
- Ergogenic Aid
An ergogenic aid is any training technique, mechanical device, nutritional practice, pharmacological method, or psychological technique that can improve exercise performance capacity and/or enhance training adaptations [ 1 – 3 ]. This includes aids that may help prepare an individual to exercise, improve the efficiency of exercise, and/or enhance recovery from exercise. Ergogenic aids may also allow an individual to tolerate heavy training to a greater degree by helping them recover faster or help them stay injury-free and/or healthy during intense training. Although this definition seems rather straightforward, there is considerable debate regarding the ergogenic value of various nutritional supplements. Some sports nutrition specialists only consider a supplement ergogenic if studies show that the supplement significantly enhances exercise performance (e.g., helps you run faster, lift more weight, and/or perform more work during a given exercise task). On the other hand, some feel that if a supplement helps prepare an athlete to perform or enhances recovery from exercise, it has the potential to improve training adaptations and therefore should be considered ergogenic. In the view of the ISSN, one should take a broader view about the ergogenic value of supplements. While we are interested in determining the performance enhancement effects of a supplement on a single bout of exercise, we also realize that one of the goals of training is to help people tolerate a greater degree of training. Individuals who better adapt to high levels of training usually experience greater gains from training over time which can lead to improved performance. Consequently, employing nutritional practices that help prepare individuals to perform and/or enhance recovery from exercise should also be viewed as ergogenic.
Definition and Regulation of Dietary Supplements
As described in Exercise and Sports Nutrition: Principles, Promises, Science & Recommendations [ 3 ]; according to the Food and Drug Administration (FDA), dietary supplements were regulated in the same manner as food prior to 1994 [ 4 ]. Consequently, the FDA monitored the manufacturing processes, quality, and labeling of dietary supplements. However, many people felt that the FDA was too restrictive in regulating dietary supplements. As a result, Congress passed the Dietary Supplement Health and Education Act (DSHEA) in 1994 which placed dietary supplements in a special category of "foods". In October 1994, President Clinton signed DSHEA into law. The law defined a "dietary supplement" as a product taken by mouth that contains a "dietary ingredient" intended to supplement the diet. "Dietary ingredients" may include vitamins, minerals, herbs or other botanicals, amino acids, and substances (e.g., enzymes, organ tissues, glandular, and metabolites). Dietary supplements may also be extracts or concentrates from plants or foods. Dietary supplements are typically sold in the form of tablets, capsules, soft gels, liquids, powders, and bars. Products sold as dietary supplements must be clearly labeled as a dietary supplement.
According to DSHEA, dietary supplements are not drugs. Dietary supplement ingredients that were lawfully sold prior to 1994, have been "grandfathered" into the Act, meaning that a manufacturer is not required to submit to FDA the evidence it relies upon to substantiate safety or effectiveness before or after it markets these ingredients. The rationale for this exclusion is based on a long history of safe use; hence there is no need to require additional safety data. However, DSHEA grants FDA greater control over supplements containing new dietary ingredients. A new dietary ingredient is deemed adulterated and subject to FDA enforcement sanctions unless it meets one of two exemption criteria: either 1.) the supplement in question contains "only dietary ingredients which have been present in the food supply as an article used for food in a form in which the food has not been chemically altered"; or 2.) there is a "history of use or other evidence of safety" provided by the manufacturer or distributor to FDA at least 75 days before introducing the product into interstate commerce. The second criterion, applicable only to new dietary ingredients that have not been present in the food supply, requires manufacturers and distributors of a new dietary ingredient or a product containing a new dietary ingredient to submit pre-market notification to the FDA. This notification, which must be submitted at least 75 days before the product is introduced into interstate commerce, must contain information that provides a history of use or other evidence of safety establishing that the dietary ingredient, when used under the conditions recommended or suggested in the labeling of the dietary supplement will "reasonably be expected to be safe." This may include conducting in vitro toxicology testing, long-term toxicity studies using varying doses in animals to see if there are any toxic effects, providing manufacturing and quality assurance data showing purity, and provision of clinical studies conducted in humans showing safety. The FTC also requires that any representations or claims made about the supplement be substantiated by adequate evidence to show that they are not false or misleading, a policy which is also shared by the FDA. This involves, for example, providing at least two clinical trials showing efficacy of the actual product, within a population of subjects relevant to the target market, supporting the structure/function claims that are made. Structure/function claims may include several categories. They may describe the role of a nutrient or dietary ingredient intended to affect normal structure or function in humans, they may characterize the means by which a nutrient or dietary ingredient acts to maintain such structure or function, they may describe general well-being from consumption of a nutrient or dietary ingredient or they may describe a benefit related to a nutrient deficiency disease, as long as the statement also tells how widespread such a disease is in the United States. Manufacturers of dietary supplements that make structure/function claims on labels or in labeling must submit a notification to FDA no later than 30 days after marketing the dietary supplement that includes the text of the structure/function claim. DSHEA also requires supplement manufacturers to include on any label displaying structure/function claims the disclaimer "This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease" . Opponents of dietary supplements often cite this statement as evidence that the FDA did not review or approve the dietary supplement when in fact most dietary ingredients have been grandfathered in due to a long history of safe sale; whereas those products containing a new dietary ingredient which is not present in the food supply as an article used for food in a form in which the food has not been chemically altered are subject to pre-market notification to FDA regarding history of use or other evidence of safety. Unfortunately, a large number of new dietary ingredients requiring pre-market notification have been introduced into dietary supplements since October 1994 without the requisite notification.
According to the 1994 Nutrition Labeling and Education Act (NLEA), the FDA has the ability to review and approve health claims for dietary ingredients and foods. However, since the law was passed it has only approved a few claims. The delay in reviewing health claims of dietary supplements resulted in a lawsuit filed by Pearson & Shaw et al v. Shalala et al in 1993. After years of litigation, the U.S. Court of Appeals for the District of Columbia Circuit ruled in 1999 that qualified health claims may now be made about dietary supplements with approval by FDA as long as the statements are truthful and based on science. Supplement or food companies wishing to make health claims about supplements can submit research evidence to the FDA for approval of a health claim. Additionally, companies must also submit an Investigational New Drug (IND) application to FDA if a research study on a nutrient or multiple dietary ingredient composition is designed to treat an illness and/or medical affliction and/or the company hopes to one day obtain approval for making a qualified health claim as a prescription or orphan drug if the outcome of the study supports the claim. Studies investigating structure/function claims, however, do not need to be submitted to the FDA as an IND. The 1997 Food and Drug Administration Modernization Act (FDAMA) provided for health claims based on an authoritative statement of a scientific body of the U.S. Government or the National Academy of Sciences; such claims may be used after submission of a health claim notification to FDA; and the 2003 FDA Consumer Health Information for Better Nutrition Initiative provided for qualified health claims where the quality and strength of the scientific evidence falls below that required for FDA to issue an authorizing regulation. Such health claims must be qualified to assure accuracy and non-misleading presentation to consumers. More recently, the U.S. Senate passed legislation (Senate Bill 1082) that established the Reagan-Udall Foundation for the FDA. The purpose of this non-profit foundation is to lead collaborations among the FDA, academic research institutions, and industry to enhance research in evaluating the safety and efficacy of dietary supplements as well as to improve the quality and management of these products.
For many years, manufacturers and distributors of dietary supplements were not required to record, investigate or forward to FDA any reports they receive on injuries or illnesses that may be related to the use of their products. However, companies are now required by the Dietary and Supplement and Nonprescription Drug Consumer Act (Public Law 109-462 109th Congress Dec. 22, 2006) to record all adverse event complaints about their products and make them available to the FDA pursuant to an inspection. Reports of "serious" adverse events (i.e., adverse events which results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, or a congenital anomaly or birth defect; or requires, based on a reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above) must be reported to FDA within 15 business days. While these reports are unsubstantiated; can be influenced by media attention to a particular supplement; and do not necessarily show a cause and effect: they can be used by the company and FDA to monitor trends and "signals" that may suggest a problem. Once a dietary supplement product is marketed, the FDA has the responsibility for showing that the dietary supplement is unsafe before it can take action to restrict the product's use or removal from the marketplace. The FTC maintains responsibility to make sure manufacturers are truthful and not misleading regarding claims they make about dietary supplements. The FDA has the power to remove supplements from the market if it has sufficient scientific evidence to show the supplement is unsafe. Once they do, they must have sufficient evidence to meet review by the Office of General Accounting (OGA) and/or legal challenges. In the past, the FDA has acted to remove dietary supplements from the market only to be concluded by the OGA and/or federal courts to have overstepped their authority. Additionally, the FTC has the power to act against companies who make false and/or misleading marketing claims about a specific product. This includes acting against companies if the ingredients found in the supplement do not match label claims or in the event undeclared, drug ingredients are present (e.g., analogs of weight loss drugs, diuretic drugs). While this does not ensure the safety of dietary supplements, it does provide a means for governmental oversight of the dietary supplement industry if adequate resources are provided to enforce DSHEA. Since the inception of DSHEA, the FDA has required a number of supplement companies to submit evidence showing safety of their products and acted to remove a number of products sold as dietary supplements from sale in the United States due to safety concerns. Additionally, the FTC has acted against a number of supplement companies for misleading advertisements and/or structure and function claims.
As demonstrated, while some argue that the dietary supplement industry is "unregulated" and/or may have suggestions for additional regulation, manufacturers of dietary supplements must adhere to a number of federal regulations before a product can go to market. Further, they must have evidence that the ingredients sold in their supplements are generally safe if requested to do so by the FDA. For this reason, over the last 20 years, a number of quality supplement companies have employed research and development directors who help educate the public about nutrition and exercise, provide input on product development, conduct preliminary research on products, and/or assist in coordinating research trials conducted by independent research teams (e.g., university based researchers or clinical research sites). They also consult with marketing and legal teams with the responsibility to ensure structure and function claims do not misrepresent results of research findings. This has increased job opportunities for sports nutrition specialists as well as enhanced external funding opportunities for research groups interested in exercise and nutrition research.
While it is true that a number of companies falsely attribute research on different dietary ingredients or dietary supplements to their own, suppress negative findings, and/or exaggerate results from research studies; the trend in the nutrition industry has been to develop scientifically sound supplements. This trend toward greater research support is the result of: 1.) Attempts to honestly and accurately inform the public about results; 2.) Efforts to have data to support safety and efficacy on products for FDA and the FTC; and/or, 3.) To provide scientific evidence to support advertising claims and increase sales. This trend is due in part to greater scrutiny from the FDA and FTC, but also in response to an increasingly competitive marketplace where established safety and efficacy attracts more consumer loyalty and helps ensure a longer lifespan for the product in commerce. In our experience, companies who adhere to these ethical standards prosper while those who do not struggle to comply with FDA and FTC guidelines and rapidly lose consumer confidence, signaling an early demise for the product.
Product Development and Quality Assurance
One of the most common questions raised by athletes, parents, and professionals regarding dietary supplements relates to how they are manufactured and consumer awareness of supplement quality. In a number of cases, reputable companies who develop dietary supplements have research teams who scour the medical and scientific literature looking for potentially effective nutrients. These research teams often attend scientific meetings and review the latest patents, research abstracts presented at scientific meetings, and research publications. They may also consult with leading researchers to discuss ideas about dietary supplements that can be commercialized. Leading companies invest in basic research on nutrients before developing their supplement formulations. Others wait until research has been presented in patents, research abstracts, or publications before developing nutritional formulations featuring the nutrient. Once a new nutrient or formulation has been identified, the next step is to contact raw ingredient suppliers to see if the nutrient can be obtained in a highly pure source and/or if it's affordable. Sometimes, companies develop and patent new processing and purification processes because the nutrient has not yet been extracted in a pure form or is not available in large quantities. Reputable raw material manufacturers conduct extensive tests to examine purity of their raw ingredients. If the company is working on a new ingredient, they often conduct toxicity studies on the new nutrient once a purified source has been identified. They would then compile a safety dossier and communicate it to the FDA as a New Dietary Ingredient submission, with the hopes of it being allowed for lawful sale.
When a powdered formulation is designed, the list of ingredients and raw materials are typically sent to a flavoring house and packaging company to identify the best way to flavor and package the supplement. In the nutrition industry, there are several main flavoring houses and packaging companies who make a large number of dietary supplements for dietary supplement companies. Most reputable dietary supplement manufacturers submit their production facilities to inspection from the FDA and adhere to good manufacturing practices (GMP's), which represent industry standards for good manufacturing of dietary supplements. Some companies also submit their products for independent testing by third-party companies to certify that their products meet label claims. For example, NSF's certification service includes product testing, GMP inspections, ongoing monitoring and use of the NSF Mark indicating products comply with inspection standards, and screening for contaminants. More recently, companies have subjected their products for testing by third party companies to inspect for banned or unwanted substances. These types of tests help ensure that each batch of the dietary supplement does not contained substances banned by the International Olympic Committee or other athletic governing bodies (e.g., NFL). While third-party testing does not guarantee that a supplement is void of banned substances, the likelihood is much less (e.g., Banned Substances Control Group, Informed Choice, etc). Moreover, consumers can request copies of results of these tests. In our experience, companies who are not willing to provide copies of test results are not worth purchasing.
Evaluation of Nutritional Ergogenic Aids
The ISSN recommends going through a process of evaluating the validity and scientific merit of claims made when assessing the ergogenic value of a dietary supplement/technique [ 3 ]. This can be accomplished by examining the theoretical rationale behind the supplement/technique and determining whether there is any well-controlled data showing the supplement/technique works. Supplements based on sound scientific rationale with direct, supportive research showing effectiveness may be worth trying and/or recommending. However, those based on unsound scientific results and/or little to no data supporting the ergogenic value of the actual supplement/technique may not be worthwhile. The sports nutrition specialist should be a resource to help their clients interpret the scientific and medical research that may impact their welfare and/or help them train more wisely and effectively. The following are recommended questions to ask when evaluating the potential ergogenic value of a supplement.
Does The Theory Make Sense?
Most supplements that have been marketed to improve health and/or exercise performance are based on theoretical applications derived from basic and/or clinical research studies. Based on these preliminary studies, a training device or supplement is often marketed to people proclaiming the benefits observed in these basic research studies. Although the theory may appear relevant, critical analysis of this process often reveals flaws in scientific logic and/or that the claims made don't quite match up with the literature cited. By evaluating the literature on your own you can discern whether a supplement has been based on sound scientific evidence or not. To do so, it is suggested you read reviews about the training method, nutrient, and/or supplement from researchers who have been intimately involved in this line of research and/or consult reliable references about nutritional and herbal supplements, such as the JISSN [ 3 , 5 ]. We also suggest doing a search on the nutrient/supplement on the National Library of Medicine's Pub Med Online http://www.ncbi.nlm.nih.gov . A quick look at these references will often help determine if the theory is plausible or not. In our experience, proponents of ergogenic aids often overstate claims made about training devices and/or dietary supplements while opponents of dietary supplements and ergogenic aids are either unaware and/or ignorant of research supporting their use. The sports nutrition specialist has the responsibility to know the literature and/or search available databases to evaluate whether there is merit or not to a proposed ergogenic aid.
Is There Any Scientific Evidence Supporting The Ergogenic Value?
The next question to ask is whether there is any well-controlled data showing effectiveness of the proposed ergogenic aid works as claimed in athletes or people involved in training. The first place to look is the list of references cited in marketing material supporting their claims. We look to see if the abstracts or articles cited are general references or specific studies that have evaluated the efficacy of the nutrient/supplement. We then critically evaluate the abstracts and articles by asking a series of questions.
Are the studies basic research done in animals/clinical populations or have the studies been conducted on athletes/trained subjects? Studies reporting improved performance in rats or persons with type 2 diabetes may be insightful but research conducted on non-diabetic athletes is much more practical and relevant.
Were the studies well controlled? For ergogenic aid research, the study should be a placebo controlled, double-blind, and randomized clinical trial if possible. This means that neither the researcher's nor the subject's were aware which group received the supplement or the placebo during the study and that the subjects were randomly assigned into the placebo or supplement group. An additional element of rigor is called a cross-over design, where each subject, at different times (separated by an interval known as a "washout period"), is exposed to each of the treatments. While utilization of a cross-over design is not always feasible, it removes the element of variability between subjects and increases the strength of the findings. At times, supplement claims have been based on poorly designed studies (i.e., small groups of subjects, no control group, use of unreliable tests, etc) and/or testimonials which make interpretation much more difficult. Well-controlled clinical trials provide stronger evidence as to the potential ergogenic value.
Do the studies report statistically significant results or are claims being made on non-significant means or trends reported? Appropriate statistical analysis of research results allows for an unbiased interpretation of data. Although studies reporting statistical trends may be of interest and lead researchers to conduct additional research, studies reporting statistically significant results are obviously more convincing. With this said, a sports nutrition specialist must be careful not to commit type II statistical errors (i.e., indicating that no differences were observed when a true effect was seen but not detected statistically). Since many studies on ergogenic aids (particularly in high level athletes) evaluate small numbers of subjects, results may not reach statistical significance even though large mean changes were observed. In these cases, additional research is warranted to further examine the potential ergogenic aid before conclusions can be made.
Do the results of the studies cited match the claims made about the supplement? It is not unusual for marketing claims to greatly exaggerate the results found in the actual studies. Additionally, it is not uncommon for ostensibly compelling results, that may indeed by statistically significant, to be amplified while other relevant findings of significant consumer interest are obscured or omitted (e.g. a dietary supplement showing statistically significant increases in circulating testosterone yet changes in body composition or muscular performance were not superior to a placebo). The only way to determine this is to read the entire article, and not just the abstract or even the article citation, and compare results observed in the studies to marketing claims. Reputable companies accurately and completely report results of studies so that consumers can make informed decisions about whether to try a product or not.
Were results of the study presented at a reputable scientific meeting and/or published in a peer-reviewed scientific journal? At times, claims are based on research that has either never been published or only published in an obscure journal. The best research is typically presented at respected scientific meetings and/or published in reputable peer-reviewed journals. Two ways to determine a journal's reputation is either identifying the publisher or the "impact factor" of the journal. A number of "peer-reviewed" journals are published by companies with ties to, or are actually owned by, nutritional products companies (even though they may be available on PubMed). Therefore, we recommend looking up the publisher's website and see how many other journals they publish. If you see only a few other journals this is a suggestion that the journal is not a reputable journal. Alternatively, inquire about the impact factor, a qualitative ranking determined by the number of times a journal's articles are cited. Impact factors are determined and published by Thomson Reuters under Journal Citation Reports ® (a subscription service available at most university libraries). Most journals list their impact factor on the journal home page. The most significant and erudite scientific articles are typically the most read and the most cited.
Have the research findings been replicated at several different labs? The best way to know an ergogenic aid works is to see that results have been replicated in several studies preferably by a number of separate, distinct research groups. The most reliable ergogenic aids are those in which a number of studies, conducted at different labs, have reported similar results of safety and efficacy. Additionally, replication of results by different, unaffiliated labs with completely different authors also removes or reduces the potentially confounding element of publication bias (publication of studies showing only positive results) and conflicts of interest. A notable number of studies on ergogenic aids are conducted in collaboration with one or more research scientists or co-investigators that have a real or perceived economic interest in the outcome of the study. This could range from being a co-inventor on a patent application that is the subject of the ergogenic aid, being paid or receiving royalties from the creation of a dietary supplement formulation, or having stock options or shares in a company that owns or markets the ergogenic aid described in the study. An increasing number of journals require disclosures by all authors of scientific articles, and including such disclosures in published articles. This is driven by the aim of providing greater transparency and research integrity. Disclosure of a conflict of interest does not alone discredit or dilute the merits of a research study. The primary thrust behind public disclosures of potential conflicts of interest is the prevention of a later revelation of an interest that has the potential of discrediting the study in question, the authors, and even the research center or institution where the study was conducted.
Is The Supplement Legal And Safe?
The final question that should be asked is whether the supplement is legal and/or safe. Some athletic associations have banned the use of various nutritional supplements (e.g., prohormones, Ephedra that contains ephedrine, "muscle building" supplements, etc). Obviously, if the supplement is banned, the sports nutrition specialist should discourage its use. In addition, many supplements have not been studied for long-term safety. People who consider taking nutritional supplements should be well aware of the potential side effects so that they can make an informed decision regarding whether to use a supplement or not. Additionally, they should consult with a knowledgeable physician to see if there are any underlying medical problems that may contraindicate use. When evaluating the safety of a supplement, we suggest looking to see if any side effects have been reported in the scientific or medical literature. In particular, we suggest determining how long a particular supplement has been studied, the dosages evaluated, and whether any side effects were observed. We also recommend consulting the Physician's Desk Reference (PDR) for nutritional supplements and herbal supplements to see if any side effects have been reported and/or if there are any known drug interactions. If no side effects have been reported in the scientific/medical literature, we generally will view the supplement as safe for the length of time and dosages evaluated.
Classifying and Categorizing Supplements
Dietary supplements may contain carbohydrate, protein, fat, minerals, vitamins, herbs, enzymes, metabolic intermediates (like amino acids), and/or various plant/food extracts. Supplements can generally be classified as convenience supplements (e.g., energy bars, meal replacement powders, ready to drink supplements) designed to provide a convenient means of meeting caloric needs and/or managing caloric intake, weight gain, weight loss, and/or performance enhancement. Based on the above criteria, we generally categorize nutritional supplements into the following categories:
Apparently Effective . Supplements that help people meet general caloric needs and/or the majority of research studies in relevant populations show is effective and safe.
Possibly Effective . Supplements with initial studies supporting the theoretical rationale but requiring more research to determine how the supplement may affect training and/or performance.
Too Early To Tell . Supplements with sensible theory but lacking sufficient research to support its current use.
Apparently Ineffective . Supplements that lack a sound scientific rationale and/or research has clearly shown to be ineffective.
When a sports nutrition specialist counsels people who train, they should first evaluate their diet and training program. They should make sure that the athlete is eating an energy balanced, nutrient dense diet and that they are training intelligently. This is the foundation to build a good program. Following this, we suggest that they generally only recommend supplements in category I (i.e., 'Apparently Effective). If someone is interested in trying supplements in category II (i.e., 'Possibly Effective'), they should make sure that they understand that these supplements are more experimental and that they may or may not see the type of results claimed. We recommend discouraging people from trying supplements in category III (i.e., 'Too Early to Tell') because there isn't enough data available on their ergogenic value. However, if someone wants to try one of these supplements, they should understand that although there is some theoretical rationale, there is little evidence to support use at this time. Obviously, we do not support athletes taking supplements in categories IV (i.e., 'Apparently Ineffective'). We believe that this approach is a more scientifically supportable and balanced view than simply dismissing the use of all dietary supplements out of hand.
General Dietary Guidelines for Active Individuals
A well-designed diet that meets energy intake needs and incorporates proper timing of nutrients is the foundation upon which a good training program can be developed. Research has clearly shown that not ingesting a sufficient amount of calories and/or enough of the right type of macronutrients may impede an athlete's training adaptations while athletes who consume a balanced diet that meets energy needs can augment physiological training adaptations. Moreover, maintaining an energy deficient diet during training may lead to loss of muscle mass and strength, increased susceptibility to illness, and increased prevalence of overreaching and/or overtraining. Incorporating good dietary practices as part of a training program is one way to help optimize training adaptations and prevent overtraining. The following overviews energy intake and major nutrient needs of active individuals.
Energy Intake
The first component to optimize training and performance through nutrition is to ensure the athlete is consuming enough calories to offset energy expenditure [ 1 , 6 – 8 ]. People who participate in a general fitness program (e.g., exercising 30 - 40 minutes per day, 3 times per week) can typically meet nutritional needs following a normal diet (e.g., 1,800 - 2,400 kcals/day or about 25 - 35 kcals/kg/day for a 50 - 80 kg individual) because their caloric demands from exercise are not too great (e.g., 200 - 400 kcals/session) [ 1 ]. However, athletes involved in moderate levels of intense training (e.g., 2-3 hours per day of intense exercise performed 5-6 times per week) or high volume intense training (e.g., 3-6 hours per day of intense training in 1-2 workouts for 5-6 days per week) may expend 600 - 1,200 kcals or more per hour during exercise [ 1 , 9 ]. For this reason, their caloric needs may approach 50 - 80 kcals/kg/day (2,500 - 8,000 kcals/day for a 50 - 100 kg athlete). For elite athletes, energy expenditure during heavy training or competition may be enormous. For example, energy expenditure for cyclists to compete in the Tour de France has been estimated as high as 12,000 kcals/day (150 - 200 kcals/kg/d for a 60 - 80 kg athlete) [ 9 – 11 ]. Additionally, caloric needs for large athletes (i.e., 100 - 150 kg) may range between 6,000 - 12,000 kcals/day depending on the volume and intensity of different training phases [ 9 ].
Although some argue that athletes can meet caloric needs simply by consuming a well-balanced diet, it is often very difficult for larger athletes and/or athletes engaged in high volume/intense training to be able to eat enough food in order to meet caloric needs [ 1 , 7 , 9 , 10 , 12 ]. Maintaining an energy deficient diet during training often leads to significant weight loss (including muscle mass), illness, onset of physical and psychological symptoms of overtraining, and reductions in performance [ 8 ]. Nutritional analyses of athletes' diets have revealed that many are susceptible to maintaining negative energy intakes during training. Susceptible populations include runners, cyclists, swimmers, triathletes, gymnasts, skaters, dancers, wrestlers, boxers, and athletes attempting to lose weight too quickly [ 7 ]. Additionally, female athletes have been reported to have a high incidence of eating disorders [ 7 ]. Consequently, it is important for the sports nutrition specialist working with athletes to ensure that athletes are well-fed and consume enough calories to offset the increased energy demands of training, and maintain body weight. Although this sounds relatively simple, intense training often suppresses appetite and/or alters hunger patterns so that many athletes do not feel like eating [ 7 ]. Some athletes do not like to exercise within several hours after eating because of sensations of fullness and/or a predisposition to cause gastrointestinal distress. Further, travel and training schedules may limit food availability and/or the types of food athletes are accustomed to eating. This means that care should be taken to plan meal times in concert with training, as well as to make sure athletes have sufficient availability of nutrient dense foods throughout the day for snacking between meals (e.g., drinks, fruit, carbohydrate/protein bars, etc) [ 1 , 6 , 7 ]. For this reason, sports nutritionists' often recommend that athletes consume 4-6 meals per day and snacks in between meals in order to meet energy needs. Use of nutrient dense energy bars and high calorie carbohydrate/protein supplements provides a convenient way for athletes to supplement their diet in order to maintain energy intake during training.
Carbohydrate
The second component to optimizing training and performance through nutrition is to ensure that athletes consume the proper amounts of carbohydrate (CHO), protein (PRO) and fat in their diet. Individuals engaged in a general fitness program can typically meet macronutrient needs by consuming a normal diet (i.e., 45-55% CHO [3-5 grams/kg/day], 10-15% PRO [0.8 - 1.0 gram/kg/day], and 25-35% fat [0.5 - 1.5 grams/kg/day]). However, athletes involved in moderate and high volume training need greater amounts of carbohydrate and protein in their diet to meet macronutrient needs. For example, in terms of carbohydrate needs, athletes involved in moderate amounts of intense training (e.g., 2-3 hours per day of intense exercise performed 5-6 times per week) typically need to consume a diet consisting of 55-65% carbohydrate (i.e., 5-8 grams/kg/day or 250 - 1,200 grams/day for 50 - 150 kg athletes) in order to maintain liver and muscle glycogen stores [ 1 , 6 ]. Research has also shown that athletes involved in high volume intense training (e.g., 3-6 hours per day of intense training in 1-2 workouts for 5-6 days per week) may need to consume 8-10 grams/day of carbohydrate (i.e., 400 - 1,500 grams/day for 50 - 150 kg athletes) in order to maintain muscle glycogen levels [ 1 , 6 ]. This would be equivalent to consuming 0.5 - 2.0 kg of spaghetti. Preferably, the majority of dietary carbohydrate should come from complex carbohydrates with a low to moderate glycemic index (e.g., whole grains, vegetables, fruit, etc). However, since it is physically difficult to consume that much carbohydrate per day when an athlete is involved in intense training, many nutritionists and the sports nutrition specialist recommend that athletes consume concentrated carbohydrate juices/drinks and/or consume high carbohydrate supplements to meet carbohydrate needs.
While consuming this amount of carbohydrate is not necessary for the fitness minded individual who only trains 3-4 times per week for 30-60 minutes, it is essential for competitive athletes engaged in intense moderate to high volume training. The general consensus in the scientific literature is the body can oxidize 1 - 1.1 gram of carbohydrate per minute or about 60 grams per hour [ 13 ]. The American College of Sports Medicine (ACSM) recommends ingesting 0.7 g/kg/hr during exercise in a 6-8% solution (i.e., 6-8 grams per 100 ml of fluid). Harger-Domitrovich et al [ 14 ] reported that 0.6 g/kg/h of maltodextrin optimized carbohydrate utilization [ 14 ]. This would be about 30 - 70 grams of CHO per hour for a 50 - 100 kg individual [ 15 – 17 ]. Studies also indicate that ingestion of additional amounts of carbohydrate does not further increase carbohydrate oxidation.
It should also be noted that exogenous carbohydrate oxidation rates have been shown to differ based on the type of carbohydrate consumed because they are taken up by different transporters [ 18 – 20 ]. For example, oxidation rates of disaccharides and polysaccharides like sucrose, maltose, and maltodextrins are high while fructose, galactose, trehalose, and isomaltulose are lower [ 21 , 22 ]. Ingesting combinations of glucose and sucrose or maltodextrin and fructose have been reported to promote greater exogenous carbohydrate oxidation than other forms of carbohydrate [ 18 – 26 ]. These studies generally indicate a ratio of 1-1.2 for maltodextrin to 0.8-1.0 fructose. For this reason, we recommend that care should be taken to consider the type of carbohydrate to ingest prior to, during, and following intense exercise in order to optimize carbohydrate availability.
There has been considerable debate regarding protein needs of athletes [ 27 – 31 ]. Initially, it was recommended that athletes do not need to ingest more than the RDA for protein (i.e., 0.8 to 1.0 g/kg/d for children, adolescents and adults). However, research over the last decade has indicated that athletes engaged in intense training need to ingest about two times the RDA of protein in their diet (1.5 to 2.0 g/kg/d) in order to maintain protein balance [ 27 , 28 , 30 , 32 , 33 ]. If an insufficient amount of protein is obtained from the diet, an athlete will maintain a negative nitrogen balance, which can increase protein catabolism and slow recovery. Over time, this may lead to muscle wasting and training intolerance [ 1 , 8 ].
For people involved in a general fitness program, protein needs can generally be met by ingesting 0.8 - 1.0 grams/kg/day of protein. Older individuals may also benefit from a higher protein intake (e.g., 1.0 - 1.2 grams/kg/day of protein) in order to help prevent sarcopenia. It is recommended that athletes involved in moderate amounts of intense training consume 1 - 1.5 grams/kg/day of protein (50 - 225 grams/day for a 50 - 150 kg athlete) while athletes involved in high volume intense training consume 1.5 - 2.0 grams/kg/day of protein (75 - 300 grams/day for a 50 - 150 kg athlete) [ 34 ]. This protein need would be equivalent to ingesting 3 - 11 servings of chicken or fish per day for a 50 - 150 kg athlete [ 34 ]. Although smaller athletes typically can ingest this amount of protein in their normal diet, larger athletes often have difficulty consuming this much dietary protein. Additionally, a number of athletic populations have been reported to be susceptible to protein malnutrition (e.g., runners, cyclists, swimmers, triathletes, gymnasts, dancers, skaters, wrestlers, boxers, etc). Therefore, care should be taken to ensure that athletes consume a sufficient amount of quality protein in their diet in order to maintain nitrogen balance (e.g., 1.5 - 2 grams/kg/day).
However, it should be noted that not all protein is the same. Proteins differ based on the source that the protein was obtained, the amino acid profile of the protein, and the methods of processing or isolating the protein [ 35 ]. These differences influence availability of amino acids and peptides that have been reported to possess biological activity (e.g., α-lactalbumin, β-lactoglobulin, glycomacropeptides, immunoglobulins, lactoperoxidases, lactoferrin, etc). Additionally, the rate of digestion and/or absorption and metabolic activity of the protein also are important considerations [ 35 ]. For example, different types of proteins (e.g., casein and whey) are digested at different rates, which directly affect whole body catabolism and anabolism [ 35 – 38 ]. Therefore, care should be taken not only to make sure the athlete consumes enough protein in their diet but also that the protein is high quality. The best dietary sources of low fat, high quality protein are light skinless chicken, fish, egg white and skim milk (casein and whey) [ 35 ]. The best sources of high quality protein found in nutritional supplements are whey, colostrum, casein, milk proteins and egg protein [ 34 , 35 ]. Although some athletes may not need to supplement their diet with protein and some sports nutrition specialists may not think that protein supplements are necessary, it is common for a sports nutrition specialist to recommend that some athletes supplement their diet with protein in order to meet dietary protein needs and/or provide essential amino acids following exercise in order to optimize protein synthesis.
The ISSN has recently adopted a position stand on protein that highlights the following points [ 39 ]:
Exercising individuals need approximately 1.4 to 2.0 grams of protein per kilogram of bodyweight per day.
Concerns that protein intake within this range is unhealthy are unfounded in healthy, exercising individuals.
An attempt should be made to obtain protein requirements from whole foods, but supplemental protein is a safe and convenient method of ingesting high quality dietary protein.
The timing of protein intake in the time period encompassing the exercise session has several benefits including improved recovery and greater gains in fat free mass.
Protein residues such as branched chain amino acids have been shown to be beneficial for the exercising individual, including increasing the rates of protein synthesis, decreasing the rate of protein degradation, and possibly aiding in recovery from exercise.
Exercising individuals need more dietary protein than their sedentary counterparts
The dietary recommendations of fat intake for athletes are similar to or slightly greater than those recommended for non-athletes in order to promote health. Maintenance of energy balance, replenishment of intramuscular triacylglycerol stores and adequate consumption of essential fatty acids are of greater importance among athletes and allow for somewhat increased intake [ 40 ]. This depends on the athlete's training state and goals. For example, higher-fat diets appear to maintain circulating testosterone concentrations better than low-fat diets [ 41 – 43 ]. This has relevance to the documented testosterone suppression which can occur during volume-type overtraining [ 44 ]. Generally, it is recommended that athletes consume a moderate amount of fat (approximately 30% of their daily caloric intake), while increases up to 50% of kcal can be safely ingested by athletes during regular high-volume training [ 40 ]. For athletes attempting to decrease body fat, however, it has been recommended that they consume 0.5 to 1 g/kg/d of fat [ 1 ]. The reason for this is that some weight loss studies indicate that people who are most successful in losing weight and maintaining the weight loss are those who ingest less than 40 g/d of fat in their diet [ 45 , 46 ] although this is not always the case [ 47 ]. Certainly, the type of dietary fat (e.g. n-6 versus n-3; saturation state) is a factor in such research and could play an important role in any discrepancies [ 48 , 49 ]. Strategies to help athletes manage dietary fat intake include teaching them which foods contain various types of fat so that they can make better food choices and how to count fat grams [ 1 , 7 ].
Strategic Eating and Refueling
In addition to the general nutritional guidelines described above, research has also demonstrated that timing and composition of meals consumed may play a role in optimizing performance, training adaptations, and preventing overtraining [ 1 , 6 , 33 , 50 ]. In this regard, it takes about 4 hours for carbohydrate to be digested and begin being stored as muscle and liver glycogen. Consequently, pre-exercise meals should be consumed about 4 to 6 h before exercise [ 6 ]. This means that if an athlete trains in the afternoon, breakfast is the most important meal to top off muscle and liver glycogen levels. Research has also indicated that ingesting a light carbohydrate and protein snack 30 to 60 min prior to exercise (e.g., 50 g of carbohydrate and 5 to 10 g of protein) serves to increase carbohydrate availability toward the end of an intense exercise bout [ 51 , 52 ]. This also serves to increase availability of amino acids and decrease exercise-induced catabolism of protein [ 33 , 51 , 52 ].
When exercise lasts more than one hour, athletes should ingest glucose/electrolyte solution (GES) drinks in order to maintain blood glucose levels, help prevent dehydration, and reduce the immunosuppressive effects of intense exercise [ 6 , 53 – 58 ]. Following intense exercise, athletes should consume carbohydrate and protein (e.g., 1 g/kg of carbohydrate and 0.5 g/kg of protein) within 30 min after exercise as well as consume a high carbohydrate meal within two hours following exercise [ 1 , 31 , 50 ]. This nutritional strategy has been found to accelerate glycogen resynthesis as well as promote a more anabolic hormonal profile that may hasten recovery [ 59 – 61 ]. Finally, for 2 to 3 days prior to competition, athletes should taper training by 30 to 50% and consume 200 to 300 g/d of extra carbohydrate in their diet. This carbohydrate loading technique has been shown to supersaturate carbohydrate stores prior to competition and improve endurance exercise capacity [ 1 , 6 , 50 ]. Thus, the type of meal and timing of eating are important factors in maintaining carbohydrate availability during training and potentially decreasing the incidence of overtraining. The ISSN has a adopted a position stand on nutrient timing [ 13 ] that was summarized with the following points:
Prolonged exercise (> 60 - 90 min) of moderate to high intensity exercise will deplete the internal stores of energy, and prudent timing of nutrient delivery can help offset these changes.
During intense exercise, regular consumption (10 - 15 fl oz.) of a carbohydrate/electrolyte solution delivering 6 - 8% CHO (6 - 8 g CHO/100 ml fluid) should be consumed every 15 - 20 min to sustain blood glucose levels.
Glucose, fructose, sucrose and other high-glycemic CHO sources are easily digested, but fructose consumption should be minimized as it is absorbed at a slower rate and increases the likelihood of gastrointestinal problems.
The addition of PRO (0.15 - 0.25 g PRO/kg/day) to CHO at all time points, especially post-exercise, is well tolerated and may promote greater restoration of muscle glycogen when carbohydrate intakes are suboptimal.
Ingestion of 6 - 20 grams of essential amino acids (EAA) and 30 - 40 grams of high-glycemic CHO within three hours after an exercise bout and immediately before exercise has been shown to significantly stimulate muscle PRO synthesis.
Daily post-exercise ingestion of a CHO + PRO supplement promotes greater increases in strength and improvements in lean tissue and body fat % during regular resistance training.
Milk PRO sources (e.g. whey and casein) exhibit different kinetic digestion patterns and may subsequently differ in their support of training adaptations.
Addition of creatine monohydrate to a CHO + PRO supplement in conjunction with regular resistance training facilitates greater improvements in strength and body composition as compared with when no creatine is consumed.
Dietary focus should center on adequate availability and delivery of CHO and PRO. However, including small amounts of fat does not appear to be harmful, and may help to control glycemic responses during exercise.
Irrespective of timing, regular ingestion of snacks or meals providing both CHO and PRO (3:1 CHO: PRO ratio) helps to promote recovery and replenishment of muscle glycogen when lesser amounts of carbohydrate are consumed.
Vitamins are essential organic compounds that serve to regulate metabolic processes, energy synthesis, neurological processes, and prevent destruction of cells. There are two primary classifications of vitamins: fat and water soluble. The fat soluble vitamins include vitamins A, D, E, & K. The body stores fat soluble vitamins and therefore excessive intake may result in toxicity. Water soluble vitamins are B vitamins and vitamin C. Since these vitamins are water soluble, excessive intake of these vitamins are eliminated in urine, with few exceptions (e.g. vitamin B6, which can cause peripheral nerve damage when consumed in excessive amounts). Table 1 describes RDA, proposed ergogenic benefit, and summary of research findings for fat and water soluble vitamins. Although research has demonstrated that specific vitamins may possess some health benefit (e.g., Vitamin E, niacin, folic acid, vitamin C, etc), few have been reported to directly provide ergogenic value for athletes. However, some vitamins may help athletes tolerate training to a greater degree by reducing oxidative damage (Vitamin E, C) and/or help to maintain a healthy immune system during heavy training (Vitamin C). Theoretically, this may help athletes tolerate heavy training leading to improved performance. The remaining vitamins reviewed appear to have little ergogenic value for athletes who consume a normal, nutrient dense diet. Since dietary analyses of athletes have found deficiencies in caloric and vitamin intake, many sports nutritionists' recommend that athletes consume a low-dose daily multivitamin and/or a vitamin enriched post-workout carbohydrate/protein supplement during periods of heavy training. An article in the Journal of the American Medical Association also recently evaluated the available medical literature and recommended that Americans consume a one-a-day low-dose multivitamin in order to promote general health. Suggestions that there is no benefit of vitamin supplementation for athletes and/or it is unethical for an sports nutrition specialist to recommend that their clients take a one-a-day multi-vitamin and/or suggest taking other vitamins that may raise HDL cholesterol levels and decrease risk of heart disease (niacin), serve as antioxidants (Vitamin E), preserve musculoskeletal function and skeletal mass (vitamin D), or may help maintain a health immune system (Vitamin C) is not consistent with current available literature.
Minerals are essential inorganic elements necessary for a host of metabolic processes. Minerals serve as structure for tissue, important components of enzymes and hormones, and regulators of metabolic and neural control. Some minerals have been found to be deficient in athletes or become deficient in response to training and/or prolonged exercise. When mineral status is inadequate, exercise capacity may be reduced. Dietary supplementation of minerals in deficient athletes has generally been found to improve exercise capacity. Additionally, supplementation of specific minerals in non-deficient athletes has also been reported to affect exercise capacity. Table 2 describes minerals that have been purported to affect exercise capacity in athletes. Of the minerals reviewed, several appear to possess health and/or ergogenic value for athletes under certain conditions. For example, calcium supplementation in athletes susceptible to premature osteoporosis may help maintain bone mass. There is also recent evidence that dietary calcium may help manage body composition. Iron supplementation in athletes prone to iron deficiencies and/or anaemia has been reported to improve exercise capacity. Sodium phosphate loading has been reported to increase maximal oxygen uptake, anaerobic threshold, and improve endurance exercise capacity by 8 to 10%. Increasing dietary availability of salt (sodium chloride) during the initial days of exercise training in the heat has been reported to help maintain fluid balance and prevent dehydration. ACSM recommendations for sodium levels (340 mg) represent the amount of sodium in less than 1/8 teaspoon of salt and meet recommended guidelines for sodium ingestion during exercise (300 - 600 mg per hour or 1.7 - 2.9 grams of salt during a prolonged exercise bout) [ 62 – 65 ]. Finally, zinc supplementation during training has been reported to decrease exercise-induced changes in immune function. Consequently, somewhat in contrast to vitamins, there appear to be several minerals that may enhance exercise capacity and/or training adaptations for athletes under certain conditions. However, although ergogenic value has been purported for remaining minerals, there is little evidence that boron, chromium, magnesium, or vanadium affect exercise capacity or training adaptations in healthy individuals eating a normal diet. Suggestions that there is no benefit of mineral supplementation for athletes and/or it is unethical for a sports nutrition specialist to recommend that their clients take minerals for health and/or performance benefit is not consistent with current available literature.
The most important nutritional ergogenic aid for athletes is water. Exercise performance can be significantly impaired when 2% or more of body weight is lost through sweat. For example, when a 70-kg athlete loses more than 1.4 kg of body weight during exercise (2%), performance capacity is often significantly decreased. Further, weight loss of more than 4% of body weight during exercise may lead to heat illness, heat exhaustion, heat stroke, and possibly death [ 58 ]. For this reason, it is critical that athletes consume a sufficient amount of water and/or GES sports drinks during exercise in order to maintain hydration status. The normal sweat rate of athletes ranges from 0.5 to 2.0 L/h depending on temperature, humidity, exercise intensity, and their sweat response to exercise [ 58 ]. This means that in order to maintain fluid balance and prevent dehydration, athletes need to ingest 0.5 to 2 L/h of fluid in order to offset weight loss. This requires frequent ingestion of 6-8 oz of cold water or a GES sports drink every 5 to 15-min during exercise [ 58 , 66 – 69 ]. Athletes and should not depend on thirst to prompt them to drink because people do not typically get thirsty until they have lost a significant amount of fluid through sweat. Additionally, athletes should weigh themselves prior to and following exercise training to ensure that they maintain proper hydration [ 58 , 66 – 69 ]. The athlete should consume 3 cups of water for every pound lost during exercise in order adequately rehydrate themselves [ 58 ]. Athletes should train themselves to tolerate drinking greater amounts of water during training and make sure that they consume more fluid in hotter/humid environments. Preventing dehydration during exercise is one of the most effective ways to maintain exercise capacity. Finally, inappropriate and excessive weight loss techniques (e.g., cutting weight in saunas, wearing rubber suits, severe dieting, vomiting, using diuretics, etc) are extremely dangerous and should be prohibited. Sports nutrition specialists can play an important role in educating athletes and coaches about proper hydration methods and supervising fluid intake during training and competition.
Dietary Supplements and Athletes
Most of the work we do with athletes regarding sports nutrition is to teach them and their coaches how to structure their diet and time food intake to optimize performance and recovery. Dietary supplements can play a meaningful role in helping athletes consume the proper amount of calories, carbohydrate, and protein in their diet. However, they should be viewed as supplements to the diet, not replacements for a good diet. While it is true that most dietary supplements available for athletes have little scientific data supporting their potential role to enhance training and/or performance, it is also true that a number of nutrients and/or dietary supplements have been shown to help improve performance and/or recovery. Supplementation with these nutrients can help augment the normal diet to help optimize performance. Sports nutrition specialists must be aware of the current data regarding nutrition, exercise, and performance and be honest about educating their clients about results of various studies (whether pro or con). With the proliferation of information available about nutritional supplements to the consumer, the sports nutrition specialist, nutritionist, and nutrition industry lose credibility when they do not accurately describe results of various studies to the public. The following outlines several classifications of nutritional supplements that are often taken by athletes and categorizes them into 'apparently effective', 'possibly effective', 'too early to tell', and 'apparently ineffective' supplements based on interpretation of the literature. It should be noted that this analysis focuses primarily on whether the proposed nutrient has been found to affect exercise and/or training adaptations based on the current available literature. Additional research may or may not reveal ergogenic value, possibly altering its classification. It should be also noted that although there may be little ergogenic value to some nutrients, there may be some potential health benefits that may be helpful for some populations. Therefore, just because a nutrient does not appear to affect performance and/or training adaptations, that does not mean it does not have possible health benefits for athletes.
Convenience Supplements
Convenience supplements are meal replacement powders (MRP's), ready to drink supplements (RTD's), energy bars, and energy gels. They currently represent the largest segment of the dietary supplement industry representing 50 - 75% of most company's sales. They are typically fortified with vitamins and minerals and differ on the amount of carbohydrate, protein, and/or fat they contain. They may also vary based whether they are fortified with various nutrients purported to promote weight gain, enhance weight loss, and/or improve performance. Most people view these supplements as a nutrient dense snack and/or use them to help control caloric intake when trying to gain and/or lose weight. In our view, MRP's, RTD's, and energy bars/gels can provide a convenient way for people to meet specific dietary needs and/or serve as good alternatives to fast food other foods of lower nutritional value. Use of these types of products can be particularly helpful in providing carbohydrate, protein, and other nutrients prior to and/or following exercise in an attempt to optimize nutrient intake when an athlete doesn't have time to sit down for a good meal or wants to minimize food volume. However, they should be used to improve dietary availability of macronutrients - not as a replacement for a good diet. Care should also be taken to make sure they do not contain any banned or prohibited nutrients.
Muscle Building Supplements
The following provides an analysis of the literature regarding purported weight gain supplements and our general interpretation of how they should be categorized based on this information. Table 3 summarizes how we currently classify the ergogenic value of a number of purported performance-enhancing, muscle building, and fat loss supplements based on an analysis of the available scientific evidence.
Apparently Effective
Weight gain powders.
One of the most common means athletes have employed to increase muscle mass is to add extra calories to the diet. Most athletes "bulk up" in this manner by consuming extra food and/or weight gain powders. In order to increase skeletal muscle mass, there must be adequate energy intake (anabolic reactions are endergonic and therefore require adequate energy intake). Studies have consistently shown that simply adding an extra 500 - 1,000 calories per day to your diet in conjunction with resistance training will promote weight gain [ 31 , 33 ]. However, only about 30 - 50% of the weight gained on high calorie diets is muscle while the remaining amount of weight gained is fat. Consequently, increasing muscle mass by ingesting a high calorie diet can help build muscle but the accompanying increase in body fat may not be desirable for everyone. Therefore, we typically do not recommend this type of weight gain approach [ 39 ].
Creatine monohydrate
In our view, the most effective nutritional supplement available to athletes to increase high intensity exercise capacity and muscle mass during training is creatine monohydrate. Numerous studies have indicated that creatine supplementation increases body mass and/or muscle mass during training [ 70 ] Gains are typically 2 - 5 pounds greater than controls during 4 - 12 weeks of training [ 71 ]. The gains in muscle mass appear to be a result of an improved ability to perform high intensity exercise enabling an athlete to train harder and thereby promote greater training adaptations and muscle hypertrophy [ 72 – 75 ]. The only clinically significant side effect occasionally reported from creatine monohydrate supplementation has been the potential for weight gain [ 71 , 76 – 78 ] Although concerns have been raised about the safety and possible side effects of creatine supplementation [ 79 , 80 ], recent long-term safety studies have reported no apparent side effects [ 78 , 81 , 82 ] and/or that creatine monohydrate may lessen the incidence of injury during training [ 83 – 85 ]. Additionally a recent review was published which addresses some of the concerns and myths surrounding creatine monohydrate supplementation [ 86 ]. Consequently, supplementing the diet with creatine monohydrate and/or creatine containing formulations seems to be a safe and effective method to increase muscle mass. The ISSN position stand on creatine monohydrate [ 87 ] summarizes their findings as this:
Creatine monohydrate is the most effective ergogenic nutritional supplement currently available to athletes in terms of increasing high-intensity exercise capacity and lean body mass during training.
Creatine monohydrate supplementation is not only safe, but possibly beneficial in regard to preventing injury and/or management of select medical conditions when taken within recommended guidelines.
There is no compelling scientific evidence that the short- or long-term use of creatine monohydrate has any detrimental effects on otherwise healthy individuals.
If proper precautions and supervision are provided, supplementation in young athletes is acceptable and may provide a nutritional alternative to potentially dangerous anabolic drugs.
At present, creatine monohydrate is the most extensively studied and clinically effective form of creatine for use in nutritional supplements in terms of muscle uptake and ability to increase high-intensity exercise capacity.
The addition of carbohydrate or carbohydrate and protein to a creatine supplement appears to increase muscular retention of creatine, although the effect on performance measures may not be greater than using creatine monohydrate alone.
The quickest method of increasing muscle creatine stores appears to be to consume ~0.3 grams/kg/day of creatine monohydrate for at least 3 days followed by 3-5 g/d thereafter to maintain elevated stores. Ingesting smaller amounts of creatine monohydrate (e.g., 2-3 g/d) will increase muscle creatine stores over a 3-4 week period, however, the performance effects of this method of supplementation are less supported.
Creatine monohydrate has been reported to have a number of potentially beneficial uses in several clinical populations, and further research is warranted in these areas.
As previously described, research has indicated that people undergoing intense training may need additional protein in their diet to meet protein needs (i.e., 1.4 - 2.0 grams/day [ 13 , 39 ] . People who do not ingest enough protein in their diet may exhibit slower recovery and training adaptations [ 33 ]. Protein supplements offer a convenient way to ensure that athletes consume quality protein in the diet and meet their protein needs. However, ingesting additional protein beyond that necessary to meet protein needs does not appear to promote additional gains in strength and muscle mass. The research focus over recent years has been to determine whether different types of protein (e.g., whey, casein, soy, milk proteins, colostrum, etc) and/or various biologically active protein subtypes and peptides (e.g., α-lactalbumin, β-lactoglobulin, glycomacropeptides, immunoglobulins, lactoperoxidases, lactoferrin, etc) have varying effects on the physiological, hormonal, and/or immunological responses to training [ 88 – 91 ]. In addition, a significant amount of research has examined whether timing of protein intake and/or provision of specific amino acids may play a role in protein synthesis and/or training adaptations, conducted mostly in untrained populations [ 92 – 105 ]. Although more research is necessary in this area, evidence clearly indicates that protein needs of individuals engaged in intense training are elevated, different types of protein have varying effects on anabolism and catabolism, that different types of protein subtypes and peptides have unique physiological effects, and timing of protein intake may play an important role in optimizing protein synthesis following exercise. Therefore, it is simplistic and misleading to suggest that there is no data supporting contentions that athletes need more protein in their diet and/or there is no potential ergogenic value of incorporating different types of protein into the diet. It is the position stand of ISSN that exercising individuals need approximately 1.4 to 2.0 grams of protein per kilogram of bodyweight per day. This is greater than the RDA recommendations for sedentary individuals. According to the current literature we know that the addition of protein and or BCAA before or after resistance training can increase protein synthesis and gains in lean mass beyond normal adaptation. However, it should be noted that gains have primarily been observed in untrained populations unless the supplement contained other nutrients like creatine monohydrate [ 13 , 39 ].
Essential Amino Acids (EAA)
Recent studies have indicated that ingesting 3 to 6 g of EAA prior to [ 105 , 106 ] and/or following exercise stimulates protein synthesis [ 92 , 93 , 98 – 101 , 105 ]. Theoretically, this may enhance gains in muscle mass during training. To support this theory, a study by Esmarck and colleagues [ 107 ] found that ingesting EAA with carbohydrate immediately following resistance exercise promoted significantly greater training adaptations in elderly, untrained men, as compared to waiting until 2-hours after exercise to consume the supplement. Although more data is needed, there appears to be strong theoretical rationale and some supportive evidence that EAA supplementation may enhance protein synthesis and training adaptations. Because EAA's include BCAA's, it is probable that positive effects on protein synthesis from EAA ingestion are likely due to the BCAA content [ 108 , 109 ]. Garlick and Grant [ 109 ] infused glucose into growing rats to achieve a concentration of insulin secretion that was insufficient to stimulate protein synthesis by itself. In addition to this, all eight essential amino acids with glucose was infused into another group and then in a third group the investigators only infused the BCAA's along with the glucose. Compared with the glucose infusion alone, protein synthesis was stimulated equally by the essential amino acids and the BCAAs. This demonstrates that the BCAAs are the key amino acids that stimulate protein synthesis. The ISSN position stand on protein concluded that BCAAs have been shown to acutely stimulate protein synthesis, aid in glycogen resynthesis, delaying the onset of fatigue, and help maintain mental function in aerobic-based exercise. It was concluded that consuming BCAAs (in addition to carbohydrates) before, during, and following an exercise bout would be recommended safe and effective [ 39 ].
Possibly Effective
Β-hydroxy β-methylbutyrate (hmb).
HMB is a metabolite of the amino acid leucine. Leucine and metabolites of leucine have been reported to inhibit protein degradation [ 110 ]. Supplementing the diet with 1.5 to 3 g/d of calcium HMB during training has been typically reported to increase muscle mass and strength particularly among untrained subjects initiating training [ 111 – 116 ] and the elderly [ 117 ]. Gains in muscle mass are typically 0.5 to 1 kg greater than controls during 3 - 6 weeks of training. There is also evidence that HMB may lessen the catabolic effects of prolonged exercise [ 118 , 119 ] and that there may be additive effects of co-ingesting HMB with creatine [ 120 , 121 ]. However, the effects of HMB supplementation in athletes are less clear. Most studies conducted on trained subjects have reported non-significant gains in muscle mass possibly due to a greater variability in response of HMB supplementation among athletes [ 122 – 124 ]. Consequently, there is fairly good evidence showing that HMB may enhance training adaptations in individuals initiating training. However, additional research is necessary to determine whether HMB may enhance training adaptations in trained athletes.
Branched Chain Amino Acids (BCAA)
BCAA supplementation has been reported to decrease exercise-induced protein degradation and/or muscle enzyme release (an indicator of muscle damage) possibly by promoting an anti-catabolic hormonal profile [ 31 , 51 , 125 ]. Theoretically, BCAA supplementation during intense training may help minimize protein degradation and thereby lead to greater gains in fat-free mass. There is some evidence to support this hypothesis. For example, Schena and colleagues [ 126 ] reported that BCAA supplementation (~10 g/d) during 21-days of trekking at altitude increased fat free mass (1.5%) while subjects ingesting a placebo had no change in muscle mass. Bigard and associates [ 127 ] reported that BCAA supplementation appeared to minimize loss of muscle mass in subjects training at altitude for 6-weeks. Finally, Candeloro and coworkers [ 128 ] reported that 30 days of BCAA supplementation (14 grams/day) promoted a significant increase in muscle mass (1.3%) and grip strength (+8.1%) in untrained subjects. A recent published abstract [ 129 ] reported that resistance trained subjects ingesting 14 grams of BCAA during 8 weeks of resistance training experienced a significantly greater gain in body weight and lean mass as compared to a whey protein supplemented group and a carbohydrate placebo group. Specifically, the BCAA group gained 2 kg of body mass and 4 kg of lean body mass. In contrast, the whey protein and carbohydrate groups both gained an additional 1 kg of body mass and 2 kg and 1 kg of lean body mass, respectively. It cannot be overstated that this investigation was published as an abstract, was conducted in a gym setting, and has not undergone the rigors of peer review at this time. Although more research is necessary, these findings suggest that BCAA supplementation may have some impact on body composition.
Too Early to Tell
Α-ketoglutarate (α-kg).
α-KG is an intermediate in the Krebs cycle that is involved in aerobic energy metabolism. There is some clinical evidence that α-KG may serve as an anticatabolic nutrient after surgery [ 130 , 131 ]. However, it is unclear whether α-KG supplementation during training may affect training adaptations.
α-Ketoisocaproate (KIC)
KIC is a branched-chain keto acid that is a metabolite of leucine metabolism. In a similar manner as HMB, leucine and metabolites of leucine are believed to possess anticatabolic properties [ 132 ]. There is some clinical evidence that KIC may spare protein degradation in clinical populations [ 133 , 134 ]. Theoretically, KIC may help minimize protein degradation during training possibly leading to greater training adaptations. However, we are not aware of any studies that have evaluated the effects of KIC supplementation during training on body composition.
Ecdysterones
Ecdysterones (also known as ectysterone, 20 Beta-Hydroxyecdysterone, turkesterone, ponasterone, ecdysone, or ecdystene) are naturally derived phytoecdysteroids (i.e., insect hormones). They are typically extracted from the herbs Leuza rhaptonticum sp., Rhaponticum carthamoides , or Cyanotis vaga . They can also be found in high concentrations in the herb Suma (also known as Brazilian Ginseng or Pfaffia). Research from Russia and Czechoslovakia conducted over the last 30 years indicates that ecdysterones may possess some potentially beneficial physiological effects in insects and animals [ 135 – 140 ]. However, since most of the data on ecdysterones have been published in obscure journals, results are difficult to interpret. Wilborn and coworkers [ 141 ] gave resistance trained males 200 mg of 20-hydroxyecdysone per day during 8-weeks of resistance training. It was reported that the 20-hydroxyecdysone supplementation had no effect on fat free mass or anabolic/catabolic hormone status [ 141 ]. Due to the findings of this well controlled study in humans, ecdysterone supplementation at a dosage of 200 mg per day appears to be ineffective in terms of improving lean muscle mass. While future studies may find some ergogenic value of ecdysterones, it is our view that it is too early to tell whether phytoecdysteroids serve as a safe and effective nutritional supplement for athletes.
Growth Hormone Releasing Peptides (GHRP) and Secretagogues
Research has indicated that growth hormone releasing peptides (GHRP) and other non-peptide compounds (secretagogues) appear to help regulate growth hormone (GH) release [ 142 , 143 ]. These observations have served as the basis for development of nutritionally-based GH stimulators (e.g., amino acids, pituitary peptides, "pituitary substances", Macuna pruriens , broad bean, alpha-GPC, etc). Although there is clinical evidence that pharmaceutical grade GHRP's and some non-peptide secretagogues can increase GH and IGF-1 levels at rest and in response to exercise, it has not been demonstrated that such increases lead to an increase in skeletal muscle mass [ 144 ].
Ornithine-α-ketoglutarate (OKG)
OKG (via enteral feeding) has been shown to significantly shorten wound healing time and improve nitrogen balance in severe burn patients [ 145 , 146 ]. Because of its ability to improve nitrogen balance, OKG may provide some value for athletes engaged in intense training. A study by Chetlin and colleagues [ 147 ] reported that OKG supplementation (10 grams/day) during 6-weeks of resistance training promoted greater gains in bench press. However, no significant differences were observed in squat strength, training volume, gains in muscle mass, or fasting insulin and growth hormone. Therefore, additional research is needed before conclusions can be drawn.
Zinc/Magnesium Aspartate (ZMA)
The main ingredients in ZMA formulations are zinc monomethionine aspartate, magnesium aspartate, and vitamin B-6. The rationale of ZMA supplementation is based on studies suggesting that zinc and magnesium deficiency may reduce the production of testosterone and insulin like growth factor (IGF-1). ZMA supplementation has been theorized to increase testosterone and IGF-1 leading to greater recovery, anabolism, and strength during training. In support of this theory, Brilla and Conte [ 148 ] reported that a zinc-magnesium formulation increased testosterone and IGF-1 (two anabolic hormones) leading to greater gains in strength in football players participating in spring training. In another study conducted by Wilborn et al. [ 149 ], resistance trained males ingested a ZMA supplement and found no such increases in either total or free testosterone. In addition, this investigation also assessed changes in fat free mass and no significant differences were observed in relation to fat free mass in those subjects taking ZMA. The discrepancies concerning the two aforementioned studies may be explained by deficiencies of these minerals. Due to the role that zinc deficiency plays relative to androgen metabolism and interaction with steroid receptors [ 150 ], when there are deficiencies of this mineral, testosterone production may suffer. In the study showing increases in testosterone levels [ 148 ], there were depletions of zinc and magnesium in the placebo group over the duration of the study. Hence, increases in testosterone levels could have been attributed to impaired nutritional status rather than a pharmacologic effect. More research is needed to further evaluate the role of ZMA on body composition and strength during training before definitive conclusions can be drawn.
Apparently Ineffective
Glutamine is the most plentiful non-essential amino acid in the body and plays a number of important physiological roles [ 31 , 108 , 109 ] Glutamine has been reported to increase cell volume and stimulate protein [ 151 , 152 ] and glycogen synthesis [ 153 ]. Despite its important role in physiological roles, there is no compelling evidence to support glutamine supplementation in terms of increasing lean body mass. One study that is often cited in support of glutamine supplementation and its role in increasing muscle mass was published by Colker and associates [ 154 ]. It was reported that subjects who supplemented their diet with glutamine (5 grams) and BCAA (3 grams) enriched whey protein during training promoted about a 2 pound greater gain in muscle mass and greater gains in strength than ingesting whey protein alone. While a 2 pound increase in lean body mass was observed, it is likely that these gains were due to the BCAAs that were added to the whey protein. In a well-designed investigation, Candow and co-workers [ 155 ] studied the effects of oral glutamine supplementation combined with resistance training in young adults. Thirty-one participants were randomly allocated to receive either glutamine (0.9 g/kg of lean tissue mass) or a maltodextrin placebo (0.9 g/kg of lean tissue mass) during 6 weeks of total body resistance training. At the end of the 6-week intervention, the authors concluded glutamine supplementation during resistance training had no significant effect on muscle performance, body composition or muscle protein degradation in young healthy adults. While there may be other beneficial uses for glutamine supplementation, there does not appear to be any scientific evidence that it supports increases in lean body mass or muscular performance.
Smilax officinalis (SO)
SO is a plant that contains plant sterols purported to enhance immunity as well as provide an androgenic effect on muscle growth [ 1 ]. Some data supports the potential immune enhancing effects of SO. However, we are not aware of any data that show that SO supplementation increases muscle mass during training.
Isoflavones
Isoflavones are naturally occurring non-steroidal phytoestrogens that have a similar chemical structure as ipriflavone (a synthetic flavonoid drug used in the treatment of osteoporosis) [ 156 – 158 ]. For this reason, soy protein (which is an excellent source of isoflavones) and isoflavone extracts have been investigated in the possible treatment of osteoporosis. Results of these studies have shown promise in preventing declines in bone mass in post-menopausal women as well as reducing risks to side effects associated with estrogen replacement therapy. More recently, the isoflavone extracts 7-isopropoxyisoflavone (ipriflavone) and 5-methyl-7-methoxy-isoflavone (methoxyisoflavone) have been marketed as "powerful anabolic" substances. These claims have been based on research described in patents filed in Hungary in the early 1970s [ 159 , 160 ]. Aubertin-Leheudre M, et al. [ 161 ] investigated the effects that isoflavone supplementation would have on fat-free mass in obese, sarcopenic postmenopausal women. Eighteen sarcopenic-obese women ingested 70 mg of isoflavones per day (44 mg of daidzein, 16 mg glycitein and 10 mg genistein) or a placebo for six months. There was no exercise intervention in the investigation, only the isoflavone supplementation. At the end of the six month intervention, it was reported that there was no difference in total body fat free mass between the isoflavone and placebo groups, but there was a significant increase in the appendicular (arms and legs) fat free mass in the isoflavone supplemented group but not the placebo group. Findings from this study have some applications to sedentary, postmenopausal women. However, there are currently no peer-reviewed data indicating that isoflavone supplementation affects exercise, body composition, or training adaptations in physically active individuals.
Sulfo-Polysaccharides (Myostatin Inhibitors)
Myostatin or growth differentiation factor 8 (GDF-8) is a transforming growth factor that has been shown to serve as a genetic determinant of the upper limit of muscle size and growth [ 162 ]. Recent research has indicated that eliminating and/or inhibiting myostatin gene expression in mice [ 163 ] and cattle [ 164 – 166 ] promotes marked increases in muscle mass during early growth and development. The result is that these animals experience what has been termed as a "double-muscle" phenomenon apparently by allowing muscle to grow beyond its normal genetic limit. In agriculture research, eliminating and/or inhibiting myostatin may serve as an effective way to optimize animal growth leading to larger, leaner, and a more profitable livestock yield. In humans, inhibiting myostatin gene expression has been theorized as a way to prevent or slow down muscle wasting in various diseases, speed up recovery of injured muscles, and/or promote increases in muscle mass and strength in athletes [ 167 ]. While these theoretical possibilities may have great promise, research on the role of myostatin inhibition on muscle growth and repair is in the very early stages - particularly in humans. There is some evidence that myostatin levels are higher in the blood of HIV positive patients who experience muscle wasting and that myostatin levels negatively correlate with muscle mass [ 162 ]. There is also evidence that myostatin gene expression may be fiber specific and that myostatin levels may be influenced by immobilization in animals [ 168 ]. Additionally, a study by Ivey and colleagues [ 167 ] reported that female athletes with a less common myostatin allele (a genetic subtype that may be more resistant to myostatin) experienced greater gains in muscle mass during training and less loss of muscle mass during detraining. No such pattern was observed in men with varying amounts of training histories and muscle mass. These early studies suggest that myostatin may play a role in regulating muscle growth to some degree. Some nutrition supplement companies have marketed sulfo-polysaccharides (derived from a sea algae called Cytoseira canariensis ) as a way to partially bind the myostatin protein in serum. When untrained males supplemented with 1200 mg/day of Cystoseira canariensis in conjunction with a twelve week resistance training regimen, it was reported that there were no differences between the supplemented group and the placebo group in relation to fat-free mass, muscle strength, thigh volume/mass, and serum myostatin [ 169 ]. Interestingly, a recent paper by Seremi and colleagues [ 170 ] reported that resistance training reduced serum myostatin levels and that creatine supplementation in conjunction with resistance training promoted further reductions. Nevertheless, though the research is limited, there is currently no published data supporting the use of sulfo-polysaccharides as a muscle building supplement.
Boron is a trace mineral proposed to increase testosterone levels and promote anabolism. Several studies have evaluated the effects of boron supplementation during training on strength and body composition alterations. These studies (conducted on male bodybuilders) indicate that boron supplementation (2.5 mg/d) appears to have no impact on muscle mass or strength [ 171 , 172 ].
Chromium is a trace mineral that is involved in carbohydrate and fat metabolism. Clinical studies have suggested that chromium may enhance the effects of insulin particularly in diabetic populations. Since insulin is an anti-catabolic hormone and has been reported to affect protein synthesis, chromium supplementation has been theorized to serve as an anabolic nutrient. Theoretically, this may increase anabolic responses to exercise. Although some initial studies reported that chromium supplementation increased gains in muscle mass and strength during training particularly in women [ 173 – 175 ], most well-controlled studies [ 176 ] that have been conducted since then have reported no benefit in healthy individuals taking chromium (200-800 mcg/d) for 4 to 16-weeks during training [ 177 – 183 ]. Consequently, it appears that although chromium supplementation may have some therapeutic benefits for diabetics, chromium does not appear to be a muscle-building nutrient for athletes.
Conjugated Linoleic Acids (CLA)
Animal studies indicate that adding CLA to dietary feed decreases body fat, increases muscle and bone mass, has anti-cancer properties, enhances immunity, and inhibits progression of heart disease [ 184 – 186 ]. Consequently, CLA supplementation in humans has been suggested to help manage body composition, delay loss of bone, and provide health benefit. Although animal studies are impressive [ 187 – 189 ] and some studies suggests benefit over time at some but not all dosages [ 190 – 192 ], there is little current evidence that CLA supplementation during training can affect lean tissue accretion [ 193 , 194 ]. As will be discussed below, there appears to be more promise of CLA as a supplement to promote general health and/or reductions in fat mass over time.
Gamma Oryzanol (Ferulic Acid)
Gamma oryzanol is a plant sterol theorized to increase anabolic hormonal responses during training [ 195 ]. Although data are limited, one study reported no effect of 0.5 g/d of gamma oryzanol supplementation on strength, muscle mass, or anabolic hormonal profiles during 9-weeks of training [ 196 ].
Prohormones
Testosterone and growth hormone are two primary hormones in the body that serve to promote gains in muscle mass (i.e., anabolism) and strength while decreasing muscle breakdown (catabolism) and fat mass [ 197 – 204 ]. Testosterone also promotes male sex characteristics (e.g., hair, deep voice, etc) [ 198 ]. Low level anabolic steroids are often prescribed by physicians to prevent loss of muscle mass for people with various diseases and illnesses [ 205 – 216 ]. It is well known that athletes have experimented with large doses of anabolic steroids in an attempt to enhance training adaptations, increase muscle mass, and/or promote recovery during intense training [ 198 – 200 , 203 , 204 , 217 ]. Research has generally shown that use of anabolic steroids and growth hormone during training can promote gains in strength and muscle mass [ 197 , 202 , 204 , 210 , 213 , 218 – 225 ]. However, a number of potentially life threatening adverse effects of steroid abuse have been reported including liver and hormonal dysfunction, hyperlipidemia (high cholesterol), increased risk to cardiovascular disease, and behavioral changes (i.e., steroid rage) [ 220 , 226 – 230 ]. Some of the adverse effects associated with the use of these agents are irreversible, particularly in women [ 227 ]. For this reason, anabolic steroids have has been banned by most sport organizations and should be avoided unless prescribed by a physician to treat an illness.
Prohormones (androstenedione, 4-androstenediol, 19-nor-4-androstenedione, 19-nor-4-androstenediol, 7-keto DHEA, and DHEA, etc) are naturally derived precursors to testosterone or other anabolic steroids. Prohormones have become popular among body builders because they believe they are natural boosters of anabolic hormones. Consequently, a number of over-the-counter supplements contain prohormones. While there is some data indicating that prohormones increase testosterone levels [ 231 , 232 ], there is virtually no evidence that these compounds affect training adaptations in younger men with normal hormone levels. In fact, most studies indicate that they do not affect testosterone and that some may actually increase estrogen levels and reduce HDL-cholesterol [ 220 , 231 , 233 – 238 ]. Consequently, although there may be some potential applications for older individuals to replace diminishing androgen levels, it appears that prohormones have no training value. Since prohormones are "steroid-like compounds", most athletic organizations have banned their use. Use of nutritional supplements containing prohormones will result in a positive drug test for anabolic steroids. Use of supplements knowingly or unknowingly containing prohormones have been believed to have contributed to a number of recent positive drug tests among athletes. Consequently, care should be taken to make sure that any supplement an athlete considers taking does not contain prohormone precursors particularly if their sport bans and tests for use of such compounds. It is noteworthy to mention that many prohormones are not lawful for sale in the USA since the passage of the Anabolic Steroid Control Act of 2004. The distinctive exception to this is DHEA, which has been the subject of numerous clinical studies in aging populations.
Rather than provide the body with a precursor to testosterone, a more recent technique to enhance endogenous testosterone has been to inhibit aromatase activity [ 239 ]. Two studies have investigated the effects of aromatase inhibitors (androst-4-ene-3,6,17-trione) [ 240 ] and (hydroxyandrost-4-ene-6,17-dioxo-3-THP ether and 3,17-diketo-androst-1,4,6-triene) [ 241 ]. In both of these investigations, it was reported that free testosterone and dihydrotesterone levels were significantly increased. Muscle mass/fat free mass was not measured in one investigation [ 240 ] and no changes were observed in fat free mass in the other investigation [ 241 ].
Tribulus terrestris
Tribulus terrestris (also known as puncture weed/vine or caltrops) is a plant extract that has been suggested to stimulate leutinizing hormone (LH) which stimulates the natural production of testosterone [ 132 ]. Consequently, Tribulus has been marketed as a supplement that can increase testosterone and promote greater gains in strength and muscle mass during training. Several recent studies have indicated that Tribulus supplementation appears to have no effects on body composition or strength during training [ 242 – 244 ].
Vanadyl Sulfate (Vanadium)
In a similar manner as chromium, vanadyl sulfate is a trace mineral that has been found to affect insulin-sensitivity and may affect protein and glucose metabolism [ 132 , 245 ]. For this reason, vanadyl sulfate has been purported to increase muscle mass and strength during training. Although there may be some clinical benefits for diabetics (with a therapeutic dose of at least 50 mg vanadyl sulfate twice daily [ 246 , 247 ], vanadyl sulfate supplementation does not appear to have any effect on strength or muscle mass during training in non-diabetic, weight training individuals [ 248 , 249 ].
Weight Loss Supplements
Although exercise and proper diet remain the best way to promote weight loss and/or manage body composition, a number of nutritional approaches have been investigated as possible weight loss methods (with or without exercise). The following overviews the major types of weight loss products available and discusses whether any available research supports their use. See Table 3 for a summary.
Low Calorie Diet Foods & Supplements
Most of the products in this category represent low fat/carbohydrate, high protein food alternatives [ 250 ]. They typically consist of pre-packaged food, bars, MRP, or RTD supplements. They are designed to provide convenient foods/snacks to help people follow a particular low calorie diet plan. In the scientific literature, diets that provide less than 1000 calories per day are known as very low calorie diets (VLCD's). Pre-packaged food, MRP's, and/or RTD's are often provided in VLCD plans to help people cut calories. In most cases, VLCD plans recommend behavioural modification and that people start a general exercise program.
Research on the safety and efficacy of people maintaining VLCD's generally indicate that they can promote weight loss. For example, Hoie et al [ 251 ] reported that maintaining a VLCD for 8-weeks promoted a 27 lbs (12.6%) loss in total body mass, a 21 lbs loss in body fat (23.8%), and a 7 lbs (5.2%) loss in lean body mass in 127 overweight volunteers. Bryner and colleagues [ 252 ] reported that addition of a resistance training program while maintaining a VLCD (800 kcal/d for 12-weeks) resulted in a better preservation of lean body mass and resting metabolic rate compared to subjects maintaining a VLCD while engaged in an endurance training program. Meckling and Sherfey [ 253 ] reported that the combination of high protein and exercise was the most effective intervention for weight loss and was superior to a low-fat, high-carbohydrate diet in promoting weight loss and nitrogen balance regardless of the presence of an exercise intervention. Recent studies indicate that high protein/low fat VLCD's may be better than high carbohydrate/low fat diets in promoting weight loss [ 46 , 253 – 260 ]. The reason for this is that typically when people lose weight about 40-50% of the weight loss is muscle which decreases resting energy expenditure. Increasing protein intake during weight loss helps preserve muscle mass and resting energy expenditure to a better degree than high carbohydrate diets [ 261 , 262 ]. These findings and others indicate that VLCD's (typically using MRP's and/or RTD's as a means to control caloric intake) can be effective particularly as part of an exercise and behavioural modification program. Most people appear to maintain at least half of the initial weight lost for 1-2 years but tend to regain most of the weight back within 2-5 years. Therefore, although these diets may help people lose weight on the short-term, it is essential people who use them follow good diet and exercise practices in order to maintain the weight loss. The addition of dietary protein whether in whole food form or meal replacement form could assist in this weight maintenance due to the fact that the retention of muscle mass is greater than in high carbohydrate/low-fat weight loss trials?
Ephedra, Caffeine, and Silicin
Thermogenics are supplements designed to stimulate metabolism thereby increasing energy expenditure and promote weight loss. They typically contain the "ECA" stack of ephedra alkaloids (e.g., Ma Haung, 1R,2S Nor-ephedrine HCl, Sida Cordifolia), caffeine (e.g., Gaurana, Bissey Nut, Kola) and aspirin/salicin (e.g., Willow Bark Extract). The first of the three traditional thermogenics is now banned by the FDA however the safety associated with the ingestion of ephedra is debated. More recently, other potentially thermogenic nutrients have been added to various thermogenic formulations. For example, thermogenic supplements may also contain synephrine (e.g., Citrus Aurantum, Bitter Orange), calcium & sodium phosphate, thyroid stimulators (e.g., guggulsterones, L-tyrosine, iodine), cayenne & black pepper, and ginger root.
A significant amount of research has evaluated the safety and efficacy of EC and ECA type supplements. According to a meta-analysis in the Journal of American Medical Association, ephedrine/ephedra promote a more substantial weight loss 0.9 kg per month in comparison to placebo in clinical trials but are associated with increased risk of psychiatric, autonomic or gastrointestinal symptoms as well as heart palpitations. Several studies have confirmed that use of synthetic or herbal sources of ephedrine and caffeine (EC) promote about 2 lbs of extra weight loss per month while dieting (with or without exercise) and that EC supplementation is generally well tolerated in healthy individuals [ 263 – 274 ]. For example, Boozer et al [ 267 ] reported that 8-weeks of ephedrine (72 mg/d) and caffeine (240 mg/d) supplementation promoted a 9 lbs loss in body mass and a 2.1% loss in body fat with minor side effects. Hackman and associates [ 275 ] reported that a 9 month clinical trial utilizing a multi-nutrient supplement containing 40 mg/d of ephedra alkaloids and 100 mg/day caffeine resulted in a loss of weight and body fat, improved metabolic parameters including insulin sensitivity without any apparent side effects. Interestingly, Greenway and colleagues [ 274 ] reported that EC supplementation was a more cost-effective treatment for reducing weight, cardiac risk, and LDL cholesterol than several weight loss drugs (fenfluramine with mazindol or phentermine). Finally, Boozer and associates [ 268 ] reported that 6-months of herbal EC supplementation promoted weight loss with no clinically significant adverse effects in healthy overweight adults. Less is known about the safety and efficacy of synephrine, thyroid stimulators, cayenne/black pepper and ginger root.
Despite these findings, the Food and Drug Administration (FDA) banned the sale of ephedra containing supplements. The rationale has been based on reports to adverse event monitoring systems and in the media suggesting a link between intake of ephedra and a number of severe medical complications (e.g., high blood pressure, elevated heart rate, arrhythmias, sudden death, heat stroke, etc) [ 276 , 277 ]. Although results of available clinical studies do not show these types of adverse events, ephedra is no longer available as an ingredient in dietary supplements and thus cannot be recommended for use. Consequently, thermogenic supplements now contain other nutrients believed to increase energy expenditure (e.g., synephrine, green tea, etc) and are sold as "ephedrine-free" types of products. Anyone contemplating taking thermogenic supplements should carefully consider the potential side effects, discuss possible use with a knowledgeable physician, and be careful not to exceed recommended dosages.
High Fiber Diets
One of the oldest and most common methods of suppressing the appetite is to consume a diet that is high in fiber. Ingesting high fiber foods (fruits, vegetables) or fiber containing supplements (e.g., glucomannan) increase the feeling of fullness (satiety) which typically allows an individual to feel full while ingesting fewer calories. Theoretically, maintaining a high fiber diet may serve to help decrease the amount of food you eat. In addition, high fiber diets/supplements help lower cholesterol and blood pressure, enhance insulin sensitivity, and promote weight loss in obese subjects [ 278 ]. A recent study found that a Mediterranean diet that was high in fiber resulted in a more dramatic weight loss that a traditional low-fat diet and had beneficial effects on glycemic control [ 279 ]. Other research on high fiber diets indicates that they provide some benefit, particularly in diabetic populations. For example, Raben et al [ 280 ] reported that subjects maintaining a low fat/high fiber diet for 11 weeks lost about 3 lbs of weight and 3.5 lbs of fat. Other studies have reported mixed results on altering body composition using various forms of higher fiber diets [ 281 – 284 ]. Consequently, although maintaining a low fat/high fiber diet that is high in fruit and vegetable content has various health benefits, these diets seem to have potential to promote weight loss as well as weight maintenance thus we can recommend high fiber diets as a safe and healthy approach to possibly improve body composition.
Several studies and recent reviews have reported that calcium supplementation alone or in combination with other ingredients does not affect weight loss or fat loss [ 285 – 290 ]. Research has indicated that calcium modulates 1,25-diydroxyvitamin D which serves to regulate intracellular calcium levels in fat cells [ 291 , 292 ]. Increasing dietary availability of calcium reduces 1,25-diydroxyvitamin D and promotes reductions in fat mass in animals [ 292 – 294 ]. Dietary calcium has been shown to suppress fat metabolism and weight gain during periods of high caloric intake [ 291 , 293 , 295 ]. Further, increasing calcium intake has been shown to increase fat metabolism and preserve thermogenesis during caloric restriction [ 291 , 293 , 295 ]. In support of this theory, Davies and colleagues [ 296 ] reported that dietary calcium was negatively correlated to weight and that calcium supplementation (1,000 mg/d) accounted for an 8 kg weight loss over a 4 yr period. Additionally, Zemel and associates [ 291 ] reported that supplemental calcium (800 mg/d) or high dietary intake of calcium (1,200 - 1,300 mg/d) during a 24-week weight loss program promoted significantly greater weight loss (26-70%) and dual energy x-ray absorptiometer (DEXA) determined fat mass loss (38-64%) compared to subjects on a low calcium diet (400-500 mg/d). These findings and others suggest a strong relationship between calcium intake and fat loss. However, more research needs to be conducted before definitive conclusions can be drawn.
Green Tea Extract
Green tea is now one of the most common herbal supplements that is being added to thermogenic products because it has been suggested to affect weight loss and is now the fourth most commonly used dietary supplement in the US [ 297 ]. Green tea contains high amounts of caffeine and catechin polyphenols. The primary catechin that is associated to the potential effects on weight loss through diet induced thermogenesis is the catechin epigallocatechin gallate, also known as EGCG [ 298 , 299 ]. Research suggests that catechin polyphenols possess antioxidant properties and the intake of tea catechins is associated with a reduced risk of cardiovascular disease [ 298 – 300 ]. In addition, green tea has also been theorized to increase energy expenditure by stimulating brown adipose tissue thermogenesis. In support of this theory, Dulloo et al [ 301 , 302 ] reported that green tea supplementation in combination with caffeine (e.g., 50 mg caffeine and 90 mg epigallocatechin gallate taken 3-times per day) significantly increased 24-hour energy expenditure and fat utilization in humans to a much greater extent than when an equivalent amount of caffeine was evaluated suggesting a synergistic effect. Recently, work by Di Pierro and colleagues [ 303 ] reported that the addition of a green tea extract to a hypocaloric diet resulted in a significant increase in weight loss (14 kg vs. 5 kg) versus a hypocaloric diet alone over a 90 day clinical trial. Maki and coworkers [ 304 ] also demonstrated that green tea catechin consumption enhanced the exercise-induced changes in abdominal fat. However, it must be noted that both human and animal studies have not supported these findings and have reported that supplementation of these extracts does not affect weight loss [ 305 , 306 ]. Theoretically, increases in energy expenditure may help individuals lose weight and/or manage body composition.
CLA is a term used to describe a group of positional and geometric isomers of linoleic acid that contain conjugated double bonds. Adding CLA to the diet has been reported to possess significant health benefits in animals [ 184 , 307 ]. In terms of weight loss, CLA feedings to animals have been reported to markedly decrease body fat accumulation [ 185 , 308 ]. Consequently, CLA has been marketed as a health and weight loss supplement since the mid 1990s. Despite the evidence in animal models, the effect of CLA supplementation in humans is less clear. There are some data suggesting that CLA supplementation may modestly promote fat loss and/or increases in lean mass [ 190 – 192 , 309 – 314 ]. Recent work suggested that CLA supplementation coupled with creatine and whey protein resulted in a increase in strength and lean-tissue mass during resistance training [ 315 ]. However, other studies indicate that CLA supplementation (1.7 to 12 g/d for 4-weeks to 6-months) has limited to no effects on body composition alterations in untrained or trained populations [ 190 , 310 , 316 – 324 ]. The reason for the discrepancy in research findings has been suggested to be due to differences in purity and the specific isomer studied. For instance, early studies in humans showing no effect used CLA that contained all 24 isomers. Today, most labs studying CLA use 50-50 mixtures containing the trans-10, cis-12 and cis-9, trans-11 isomers, the former of which being recently implicated in positively altering body composition. This has been supported by recent work indicating that CLA (50:50 cis-9, trans-11:trans-10, cis-12) plus polyunsaturated fatty acid supplementation prevented abdominal fat increases and increase fat-free mass [ 325 ]. However, it must be noted that this response only occurred in young obese individuals. Thus, CLA supplementation may have potential in the areas of general health and it is clear that research on the effects on body composition is ongoing and still quite varied. Further research is needed to determine which CLA isomer is ideal for ingestion and possibly if there are differential responses among lean or obese and old or young populations.
Gymnema Sylvestre
Gymnema Sylvestre is a supplement that is purported to regulate weight loss and blood sugar levels. It is purported to affect glucose and fat metabolism as well as inhibit sweet cravings. In support of these contentions, some recent data have been published by Shigematsu and colleagues [ 326 , 327 ] showing that short and long-term oral supplementation of gymnema sylvestre in rats fed normal and high-fat diets may have some positive effects on fat metabolism, blood lipid levels, and/or weight gain/fat deposition. More recent work in rats has shown that gymnema sylvestre supplementation promoted weight loss by reducing hyperlipidemia [ 328 ]. The only apparent clinical trial in humans showed that an herbal combination group containing 400 mg of gymnema sylvestra resulted in effective and safe weight loss while promoting improved blood lipid profiles. It should be noted that this group was not significantly different that the other active group, containing HCA, when observing these dependent variables [ 329 ]. Due to the lack of substantial positive research on the effects of gymnema sylvestre supplementation in humans, we cannot recommend gymnema sylvestre as a supplement to positively affect weight loss.
Phosphatidyl Choline (Lecithin)
Choline is considered an essential nutrient that is needed for cell membrane integrity and to facilitate the movement of fats in and out of cells. It is also a component of the neurotransmitter acetylcholine and is needed for normal brain functioning, particularly in infants. For this reason, phosphatidyl choline (PC) has been purported as a potentially effective supplement to promote fat loss as well as improve neuromuscular function. However, despite these alleged benefits of lecithin supplementation, there are no clinical trials in humans to support a potential role of lecithin supplementation affecting weight loss.
Betaine is a compound that is involved in the metabolism of choline and homocysteine. Garcia Neto et al. [ 330 ] have shown that betaine feedings can effect liver metabolism, fat metabolism, and fat deposition in chickens. Betaine supplementation may also help lower homocysteine levels which is a marker of risk to heart disease [ 331 ]. For this reason, betaine supplements have been marketed as a supplement designed to promote heart health as well as a weight loss. A recent study by Hoffman and colleagues [ 332 ] found betaine supplementation to improve muscular endurance in active college age males. Despite this, there appears to be little evidence in human models that supports the role of betaine as a supplement for weight loss and thus it is not recommended for supplementation.
Coleus Forskohlii (Forskolin)
Forskolin, which is touted as a weight loss supplement is a plant native to India that has been used for centuries in traditional Ayurvedic medicine primarily to treat skin disorders and respiratory problems [ 333 , 334 ]. A considerable amount of research has evaluated the physiological and potential medical applications of forskolin over the last 25 years. Forskolin has been reported to reduce blood pressure, increase the hearts ability to contract, help inhibit platelet aggregation, improve lung function, and aid in the treatment of glaucoma [ 333 – 335 ]. With regard to weight loss, forskolin has been reported to increase cyclic AMP and thereby stimulate fat metabolism [ 336 – 338 ]. Theoretically, forskolin may therefore serve as an effective weight loss supplement. Recent evidence has shown that forskolin supplementation had no effect on improving body composition in mildly obese women [ 339 ]. In contrast, work done by Godard et al. in 2005 reported that 250 mg of a 10% forskolin extract taken twice daily resulted in improvements in body composition in overweight and obese men [ 340 ]. Another study suggested that supplementing the diet with coleus forskohlii in overweight women helped maintain weight and was not associated with any clinically significant adverse events [ 341 ]. Currently, research is still needed on forskolin supplementation before it can be recommended as an effective weight loss supplement.
Dehydroepiandrosterone (DHEA) and 7-Keto DHEA
Dehydroepiandrosterone (DHEA) and its sulfated conjugate DHEAS represent the most abundant adrenal steroids in circulation [ 342 ]. Although, DHEA is considered a weak androgen, it can be converted to the more potent androgens testosterone and dihydrotestosterone in tissues. In addition, DHEAS can be converted into androstenedione and testosterone. DHEA levels have been reported to decline with age in humans [ 343 ]. The decline in DHEA levels with aging has been associated with increased fat accumulation and risk to heart disease [ 344 ]. Since DHEA is a naturally occurring compound, it has been suggested that dietary supplementation of DHEA may help maintain DHEA availability, maintain and/or increase testosterone levels, reduce body fat accumulation, and/or reduce risk to heart disease as one ages [ 342 , 344 ]. Although animal studies have generally supported this theory, the effects of DHEA supplementation on body composition in human trials have been mixed. For example, Nestler and coworkers [ 345 ] reported that DHEA supplementation (1,600 mg/d for 28-d) in untrained healthy males promoted a 31% reduction in percentage of body fat. However, Vogiatzi and associates [ 346 ] reported that DHEA supplementation (40 mg/d for 8 wks) had no effect on body weight, percent body fat, or serum lipid levels in obese adolescents. More recent work has supported these findings suggesting that one year of DHEA supplementation had no effect on body composition when taken at 50 mg per day [ 347 ]. 7-keto DHEA, a DHEA precursor, has been marketed as a potentially more effective form of DHEA which is believed to possess lypolytic properties. Although data are limited, Kalman and colleagues and coworkers [ 348 ] reported that 7-keto DHEA supplementation (200 mg/d) during 8-weeks of training promoted a greater loss in body mass and fat mass while increasing T3 while observing no significant effects on thyroid stimulating hormone (TSH) or T4. More recent data has shown that 7-keto DHEA supplementation can increase RMR [ 349 ] and blunt the decrease in RMR associated with 8 weeks of restricted dieting [ 350 ]. However, it must be noted that the second study did not use isolated 7-keto DHEA but used a commercial weight loss product that contained DHEA as well as other known weight loss agents (i.e. caffeine, green tea extract, citrus aurantium, etc.). Thus, these results do not directly support the use of 7-keto DHEA. Although more research is needed on the effects of supplementing DHEA by itself as a weight loss agent, these findings provide minimal support that 7-keto DHEA may serve as an effective weight loss supplement.
Psychotropic Nutrients/Herbs
Psychotropic nutrients/herbs are a new class of supplements that often contain things like St. John's Wart, Kava, Ginkgo Biloba, Ginseng, and L-Tyrosine. They are believed to serve as naturally occurring antidepressants, relaxants, and mental stimulants thus the theoretical rationale regarding weight loss is that they may help people fight depression or maintain mental alertness while dieting. There are no clinical weight loss trials that utilize any of the above nutrients/herbs as the active ingredient in the supplementation trial. Although a number of studies support potential role as naturally occurring psychotropics or stimulants, the potential value in promoting weight loss is unclear and therefore are not recommended for supplementation.
Calcium Pyruvate
Calcium Pyruvate is supplement that hit the scene about 10-15 ago with great promise. The theoretical rationale was based on studies from the early 1990s that reported that calcium pyruvate supplementation (16 - 25 g/d with or without dihydroxyacetone phosphate [DHAP]) promoted fat loss in overweight/obese patients following a medically supervised weight loss program [ 351 – 353 ]. Although the mechanism for these findings was unclear, the researchers speculated that it might be related to appetite suppression and/or altered carbohydrate and fat metabolism. Since calcium pyruvate is very expensive, several studies have attempted to determine whether ingesting smaller amounts of calcium pyruvate (6-10 g/d) affect body composition in untrained and trained populations. Results of these studies are mixed. Earlier studies have shown both a positive effect on calcium pyruvate supplementation in improving body composition [ 354 ], however, Stone and colleagues [ 355 ] reported that pyruvate supplementation did not affect hydrostatically determined body composition during 5-weeks of in-season college football training. More recently, calcium pyruvate supplementation was also shown to not have a significant effect on body composition or exercise performance. Additionally, it has been reported that supplementation may negatively affect some blood lipid levels [ 356 ]. These findings indicate that although there is some supportive data indicating that calcium pyruvate supplementation may enhance fat loss when taken at high doses (6-16 g/d), there is no evidence that ingesting the doses typically found in pyruvate supplements (0.5 - 2 g/d) has any affect on body composition. In addition, the overall quantity of research examining calcium pyruvate is minimal at best thus it is not warranted to include calcium pyruvate as a weight loss supplement.
Chitosan has been marketed as a weight loss supplement for several years as is known as a "fat trapper". It is purported to inhibit fat absorption and lower cholesterol. This notion is supported animal studies indicated by decreased fat absorption, increased fat content, and/or lower cholesterol following chitosan feedings [ 357 – 360 ]. However, the effects in humans appear to be less impressive. For example, although there is some data suggesting that chitosan supplementation may lower blood lipids in humans,[ 361 ] other studies report no effects on fat content [ 362 , 363 ]or body composition alterations [ 364 – 366 ] when administered to people following their normal diet. More recent work has shown that the effect of chitosan on fat absorption is negligible and is the equivalent of approximately 9.9 kcal/day following supplementation [ 362 ]. Other work has concluded that the insignificant amounts of fat that are trapped from supplementation would take about 7 months for a male to lose a pound of weight, and that the effect was completely ineffective in women [ 364 ]. Thus, based on the current evidence, chitosan supplementation is apparently ineffective and has no significant effects on "fat trapping" and/or on improving body composition.
Chromium supplementation is derived from its role in maintaining proper carbohydrate and fat metabolism by potentially effecting insulin signalling [ 367 ]. Initial studies reported that chromium supplementation during resistance training improved fat loss and gains in lean body mass [ 173 – 175 ]. To date, the studies using more accurate methods of assessing body composition have primarily indicate no effects on body composition in healthy non-diabetic individuals [ 176 – 183 , 368 ]. Recent work has reported that 200 mcg of chromium picolinate supplementation on individuals on a restrictive diet did not promote weight loss or body composition changes following 12 weeks of supplementation [ 368 ]. This work supports Lukaski et al [ 182 ] previous findings that 8-weeks of chromium supplementation during resistance training did not affect strength or DEXA determined body composition changes. Thus, based on the current review of the literature we cannot recommend chromium supplementation as a means of improving body composition.
Garcinia Cambogia (HCA)
HCA is a nutrient that has been hypothesized to increase fat oxidation by inhibiting citrate lypase and lipogenesis [ 369 ]. Theoretically, this may lead to greater fat burning and weight loss over time. Although there is some evidence that HCA may increase fat metabolism in animal studies, there is little to no evidence showing that HCA supplementation affects body composition in humans. For example, Ishihara et al [ 370 ] reported that HCA supplementation spared carbohydrate utilization and promoted lipid oxidation during exercise in mice. However, Kriketos and associates [ 371 ] reported that HCA supplementation (3 g/d for 3-days) did not affect resting or post-exercise energy expenditure or markers of lipolysis in healthy men. Likewise, Heymsfield and coworkers [ 372 ] reported that HCA supplementation (1.5 g/d for 12-weeks) while maintaining a low fat/high fiber diet did not promote greater weight or fat loss than subjects on placebo. Finally, Mattes and colleagues [ 373 ] reported that HCA supplementation (2.4 g/d for 12-weeks) did not affect appetite, energy intake, or weight loss. These findings suggest that HCA supplementation does not appear to promote fat loss in humans.
L-Carnitine
Carnitine serves as an important transporter of fatty acids from the cytosol into the mitochondria of the cell [ 374 ]. Increased cellular levels of carnitine would theoretically enhance transport of fats into the mitochondria and thus provide more substrates for fat metabolism. L-carnitine has been one of the most common nutrients found in various weight loss supplements. Over the years, a number of studies have been conducted on the effects of L-carnitine supplementation on fat metabolism, exercise capacity and body composition. The overwhelming conclusions of L-carnitine research indicates that L-carnitine supplementation does not affect muscle carnitine content [ 375 ], fat metabolism, aerobic- or anaerobic-exercise performance [ 375 ], and/or weight loss in overweight or trained subjects [ 376 , 377 ]. Despite the fact that L-carnitine has been shown apparently ineffective as a supplement, the research on L-carnitine has shifted to another category revolving around hypoxic stress and oxidative stress. Preliminary research has reported that L-carnitine supplementation has a minimal effect on reducing the biomarkers of exercise-induced oxidative stress [ 378 ]. While these findings are not promising, there is some recent data indicating that L-carnitine tartrate supplementation during intensified periods of training may help athletes tolerate training to a greater degree [ 379 ]. Consequently, there may be other advantages to L-carnitine supplementation than promoting fat metabolism.
The role of sodium and calcium phosphate on energy metabolism and exercise performance has been studied for decades [ 31 ]. Phosphate supplementation has also been suggested to affect energy expenditure, however, the research in this area is quite dated and no research on the effects on energy expenditure have been conducted. Some of this dated work includes the work by Kaciuba-Uscilko and colleagues [ 380 ] who reported that phosphate supplementation during a 4-week weight loss program increased resting metabolic rate (RMR) and respiratory exchange ratio (suggesting greater carbohydrate utilization and caloric expenditure) during submaximal cycling exercise. In addition, Nazar and coworkers [ 381 ] reported that phosphate supplementation during an 8-week weight loss program increased RMR by 12-19% and prevented a normal decline in thyroid hormones. Although the rate of weight loss was similar in this trial, results suggest that phosphate supplementation may influence metabolic rate possibly by affecting thyroid hormones. Despite these to dated trials, no further research has been conducted and thus the role of phosphates in regards to weight loss is inconclusive at best.
Herbal Diuretics
This is a new type of supplement recently marketed as a natural way to promote weight loss. There is limited evidence that taraxacum officinale, verbena officinalis, lithospermum officinale, equisetum arvense, arctostaphylos uva-ursi, arctium lappa and silene saxifraga infusion may affect diuresis in animals [ 382 , 383 ]. Two studies presented at the 2001 American College of Sports Medicine meeting [ 384 , 385 ] indicated that although herbal diuretics promoted a small amount of dehydration (about 0.3% in one day), they were not nearly as effective as a common diuretic drug (about 3.1% dehydration in one day). Consequently, although more research is needed, the potential value of herbal diuretics as a weight loss supplement appears limited.
Performance Enhancement Supplements
A number of nutritional supplements have been proposed to enhance exercise performance. Some of these nutrients have been described above. Table 3 categorizes the proposed ergogenic nutrients into apparently safe and effective, possibly effective, too early to tell, and apparently ineffective. Weight gain supplements purported to increase muscle mass may also have ergogenic properties if they also promote increases in strength. Similarly, some sports may benefit from reductions in fat mass. Therefore, weight loss supplements that help athletes manage body weight and/or fat mass may also possess some ergogenic benefit. The following describes which supplements may or may not affect performance that were not previously described.
Water and Sports Drinks
Preventing dehydration during exercise is one of the keys of maintaining exercise performance (particularly in hot/humid environments). People engaged in intense exercise or work in the heat need to frequently ingest water or sports drinks (e.g., 1-2 cups every 10 - 15 minutes). The goal should be not to lose more than 2% of body weight during exercise (e.g., 180 lbs × 0.02 = 3.6 lbs). Sports drinks typically contain salt and carbohydrate at scientifically engendered quantities. Studies show that ingestion of sports drinks during exercise in hot/humid environments can help prevent dehydration and improve endurance exercise capacity [[ 56 ], von Duvillard 2005), [ 386 , 387 ]]. In fact, research has shown that carbohydrate intake during team sport type activities can increase exercise performance and CNS function [ 15 , 16 , 388 ]. Consequently, frequent ingestion of water and/or sports drinks during exercise is one of the easiest and most effective ergogenic aids.
One of the best ergogenic aids available for athletes and active individuals alike, is carbohydrate. Athletes and active individuals should consume a diet high in carbohydrate (e.g., 55 - 65% of calories or 5-8 grams/kg/day) in order to maintain muscle and liver carbohydrate stores [ 1 , 3 ]. Research has clearly identified carbohydrate is an ergogenic aid that can prolong exercise [ 3 ]. Additionally, ingesting a small amount of carbohydrate and protein 30-60 minutes prior to exercise and use of sports drinks during exercise can increase carbohydrate availability and improve exercise performance. Finally, ingesting carbohydrate and protein immediately following exercise can enhance carbohydrate storage and protein synthesis [ 1 , 3 ].
Earlier we indicated that creatine supplementation is one of the best supplements available to increase muscle mass and strength during training. However, creatine has also been reported to improve exercise capacity in a variety of events [[ 71 ], Kendall 2005, [ 389 – 391 ]]. This is particularly true when performing high intensity, intermittent exercise such as multiple sets of weight lifting, repeated sprints, and/or exercise involving sprinting and jogging (e.g., soccer) [ 71 ]. Creatine has also been shown to be effective at improving high intensity interval training. A 2009 study found that in addition to high intensity interval training creatine improved critical power [ 390 ]. Although studies evaluating the ergogenic value of creatine on endurance exercise perfor mance are mixed, endurance athletes may also theoretically benefit in several ways. For example, increasing creatine stores prior to carbohydrate loading (i.e., increasing dietary carbohydrate intake before competition in an attempt to maximize carbohydrate stores) has been shown to improve the ability to store carbohydrate [ 392 – 394 ]. A 2003 study found that ingesting 20 grams of creatine for 5 days improved endurance and anaerobic performance in elite rowers [ 395 ]. Further, co ingesting creatine with carbohydrate has been shown to optimize creatine and carbohydrate loading [ 396 ]. Most endurance athletes also perform interval training (sprint or speed work) in an attempt to improve anaerobic threshold. Since creatine has been reported to enhance interval sprint performance, creatine supplementation during training may improve training adaptations in endurance athletes [ 397 , 398 ]. Finally, many endurance athletes lose weight during their competitive season. Creatine supplementation during training may help people maintain weight.
Sodium Phosphate
We previously mentioned that sodium phosphate supplementation may increase resting energy expenditure and therefore could serve as a potential weight loss nutrient. However, most research on sodium phosphate has actually evaluated the potential ergogenic value. A number of studies indicated that sodium phosphate supplementation (e.g., 1 gram taken 4 times daily for 3-6 days) can increase maximal oxygen uptake (i.e., maximal aerobic capacity) and anaerobic threshold by 5-10% [ 399 – 403 ]. These finding suggest that sodium phosphate may be highly effective in improving endurance exercise capacity. In addition to endurance enhancement, sodium phosphate loading improved mean power output and oxygen uptake in trained cyclist in a 2008 study [ 404 ]. Other forms of phosphate (i.e., calcium phosphate, potassium phosphate) do not appear to possess ergogenic value.
Sodium Bicarbonate (Baking Soda)
During high intensity exercise, acid (H+) and carbon dioxide (CO 2 ) accumulate in the muscle and blood. One of the ways you get rid of the acidity and CO 2 is to buffer the acid and CO 2 with bicarbonate ions. The acid and CO 2 are then removed in the lungs. Bicarbonate loading (e.g., 0.3 grams per kg taken 60-90 minutes prior to exercise or 5 grams taken 2 times per day for 5-days) has been shown to be an effective way to buffer acidity during high intensity exercise lasting 1-3 minutes in duration [ 405 – 408 ]. This can improve exercise capacity in events like the 400 - 800 m run or 100 - 200 m swim [ 409 ]. In elite male swimmers sodium bicarbonate supplementation significantly improved 200 m freestyle performance [ 410 ]. A 2009 study found similar improvements in performance in youth swimmers at distances of 50 to 200 m. Although bicarbonate loading can improve exercise, some people have difficulty with their stomach tolerating bicarbonate as it may cause gastrointestinal distress.
Caffeine is a naturally derived stimulant found in many nutritional supplements typically as gaurana, bissey nut, or kola. Caffeine can also be found in coffee, tea, soft drinks, energy drinks, and chocolate. It has previously been made clear that caffeine can have a positive effect on energy expenditure, weight loss, and body fat. Caffeine has also been shown to be an effective ergogenic aid. Research investigating the effects of caffeine on a time trial in trained cyclist found that caffeine improved speed, peak power, and mean power [ 411 ]. Similar results were observed in a recent study that found cyclists who ingested a caffeine drink prior to a time trial demonstrated improvements in performance [ 412 , 413 ]. Studies indicate that ingestion of caffeine (e.g., 3-9 mg/kg taken 30 - 90 minutes before exercise) can spare carbohydrate use during exercise and thereby improve endurance exercise capacity [ 406 , 414 ]. In addition to the apparent positive effects on endurance performance, caffeine has also been shown to improve repeated sprint performance benefiting the anaerobic athlete [ 415 , 416 ]. People who drink caffeinated drinks regularly, however, appear to experience less ergogenic benefits from caffeine [ 417 ]. Additionally, some concern has been expressed that ingestion of caffeine prior to exercise may contribute to dehydration although recent studies have not supported this concern [ 414 , 418 , 419 ]. Caffeine doses above 9 mg/kg can result in urinary caffeine levels that surpass the doping threshold for many sport organizations. Suggestions that there is no ergogenic value to caffeine supplementation is not supported by the preponderance of available scientific studies.
In recent years research has begun investigating the effects of β-alanine supplementation on performance. β-alanine has ergogenic potential based on its relationship with carnosine. Carnosine is a dipeptide comprised of the amino acids, histidine and β-alanine naturally occurring in large amounts in skeletal muscles. Carnosine is believed to be one of the primary muscle-buffering substances available in skeletal muscle. Studies have demonstrated that taking β-alanine orally over a 28-day period was effective in increasing carnosine levels [ 420 , 421 ]. This proposed benefit would increase work capacity and decrease time to fatigue. Researchers have found that β-alanine supplementation decreases rate of fatigue [ 422 ]. This could translate into definite strength gains and improved performance. A recent study [ 423 ] supplemented men with β-alanine for 10 weeks and showed that muscle carnosine levels were significantly increased after 4 and 10 weeks of β-alanine supplementation.
Stout et al. [ 422 ] conducted a study that examined the effects of β-alanine supplementation on physical working capacity at fatigue threshold. The results showed decreased fatigue in the subjects tested. Other studies have shown that β-alanine supplementation can increase the number of repetitions one can do [ 424 ], increased lean body mass [ 425 ], increase knee extension torque [ 426 ] and training volume [ 427 ]. In fact, one study also showed that adding β-alanine supplementation with creatine improves performance over creatine alone [ 428 ]. While it appears that β-alanine supplementation can decrease fatigue rate, raise carnosine levels, and improve performance all of the research is not as favorable. There are other studies that show no performance benefits [ 425 , 429 ]
Post-Exercise Carbohydrate and Protein
Ingesting carbohydrate and protein following exercise enhances carbohydrate storage and protein synthesis. Theoretically, ingesting carbohydrate and protein following exercise may lead to greater training adaptations. In support of this theory, Esmarck and coworkers [ 107 ] found that ingesting carbohydrate and protein immediately following exercise doubled training adaptations in comparison to waiting until 2-hours to ingest carbohydrate and protein. Additionally, Tarnopolsky and associates [ 430 ] reported that post-exercise ingestion of carbohydrate with protein promoted as much strength gains as ingesting creatine with carbohydrate during training. A recent study by Kreider and colleagues [ 431 ] found that protein and carbohydrate supplementation post workout was capable of positively supporting the post exercise anabolic response. In the last few years many studies have agreed with these findings in that post workout supplementation is vital to recovery and training adaptations [ 13 , 104 , 431 – 433 ]. These findings underscore the importance of post-exercise carbohydrate and protein ingestion to support muscle anabolism and strength. However, it is still unclear if there are direct implications of protein/carbohydrate supplementation on other markers of performance such as time to exhaustion, maximal oxygen uptake, and/or skill development.
Ingestion of 3-6 grams of EAA following resistance exercise has been shown to increase protein synthesis [ 92 , 93 , 98 – 102 , 105 , 434 ]. Theoretically, ingestion of EAA after exercise should enhance gains in strength and muscle mass during training. While there is sound theoretical rationale, it is currently unclear whether following this strategy would lead to greater training adaptations and/or whether EAA supplementation would be better than simply ingesting carbohydrate and a quality protein following exercise.
Ingestion of BCAA (e.g., 6-10 grams per hour) with sports drinks during prolonged exercise would theoretically improve psychological perception of fatigue (i.e., central fatigue). Although there is strong rationale, the effects of BCAA supplementation on exercise performance is mixed with some studies suggesting an improvement and others showing no effect [ 33 ]. More research is needed before conclusions can be drawn.
HMB supplementation has been reported to improve training adaptations in untrained individuals initiating training as well as help reduce muscle breakdown in runners. Theoretically, this should enhance training adaptations in athletes. However, most studies show little benefit of HMB supplementation in athletes. A 2004 study by Hoffman [ 435 ] found HMB supplementation to be ineffective in collegiate football players after short term supplementation. It has been hypothesized that HMB will delay or prevent muscle damage; however this has limited evidence as suggested in previous sections. There are a few studies that have been positive [ 115 ]. A 2009 study found that HMB supplementation did positively affect strength in trained men [ 436 ]. While HMB supplementation may still have some scientific rationale there is little evidence that is can directly affect performance in moderately trained subjects.
Ingesting glycerol with water has been reported to increase fluid retention [ 437 ]. Theoretically, this should help athletes prevent dehydration during prolonged exercise and improve performance particularly if they are susceptible to dehydration. Although studies indicate that glycerol can significantly enhance body fluid, results are mixed on whether it can improve exercise capacity [ 69 , 438 – 443 ]. Little research has been done on glycerol in the last five years however, a 2006 study agreed with previous findings in that glycerol has little impact on performance [ 444 ].
A number of supplements purported to enhance performance and/or training adaptation fall under this category. This includes the weight gain and weight loss supplements listed in Table 3 as well as the following supplements not previously described in this category.
Medium Chain Triglycerides (MCT)
MCT's are shorter chain fatty acids that can easily enter the mitochondria of the cell and be converted to energy through fat metabolism [ 445 ]. Studies are mixed as to whether MCT's can serve as an effective source of fat during exercise metabolism and/or improve exercise performance [ 445 – 449 ]. A 2001 study found that 60 g/day of MCT oil for two weeks was not sufficient at improving performance [ 450 ]. In fact Goedecke found that not only did MCT supplementation not improve performance, but, actually negatively affected sprint performance in trained cyclists [ 451 ]. These findings have been confirmed by others that MCT oils are not sufficient to induce positive training adaptations and may cause gastric distress [ 452 , 453 ]. It must be noted that while most studies have not been favourable, one 2009 study found that MCT oil may positively affect RPE and lactate clearance [ 454 ]. It does not appear likely that MCT can positively affect training adaptations, but further research is needed.
As described above, glutamine has been shown to influence protein synthesis and help maintain the immune system. Theoretically, glutamine supplementation during training should enhance gains in strength and muscle mass as well as help athletes tolerate training to a better degree. Although there is some evidence that glutamine supplementation with protein can improve training adaptations, more research is needed to determine the ergogenic value in athletes. There is currently no research to suggest that glutamine has a direct effect on performance.
Ribose is a 3-carbon carbohydrate that is involved in the synthesis of adenosine triphosphate (ATP) in the muscle (the useable form of energy). Clinical studies have shown that ribose supplementation can increase exercise capacity in heart patients [ 455 – 459 ]. For this reason, ribose has been suggested to be an ergogenic aid for athletes. Although more research is needed, most studies show no ergogenic value of ribose supplementation on exercise capacity in health untrained or trained populations [ 460 – 462 ]. A 2006 study [ 463 ] investigated the effects of ribose vs. dextrose on rowing performance. After eight weeks of supplementation dextrose had a better response than ribose across the subjects [ 463 ]. Kreider and associates [ 462 ] and Kersick and colleagues [ 464 ] investigated ribose supplementation on measures of anaerobic capacity in trained athletes. This research group found that ribose supplementation did not have a positive impact on performance [ 462 , 464 ]. It appears at this point that ribose supplementation does not improve aerobic or anaerobic performance.
Inosine is a building block for DNA and RNA that is found in muscle. Inosine has a number of potentially important roles that may enhance training and/or exercise performance [ 465 ]. Although there is some theoretical rationale, available studies indicate that inosine supplementation has no apparent affect on exercise performance capacity [ 466 – 468 ].
Supplements to Promote General Health
In addition to the supplements previously described, several nutrients have been suggested to help athletes stay healthy during intense training. For example, the American Medical Association recently recommended that all Americans ingest a daily low-dose multivitamin in order to ensure that people get a sufficient amount of vitamins and minerals in their diet. Although one-a-day vitamin supplementation has not been found to improve exercise capacity in athletes, it may make sense to take a daily vitamin supplement for health reasons. Glucosomine and chondroitin have been reported to slow cartilage degeneration and reduce the degree of joint pain in active individuals which may help athletes postpone and/or prevent joint problems [ 469 , 470 ]. Supplemental Vitamin C, glutamine, echinacea, and zinc have been reported to enhance immune function [ 471 – 474 ]. Consequently, some sports nutritionists recommend that athletes who feel a cold coming on take these nutrients in order to enhance immune function [ 55 , 471 – 473 ]. Similarly, although additional research is necessary, Vitamin E, Vitamin C, selenium, alpha-lipoic acid and other antioxidants may help restore overwhelmed anti-oxidant defences exhibited by athletes and reduce the risk of numerous chronic diseases in some instances [ 475 ]. Creatine, calcium β-HMB, BCAA, and L-carnitine tartrate have been shown to help athletes tolerate heavy training periods [ 31 , 118 , 125 , 126 , 128 , 379 , 476 – 478 ]. Finally, the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapantaenoic acid (EPA), in supplemental form, are now endorsed by the American Heart Association for heart health in certain individuals [ 479 ]. This supportive supplement position stems from: 1.) an inability to consume cardio-protective amounts by diet alone; and, 2.) the mercury contamination sometimes present in whole-food sources of DHA and EPA found in fatty fish. Consequently, prudent use of these types of nutrients at various times during training may help athletes stay healthy and/or tolerate training to a greater degree [ 50 ].
Maintaining an energy balance and nutrient dense diet, prudent training, proper timing of nutrient intake, and obtaining adequate rest are the cornerstones to enhancing performance and/or training adaptations. Use of a limited number of nutritional supplements that research has supported can help improve energy availability (e.g., sports drinks, carbohydrate, creatine, caffeine, β-alanine, etc) and/or promote recovery (carbohydrate, protein, essential amino acids, etc) can provide additional benefit in certain instances. The sports nutrition specialist should stay up to date regarding the role of nutrition on exercise so they can provide honest and accurate information to their students, clients, and/or athletes about the role of nutrition and dietary supplements on performance and training. Furthermore, the sports nutrition specialist should actively participate in exercise nutrition research; write unbiased scholarly reviews for journals and lay publications; help disseminate the latest research findings to the public so they can make informed decisions about appropriate methods of exercise, dieting, and/or whether various nutritional supplements can affect health, performance, and/or training; and, disclose any commercial or financial conflicts of interest during such promulgations to the public. Finally, companies selling nutritional supplements should develop scientifically based products, conduct research on their products, and honestly market the results of studies so consumers can make informed decisions.
Leutholtz B, Kreider R: Exercise and Sport Nutrition. Nutritional Health. Edited by: Wilson T, Temple N. 2001, Totowa, NJ: Humana Press, 207-39.
Chapter Google Scholar
Williams MH: Nutrition for Health, Fitness, and Sport. 1999, Dubuque, IA: ACB/McGraw-Hill
Google Scholar
Kreider R, Leutholtz B, Katch F, Katch V: Exercise & Sport Nutrition. 2009, Santa Barbara: Fitness Technologies Press
FDA: Dietary Supplements. 2003, [ http://www.cfsan.fda.gov/~dms/ds-faq.html ]
Beers MH, Berkow R: The Merck Manual. 1999, Merck Research Laboratories, 17
Sherman WM, Jacobs KA, Leenders N: Carbohydrate metabolism during endurance exercise. Overtraining in Sport. Edited by: Kreider RB, Fry AC, O'Toole ML. 1998, Champaign: Human Kinetics Publishers, 289-308.
Berning JR: Energy intake, diet, and muscle wasting. Overtraining in Sport. Edited by: Kreider RB, Fry AC, O'Toole ML. 1998, Champaign: Human Kinetics, 275-88.
Kreider RB, Fry AC, O'Toole ML: Overtraining in Sport. 1998, Champaign: Human Kinetics Publishers
Kreider RB: Physiological considerations of ultraendurance performance. Int J Sport Nutr. 1991, 1 (1): 3-27.
CAS PubMed Google Scholar
Brouns F, Saris WH, Beckers E, Adlercreutz H, Vusse van der GJ, Keizer HA, Kuipers H, Menheere P, Wagenmakers AJ, ten Hoor F: Metabolic changes induced by sustained exhaustive cycling and diet manipulation. Int J Sports Med. 1989, 10 (Suppl 1): S49-62. 10.1055/s-2007-1024954.
Article PubMed Google Scholar
Brouns F, Saris WH, Stroecken J, Beckers E, Thijssen R, Rehrer NJ, ten Hoor F: Eating, drinking, and cycling. A controlled Tour de France simulation study, Part I. Int J Sports Med. 1989, 10 (Suppl 1): S32-40. 10.1055/s-2007-1024952.
Brouns F, Saris WH, Stroecken J, Beckers E, Thijssen R, Rehrer NJ, ten Hoor F: Eating, drinking, and cycling. A controlled Tour de France simulation study Part II. Effect of diet manipulation. Int J Sports Med. 1989, 10 (Suppl 1): S41-8. 10.1055/s-2007-1024953.
Kerksick C, Harvey T, Stout J, Campbell B, Wilborn C, Kreider R, Kalman D, Ziegenfuss T, Lopez H, Landis J, Ivy JL, Antonio J: International Society of Sports Nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2008, 5: 17-10.1186/1550-2783-5-17.
Article PubMed Central PubMed CAS Google Scholar
Harger-Domitrovich SG, McClaughry AE, Gaskill SE, Ruby BC: Exogenous carbohydrate spares muscle glycogen in men and women during 10 h of exercise. Med Sci Sports Exerc. 2007, 39 (12): 2171-9. 10.1249/mss.0b013e318157a650.
Article CAS PubMed Google Scholar
Rodriguez NR, Di Marco NM, Langley S: American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009, 41 (3): 709-31. 10.1249/MSS.0b013e31890eb86.
Article PubMed CAS Google Scholar
Rodriguez NR, DiMarco NM, Langley S: Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009, 109 (3): 509-27. 10.1016/j.jada.2009.01.005.
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS: American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007, 39 (2): 377-90. 10.1249/mss.0b013e31802ca597.
Currell K, Jeukendrup AE: Superior endurance performance with ingestion of multiple transportable carbohydrates. Med Sci Sports Exerc. 2008, 40 (2): 275-81. 10.1249/mss.0b013e31815adf19.
Jeukendrup AE, Moseley L: Multiple transportable carbohydrates enhance gastric emptying and fluid delivery. Scand J Med Sci Sports. 2008
Earnest CP, Lancaster SL, Rasmussen CJ, Kerksick CM, Lucia A, Greenwood MC, Almada AL, Cowan PA, Kreider RB: Low vs. high glycemic index carbohydrate gel ingestion during simulated 64-km cycling time trial performance. J Strength Cond Res. 2004, 18 (3): 466-72. 10.1519/R-xxxxx.1.
PubMed Google Scholar
Venables MC, Brouns F, Jeukendrup AE: Oxidation of maltose and trehalose during prolonged moderate-intensity exercise. Med Sci Sports Exerc. 2008, 40 (9): 1653-9. 10.1249/MSS.0b013e318175716c.
Jentjens RL, Jeukendrup AE: Effects of pre-exercise ingestion of trehalose, galactose and glucose on subsequent metabolism and cycling performance. Eur J Appl Physiol. 2003, 88 (4-5): 459-65. 10.1007/s00421-002-0729-7.
Achten J, Jentjens RL, Brouns F, Jeukendrup AE: Exogenous oxidation of isomaltulose is lower than that of sucrose during exercise in men. J Nutr. 2007, 137 (5): 1143-8.
Jentjens RL, Venables MC, Jeukendrup AE: Oxidation of exogenous glucose, sucrose, and maltose during prolonged cycling exercise. J Appl Physiol. 2004, 96 (4): 1285-91. 10.1152/japplphysiol.01023.2003.
Jeukendrup AE, Jentjens R: Oxidation of carbohydrate feedings during prolonged exercise: current thoughts, guidelines and directions for future research. Sports Med. 2000, 29 (6): 407-24. 10.2165/00007256-200029060-00004.
Rowlands DS, Wallis GA, Shaw C, Jentjens RL, Jeukendrup AE: Glucose polymer molecular weight does not affect exogenous carbohydrate oxidation. Med Sci Sports Exerc. 2005, 37 (9): 1510-6. 10.1249/01.mss.0000177586.68399.f5.
Lemon PW, Tarnopolsky MA, MacDougall JD, Atkinson SA: Protein requirements and muscle mass/strength changes during intensive training in novice bodybuilders. J Appl Physiol. 1992, 73 (2): 767-75.
Tarnopolsky MA, MacDougall JD, Atkinson SA: Influence of protein intake and training status on nitrogen balance and lean body mass. J Appl Physiol. 1988, 64 (1): 187-93.
Tarnopolsky MA, Atkinson SA, MacDougall JD, Chesley A, Phillips S, Schwarcz HP: Evaluation of protein requirements for trained strength athletes. J Appl Physiol. 1992, 73 (5): 1986-95.
Tarnopolsky MA: Protein and physical performance. Curr Opin Clin Nutr Metab Care. 1999, 2 (6): 533-7. 10.1097/00075197-199911000-00018.
Kreider RB: Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med. 1999, 27 (2): 97-110. 10.2165/00007256-199927020-00003.
Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K: Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992, 73 (4): 1383-8.
Kreider RB: Effects of protein and amino acid supplementation on athletic performance. Sportscience. 1999, 3 (1): [ http://www.sportsci.org/jour/9901/rbk.html ]
Kreider RB, Kleiner SM: Protein supplements for athletes: need vs. convenience. Your Patient & Fitness. 2000, 14 (6): 12-8.
Bucci L, Unlu L: Proteins and amino acid supplements in exercise and sport. Energy-Yielding Macronutrients and Energy Metabolism in Sports Nutrition. Edited by: Driskell J, Wolinsky I. 2000, Boca Raton, FL: CRC Press, 191-212.
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B: Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997, 94 (26): 14930-5. 10.1073/pnas.94.26.14930.
Article PubMed Central CAS PubMed Google Scholar
Boirie Y, Beaufrere B, Ritz P: Energetic cost of protein turnover in healthy elderly humans. Int J Obes Relat Metab Disord. 2001, 25 (5): 601-5. 10.1038/sj.ijo.0801608.
Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B: Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. 1996, 271 (6 Pt 1): E1083-91.
Campbell B, Kreider RB, Ziegenfuss T, La Bounty P, Roberts M, Burke D, Landis J, Lopez H, Antonio J: International Society of Sports Nutrition position stand: protein and exercise. J Int Soc Sports Nutr. 2007, 4: 8-10.1186/1550-2783-4-8.
Article PubMed Central PubMed Google Scholar
Venkatraman JT, Leddy J, Pendergast D: Dietary fats and immune status in athletes: clinical implications. Med Sci Sports Exerc. 2000, 32 (7 Suppl): S389-95.
Dorgan JF, Judd JT, Longcope C, Brown C, Schatzkin A, Clevidence BA, Campbell WS, Nair PP, Franz C, Kahle L, Taylor PR: Effects of dietary fat and fiber on plasma and urine androgens and estrogens in men: a controlled feeding study. Am J Clin Nutr. 1996, 64 (6): 850-5.
Hamalainen EK, Adlercreutz H, Puska P, Pietinen P: Decrease of serum total and free testosterone during a low-fat high-fibre diet. J Steroid Biochem. 1983, 18 (3): 369-70. 10.1016/0022-4731(83)90117-6.
Reed MJ, Cheng RW, Simmonds M, Richmond W, James VH: Dietary lipids: an additional regulator of plasma levels of sex hormone binding globulin. J Clin Endocrinol Metab. 1987, 64 (5): 1083-5. 10.1210/jcem-64-5-1083.
Fry AC, Kraemer WJ, Ramsey LT: Pituitary-adrenal-gonadal responses to high-intensity resistance exercise overtraining. J Appl Physiol. 1998, 85 (6): 2352-9.
Miller WC, Koceja DM, Hamilton EJ: A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997, 21 (10): 941-7. 10.1038/sj.ijo.0800499.
Miller WC: Effective diet and exercise treatments for overweight and recommendations for intervention. Sports Med. 2001, 31 (10): 717-24. 10.2165/00007256-200131100-00002.
Pirozzo S, Summerbell C, Cameron C, Glasziou P: Should we recommend low-fat diets for obesity?. Obes Rev. 2003, 4 (2): 83-90. 10.1046/j.1467-789X.2003.00099.x.
Hu FB, Manson JE, Willett WC: Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001, 20 (1): 5-19.
Vessby B: Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol. 2003, 14 (1): 15-9. 10.1097/00041433-200302000-00004.
Kreider RB: Effects of creatine supplementation on performance and training adaptations. Abstracts of 6th Internationl Conference on Guanidino Compounds in Biology and Medicine. 2001
Carli G, Bonifazi M, Lodi L, Lupo C, Martelli G, Viti A: Changes in the exercise-induced hormone response to branched chain amino acid administration. Eur J Appl Physiol Occup Physiol. 1992, 64 (3): 272-7. 10.1007/BF00626291.
Cade JR, Reese RH, Privette RM, Hommen NM, Rogers JL, Fregly MJ: Dietary intervention and training in swimmers. Eur J Appl Physiol Occup Physiol. 1991, 63 (3-4): 210-5. 10.1007/BF00233850.
Nieman DC, Fagoaga OR, Butterworth DE, Warren BJ, Utter A, Davis JM, Henson DA, Nehlsen-Cannarella SL: Carbohydrate supplementation affects blood granulocyte and monocyte trafficking but not function after 2.5 h or running. Am J Clin Nutr. 1997, 66 (1): 153-9.
Nieman DC: Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc Immunol Rev. 1998, 4: 64-76.
Nieman DC: Nutrition, exercise, and immune system function. Clin Sports Med. 1999, 18 (3): 537-48. 10.1016/S0278-5919(05)70167-8.
Burke LM: Nutritional needs for exercise in the heat. Comp Biochem Physiol A Mol Integr Physiol. 2001, 128 (4): 735-48. 10.1016/S1095-6433(01)00279-3.
Burke LM: Nutrition for post-exercise recovery. Aust J Sci Med Sport. 1997, 29 (1): 3-10.
Maughan RJ, Noakes TD: Fluid replacement and exercise stress. A brief review of studies on fluid replacement and some guidelines for the athlete. Sports Med. 1991, 12 (1): 16-31. 10.2165/00007256-199112010-00003.
Zawadzki KM, Yaspelkis BB, Ivy JL: Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J Appl Physiol. 1992, 72 (5): 1854-9.
Tarnopolsky MA, Bosman M, Macdonald JR, Vandeputte D, Martin J, Roy BD: Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. J Appl Physiol. 1997, 83 (6): 1877-83.
Kraemer WJ, Volek JS, Bush JA, Putukian M, Sebastianelli WJ: Hormonal responses to consecutive days of heavy-resistance exercise with or without nutritional supplementation. J Appl Physiol. 1998, 85 (4): 1544-55.
Jeukendrup AE, Currell K, Clarke J, Cole J, Blannin AK: Effect of beverage glucose and sodium content on fluid delivery. Nutr Metab (Lond). 2009, 6: 9-10.1186/1743-7075-6-9.
Article CAS Google Scholar
Rehrer NJ: Fluid and electrolyte balance in ultra-endurance sport. Sports Med. 2001, 31 (10): 701-15. 10.2165/00007256-200131100-00001.
Sawka MN, Montain SJ: Fluid and electrolyte supplementation for exercise heat stress. Am J Clin Nutr. 2000, 72 (2 Suppl): 564S-72S.
Shirreffs SM, Armstrong LE, Cheuvront SN: Fluid and electrolyte needs for preparation and recovery from training and competition. J Sports Sci. 2004, 22 (1): 57-63. 10.1080/0264041031000140572.
Brouns F, Kovacs EM, Senden JM: The effect of different rehydration drinks on post-exercise electrolyte excretion in trained athletes. Int J Sports Med. 1998, 19 (1): 56-60. 10.1055/s-2007-971881.
Kovacs EM, Senden JM, Brouns F: Urine color, osmolality and specific electrical conductance are not accurate measures of hydration status during postexercise rehydration. J Sports Med Phys Fitness. 1999, 39 (1): 47-53.
Kovacs EM, Schmahl RM, Senden JM, Brouns F: Effect of high and low rates of fluid intake on post-exercise rehydration. Int J Sport Nutr Exerc Metab. 2002, 12 (1): 14-23.
Meyer LG, Horrigan DJ, Lotz WG: Effects of three hydration beverages on exercise performance during 60 hours of heat exposure. Aviat Space Environ Med. 1995, 66 (11): 1052-7.
Williams MH: Facts and fallacies of purported ergogenic amino acid supplements. Clin Sports Med. 1999, 18 (3): 633-49. 10.1016/S0278-5919(05)70173-3.
Kreider RB: Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem. 2003, 244 (1-2): 89-94. 10.1023/A:1022465203458.
Volek JS, Duncan ND, Mazzetti SA, Putukian M, Gomez AL, Staron RS, Kraemer WJ: Performance and muscle fiber adaptations to 12 weeks of creatine supplementation and heavy resistance training. Medicine & Science in Sports & Exercise. 1999, 31 (5):
Willoughby DS, Rosene J: Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc. 2001, 33 (10): 1674-81. 10.1097/00005768-200110000-00010.
Willoughby DS, Rosene JM: Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003, 35 (6): 923-9. 10.1249/01.MSS.0000069746.05241.F0.
Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M: Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006, 573 (Pt 2): 525-34. 10.1113/jphysiol.2006.107359.
Williams MH, Kreider R, Branch JD: Creatine: The power supplement. 1999, Champaign, IL: Human Kinetics Publishers
Kreider R, Melton C, Hunt J, Rasmussen C, Ransom J, Stroud T, Cantler E, Milnor P: Creatine does not increase incidence of cramping or injury during pre-season college football training I. Med Sci Sports Exerc. 1999, 31 (5): S355-
Article Google Scholar
Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, Milnor P, Almada AL: Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem. 2003, 244 (1-2): 95-104. 10.1023/A:1022469320296.
Graham AS, Hatton RC: Creatine: a review of efficacy and safety. J Am Pharm Assoc (Wash). 1999, 39 (6): 803-10.
CAS Google Scholar
Juhn MS, Tarnopolsky M: Potential side effects of oral creatine supplementation: a critical review. Clin J Sport Med. 1998, 8 (4): 298-304.
Taes YE, Delanghe JR, Wuyts B, Voorde Van De J, Lameire NH: Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol Dial Transplant. 2003, 18 (2): 258-64. 10.1093/ndt/18.2.258.
Schilling BK, Stone MH, Utter A, Kearney JT, Johnson M, Coglianese R, Smith L, O'Bryant HS, Fry AC, Starks M, Keith R, Stone ME: Creatine supplementation and health variables: a retrospective study. Med Sci Sports Exerc. 2001, 33 (2): 183-8.
Greenwood M, Kreider R, Greenwood L, Byars A: Creatine supplementation does not increase the incidence of injury or cramping in college baseball players. Journal of Exercise Physiology online. 2003, 6 (4): 16-22.
Greenwood M, Kreider R, Greenwood L, Earnest C, Farris J, Brown L: Effects of creatine supplementation on the incidence of cramping/injury during eighteen weeks of collegiate baseball training/competition. Med Sci Sport Exerc. 2002, 34 (S146):
Watsford ML, Murphy AJ, Spinks WL, Walshe AD: Creatine supplementation and its effect on musculotendinous stiffness and performance. J Strength Cond Res. 2003, 17 (1): 26-33. 10.1519/1533-4287(2003)017<0026:CSAIEO>2.0.CO;2.
Dalbo VJ, Roberts MD, Stout JR, Kerksick CM: Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br J Sports Med. 2008, 42 (7): 567-73. 10.1136/bjsm.2007.042473.
Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J: International Society of Sports Nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr. 2007, 4: 6-10.1186/1550-2783-4-6.
Brown EC, DiSilvestro RA, Babaknia A, Devor ST: Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutr J. 2004, 3: 22-10.1186/1475-2891-3-22.
Candow DG, Burke NC, Smith-Palmer T, Burke DG: Effect of whey and soy protein supplementation combined with resistance training in young adults. Int J Sport Nutr Exerc Metab. 2006, 16 (3): 233-44.
Flakoll PJ, Judy T, Flinn K, Carr C, Flinn S: Postexercise protein supplementation improves health and muscle soreness during basic military training in Marine recruits. J Appl Physiol. 2004, 96 (3): 951-6. 10.1152/japplphysiol.00811.2003.
Kalman D, Feldman S, Martinez M, Krieger DR, Tallon MJ: Effect of protein source and resistance training on body composition and sex hormones. J Int Soc Sports Nutr. 2007, 4: 4-10.1186/1550-2783-4-4.
Biolo G, Williams BD, Fleming RY, Wolfe RR: Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999, 48 (5): 949-57. 10.2337/diabetes.48.5.949.
Borsheim E, Tipton KD, Wolf SE, Wolfe RR: Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002, 283 (4): E648-57.
Burk A, Timpmann S, Medijainen L, Vahi M, Oopik V: Time-divided ingestion pattern of casein-based protein supplement stimulates an increase in fat-free body mass during resistance training in young untrained men. Nutr Res. 2009, 29 (6): 405-13. 10.1016/j.nutres.2009.03.008.
Cribb PJ, Williams AD, Carey MF, Hayes A: The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. 2006, 16 (5): 494-509.
Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD: Effect of protein-supplement timing on strength, power, and body-composition changes in resistance-trained men. Int J Sport Nutr Exerc Metab. 2009, 19 (2): 172-85.
Holm L, Olesen JL, Matsumoto K, Doi T, Mizuno M, Alsted TJ, Mackey AL, Schwarz P, Kjaer M: Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol. 2008, 105 (1): 274-81. 10.1152/japplphysiol.00935.2007.
Kobayashi H, Borsheim E, Anthony TG, Traber DL, Badalamenti J, Kimball SR, Jefferson LS, Wolfe RR: Reduced amino acid availability inhibits muscle protein synthesis and decreases activity of initiation factor eIF2B. Am J Physiol Endocrinol Metab. 2003, 284 (3): E488-98.
Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR: Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc. 2003, 35 (3): 449-55. 10.1249/01.MSS.0000053910.63105.45.
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR: An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000, 88 (2): 386-92.
Rasmussen BB, Wolfe RR, Volpi E: Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002, 6 (6): 358-62.
PubMed Central CAS PubMed Google Scholar
Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR: Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001, 281 (2): E197-206.
Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ: Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009, 89 (2): 608-16. 10.3945/ajcn.2008.26626.
Willoughby DS, Stout JR, Wilborn CD: Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids. 2007, 32 (4): 467-77. 10.1007/s00726-006-0398-7.
Wolfe RR: Regulation of muscle protein by amino acids. J Nutr. 2002, 132 (10): 3219S-24S.
Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR: Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003, 284 (1): E76-89.
Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M: Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001, 535 (Pt 1): 301-11. 10.1111/j.1469-7793.2001.00301.x.
Garlick PJ: The role of leucine in the regulation of protein metabolism. J Nutr. 2005, 135 (6 Suppl): 1553S-6S.
Garlick PJ, Grant I: Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988, 254 (2): 579-84.
Nair KS: Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992, 263 (5 Pt 1): E928-34.
Wilson GJ, Wilson JM, Manninen AH: Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr Metab (Lond). 2008, 5: 1. 10.1186/1743-7075-5-1.
Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW: Beta-hydroxy-beta-methylbutyrate ingestion, Part I: effects on strength and fat free mass. Med Sci Sports Exerc. 2000, 32 (12): 2109-15. 10.1097/00005768-200012000-00022.
Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW: Beta-hydroxy-beta-methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Med Sci Sports Exerc. 2000, 32 (12): 2116-9. 10.1097/00005768-200012000-00023.
Nissen S, Sharp R, Ray M: Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance exercise testing. J Am Physiol. 1996, 81: 2095-104.
Panton LB, Rathmacher JA, Baier S, Nissen S: Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition. 2000, 16 (9): 734-9. 10.1016/S0899-9007(00)00376-2.
Slater GJ, Jenkins D: Beta-hydroxy-beta-methylbutyrate (HMB) supplementation and the promotion of muscle growth and strength. Sports Med. 2000, 30 (2): 105-16. 10.2165/00007256-200030020-00004.
Vukovich MD, Stubbs NB, Bohlken RM: Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr. 2001, 131 (7): 2049-52.
Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R: Effects of beta-hydroxy-beta-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol. 2000, 89 (4): 1340-4.
Smith HJ, Wyke SM, Tisdale MJ: Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res. 2004, 64 (23): 8731-5. 10.1158/0008-5472.CAN-04-1760.
Jowko E, Ostaszewski P, Jank M, Sacharuk J, Zieniewicz A, Wilczak J, Nissen S: Creatine and beta-hydroxy-beta-methylbutyrate (HMB) additively increase lean body mass and muscle strength during a weight-training program. Nutrition. 2001, 17 (7-8): 558-66. 10.1016/S0899-9007(01)00540-8.
O'Connor DM, Crowe MJ: Effects of beta-hydroxy-beta-methylbutyrate and creatine monohydrate supplementation on the aerobic and anaerobic capacity of highly trained athletes. J Sports Med Phys Fitness. 2003, 43 (1): 64-8.
Kreider RB, Ferreira M, Wilson M, Almada AL: Effects of calcium beta-hydroxy-beta-methylbutyrate (HMB) supplementation during resistance-training on markers of catabolism, body composition and strength. Int J Sports Med. 1999, 20 (8): 503-9. 10.1055/s-1999-8835.
Slater G, Jenkins D, Logan P, Lee H, Vukovich M, Rathmacher JA, Hahn AG: Beta-hydroxy-beta-methylbutyrate (HMB) supplementation does not affect changes in strength or body composition during resistance training in trained men. Int J Sport Nutr Exerc Metab. 2001, 11 (3): 384-96.
Ransone J, Neighbors K, Lefavi R, Chromiak J: The effect of beta-hydroxy beta-methylbutyrate on muscular strength and body composition in collegiate football players. J Strength Cond Res. 2003, 17 (1): 34-9. 10.1519/1533-4287(2003)017<0034:TEOHMO>2.0.CO;2.
Coombes JS, McNaughton LR: Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J Sports Med Phys Fitness. 2000, 40 (3): 240-6.
Schena F, Guerrini F, Tregnaghi P, Kayser B: Branched-chain amino acid supplementation during trekking at high altitude. The effects on loss of body mass, body composition, and muscle power. Eur J Appl Physiol Occup Physiol. 1992, 65 (5): 394-8. 10.1007/BF00243503.
Bigard AX, Lavier P, Ullmann L, Legrand H, Douce P, Guezennec CY: Branched-chain amino acid supplementation during repeated prolonged skiing exercises at altitude. Int J Sport Nutr. 1996, 6 (3): 295-306.
Candeloro N, Bertini I, Melchiorri G, De Lorenzo A: [Effects of prolonged administration of branched-chain amino acids on body composition and physical fitness]. Minerva Endocrinol. 1995, 20 (4): 217-23.
Stoppani J, Scheett TP, Pena J, Rudolph C, Charlebois D: Consuming a supplement containing branched-chain amino acids during a resistance-training program increases lean mass, muscle strength and fat loss. Journal of The International Society of Sport Nutrition. 2009, 6 (Suppl 1):
Wernerman J, Hammarqvist F, Vinnars E: Alpha-ketoglutarate and postoperative muscle catabolism. Lancet. 1990, 335 (8691): 701-3. 10.1016/0140-6736(90)90811-I.
Hammarqvist F, Wernerman J, Ali R, Vinnars E: Effects of an amino acid solution enriched with either branched chain amino acids or ornithine-alpha-ketoglutarate on the postoperative intracellular amino acid concentration of skeletal muscle. Br J Surg. 1990, 77 (2): 214-8. 10.1002/bjs.1800770227.
Antonio J, Stout JR: Sport Supplements. 2001, Philadelphia, PA: Lippincott, Williams and Wilkins
Mitch WE, Walser M, Sapir DG: Nitrogen sparing induced by leucine compared with that induced by its keto analogue, alpha-ketoisocaproate, in fasting obese man. J Clin Invest. 1981, 67 (2): 553-62. 10.1172/JCI110066.
Van Koevering M, Nissen S: Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol. 1992, 262 (1 Pt 1): E27-31.
Slama K, Koudela K, Tenora J, Mathova A: Insect hormones in vertebrates: anabolic effects of 20-hydroxyecdysone in Japanese quail. Experientia. 1996, 52 (7): 702-6. 10.1007/BF01925578.
Slama K, Kodkoua M: Insect hormones and bioanalogues: their effect on respiratory metabolism in Dermestes vulpinus L. (Coleoptera). Biol Bull. 1975, 148 (2): 320-32. 10.2307/1540550.
Tashmukhamedova MA, Almatov KT, Syrov VN, Sultanov MB, Abidov AA: [Effect of phytoecdisteroids and anabolic steroids on liver mitochondrial respiration and oxidative phosphorylation in alloxan diabetic rats]. Nauchnye Doki Vyss Shkoly Biol Nauki. 1985 (9): 37-9.
Syrov VN: [Mechanism of the anabolic action of phytoecdisteroids in mammals]. Nauchnye Doki Vyss Shkoly Biol Nauki. 1984 (11): 16-20.
Kholodova Y: Phytoecdysteroids: biological effects, application in agriculture and complementary medicine (as presented at the 14-th Ecdysone Workshop, July, 2000, Rapperswil, Switzerland). Ukr Biokhim Zh. 2001, 73 (3): 21-9.
Toth N, Szabo A, Kacsala P, Heger J, Zador E: 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine. 2008, 15 (9): 691-8. 10.1016/j.phymed.2008.04.015.
Wilborn C, Taylor L, Campbell B, Kerksick C, Rasmussen C, Greenwood M, Kreider R: Effects of methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. Journal of the International Society of Sports Nutrition. 2006, 3 (2): 10.1186/1550-2783-3-2-19.
Bowers CY: Growth hormone-releasing peptide (GHRP). Cell Mol Life Sci. 1998, 54 (12): 1316-29. 10.1007/s000180050257.
Camanni F, Ghigo E, Arvat E: Growth hormone-releasing peptides and their analogs. Front Neuroendocrinol. 1998, 19 (1): 47-72. 10.1006/frne.1997.0158.
Zachwieja JJ, Yarasheski KE: Does growth hormone therapy in conjunction with resistance exercise increase muscle force production and muscle mass in men and women aged 60 years or older? Growth hormone-releasing peptides and their analogs. Phys Ther. 1999, 79 (1): 76-82.
Coudray-Lucas C, Le Bever H, Cynober L, De Bandt JP, Carsin H: Ornithine alpha-ketoglutarate improves wound healing in severe burn patients: a prospective randomized double-blind trial versus isonitrogenous controls. Crit Care Med. 2000, 28 (6): 1772-6. 10.1097/00003246-200006000-00012.
Donati L, Ziegler F, Pongelli G, Signorini MS: Nutritional and clinical efficacy of ornithine alpha-ketoglutarate in severe burn patients. Clin Nutr. 1999, 18 (5): 307-11. 10.1016/S0261-5614(98)80029-0.
Chetlin RD, Yeater RA, Ullrich IH, Hornsby WG, Malanga CJ, Byrner RW: The effect of ornithine alpha-ketoglutarate (OKG) on healthy, weight trained men. J Exerc Physiol Online. 2000, 3 (4): [ http://faculty.css.edu/tboone2/asep/ChetlinV2.PDF ]
Brilla L, Conte V: Effects of a novel zinc-magnesium formulation on hormones and strength. J Exerc Physiol Online. 2000, 3: 26-36.
Wilborn CD, Kerksick CM, Campbell BI, Taylor LW, Marcello BM, Rasmussen CJ, Greenwood MC, Almada A, Kreider RB: Effects of Zinc Magnesium Aspartate (ZMA) Supplementation on Training Adaptations and Markers of Anabolism and Catabolism. J Int Soc Sports Nutr. 2004, 1 (2): 12-20. 10.1186/1550-2783-1-2-12.
Om AS, Chung KW: Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J Nutr. 1996, 126 (4): 842-8.
Low SY, Taylor PM, Rennie MJ: Responses of glutamine transport in cultured rat skeletal muscle to osmotically induced changes in cell volume. J Physiol. 1996, 492: 877-85.
Rennie MJ, Ahmed A, Khogali SE, Low SY, Hundal HS, Taylor PM: Glutamine metabolism and transport in skeletal muscle and heart and their clinical relevance. J Nutr. 1996, 126 (3): 1142S-9S.
Varnier M, Leese GP, Thompson J, Rennie MJ: Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Am J Physiol. 1995, 269: E309-15.
Colker CM: Effects of supplemental protein on body composition and muscular strength in healthy athletic male adults. Curr Ther Res. 2000, 61 (1): 19-28. 10.1016/S0011-393X(00)88492-1.
Candow DG, Chilibeck PD, Burke DG, Davison KS, Smith-Palmer T: Effect of glutamine supplementation combined with resistance training in young adults. Eur J Appl Physiol. 2001, 86 (2): 142-9. 10.1007/s00421-001-0523-y.
Messina M: Soyfoods and soybean phyto-oestrogens (isoflavones) as possible alternatives to hormone replacement therapy (HRT). Eur J Cancer. 2000, 36 (Suppl 4): S71-2. 10.1016/S0959-8049(00)00233-1.
Messina M, Messina V: Soyfoods, soybean isoflavones, and bone health: a brief overview. J Ren Nutr. 2000, 10 (2): 63-8. 10.1016/S1051-2276(00)90001-3.
de Aloysio D, Gambacciani M, Altieri P, Ciaponi M, Ventura V, Mura M, Genazzani AR, Bottiglioni F: Bone density changes in postmenopausal women with the administration of ipriflavone alone or in association with low-dose ERT. Gynecol Endocrinol. 1997, 11 (4): 289-93. 10.3109/09513599709152548.
Slogoff S, Keats AS, Cooley DA, Reul GJ, Frazier OH, Ott DA, Duncan JM, Livesay JJ: Addition of papaverine to cardioplegia does not reduce myocardial necrosis. Ann Thorac Surg. 1986, 42 (1): 60-4.
Smart NA, McKenzie SG, Nix LM, Baldwin SE, Page K, Wade D, Hampson PK: Creatine supplementation does not improve repeat sprint performance in soccer players. Medicine & Science in Sports & Exercise. 1998, 30 (5): S140-10.1097/00005768-199805001-00794.
Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ: Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: a randomized double-blind controlled trial. Eur J Clin Nutr. 2007, 61 (12): 1442-4. 10.1038/sj.ejcn.1602695.
Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S: Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA. 1998, 95 (25): 14938-43. 10.1073/pnas.95.25.14938.
McPherron AC, Lawler AM, Lee SJ: Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997, 387 (6628): 83-90. 10.1038/387083a0.
McPherron AC, Lee SJ: Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997, 94 (23): 12457-61. 10.1073/pnas.94.23.12457.
Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M: A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997, 17 (1): 71-4. 10.1038/ng0997-71.
Kambadur R, Sharma M, Smith TP, Bass JJ: Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997, 7 (9): 910-6.
Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF: Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000, 55 (11): M641-8.
Carlson CJ, Booth FW, Gordon SE: Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999, 277 (2 Pt 2): R601-6.
Willoughby DS: Effects of an alleged myostatin-binding supplement and heavy resistance training on serum myostatin, muscle strength and mass, and body composition. Int J Sport Nutr Exerc Metab. 2004, 14 (4): 461-72.
Saremi A, Gharakhanloo R, Sharghi S, Gharaati MR, Larijani B, Omidfar K: Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol. 2009
Green NR, Ferrando AA: Plasma boron and the effects of boron supplementation in males. Environ Health Perspect. 1994, 102 (Suppl 7): 73-7. 10.2307/3431966.
Ferrando AA, Green NR: The effect of boron supplementation on lean body mass, plasma testosterone levels, and strength in male bodybuilders. Int J Sport Nutr. 1993, 3 (2): 140-9.
Evans GW: The effect of chromium picolinate on insulin controlled parameters in humans. Int Biosc Med Res. 1989, 11: 163-80.
Hasten DL, Rome EP, Franks BD, Hegsted M: Effects of chromium picolinate on beginning weight training students. Int J Sport Nutr. 1992, 2 (4): 343-50.
Grant KE, Chandler RM, Castle AL, Ivy JL: Chromium and exercise training: effect on obese women. Med Sci Sports Exerc. 1997, 29 (8): 992-8.
Campbell WW, Joseph LJ, Anderson RA, Davey SL, Hinton J, Evans WJ: Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int J Sport Nutr Exerc Metab. 2002, 12 (2): 125-35.
Campbell WW, Joseph LJ, Davey SL, Cyr-Campbell D, Anderson RA, Evans WJ: Effects of resistance training and chromium picolinate on body composition and skeletal muscle in older men. J Appl Physiol. 1999, 86 (1): 29-39.
Walker LS, Bemben MG, Bemben DA, Knehans AW: Chromium picolinate effects on body composition and muscular performance in wrestlers. Med Sci Sports Exerc. 1998, 30 (12): 1730-7. 10.1097/00005768-199812000-00012.
Livolsi JM, Adams GM, Laguna PL: The effect of chromium picolinate on muscular strength and body composition in women athletes. J Strength Cond Res. 2001, 15 (2): 161-6. 10.1519/1533-4287(2001)015<0161:TEOCPO>2.0.CO;2.
Volpe SL, Huang HW, Larpadisorn K, Lesser II: Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. J Am Coll Nutr. 2001, 20 (4): 293-306.
Hallmark MA, Reynolds TH, DeSouza CA, Dotson CO, Anderson RA, Rogers MA: Effects of chromium and resistive training on muscle strength and body composition. Med Sci Sports Exerc. 1996, 28 (1): 139-44. 10.1097/00005768-199601000-00025.
Lukaski HC, Bolonchuk WW, Siders WA, Milne DB: Chromium supplementation and resistance training: effects on body composition, strength, and trace element status of men. Am J Clin Nutr. 1996, 63 (6): 954-65.
Clancy SP, Clarkson PM, DeCheke ME, Nosaka K, Freedson PS, Cunningham JJ, Valentine B: Effects of chromium picolinate supplementation on body composition, strength, and urinary chromium loss in football players. Int J Sport Nutr. 1994, 4 (2): 142-53.
Pariza MW, Park Y, Cook ME: Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999, 52 (2 Suppl): 107-l10.
Pariza MW, Park Y, Cook ME: Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med. 2000, 223 (1): 8-13. 10.1046/j.1525-1373.2000.22302.x.
Pariza MW, Park Y, Cook ME: The biologically active isomers of conjugated linoleic acid. Prog Lipid Res. 2001, 40 (4): 283-98. 10.1016/S0163-7827(01)00008-X.
DeLany JP, Blohm F, Truett AA, Scimeca JA, West DB: Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am J Physiol. 1999, 276 (4 Pt 2): R1172-9.
DeLany JP, West DB: Changes in body composition with conjugated linoleic acid. J Am Coll Nutr. 2000, 19 (4): 487S-93S.
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW: Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997, 32 (8): 853-8. 10.1007/s11745-997-0109-x.
Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O: Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000, 130 (12): 2943-8.
Gaullier JM, Berven G, Blankson H, Gudmundsen O: Clinical trial results support a preference for using CLA preparations enriched with two isomers rather than four isomers in human studies. Lipids. 2002, 37 (11): 1019-25. 10.1007/s11745-002-0995-y.
Pinkoski C, Chilibeck PD, Candow DG, Esliger D, Ewaschuk JB, Facci M, Farthing JP, Zello GA: The effects of conjugated linoleic acid supplementation during resistance training. Med Sci Sports Exerc. 2006, 38 (2): 339-48. 10.1249/01.mss.0000183860.42853.15.
Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, Roy B, Doherty T: Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007, 2 (10): e991-10.1371/journal.pone.0000991.
Campbell B, Kreider RB: Conjugated linoleic acids. Curr Sports Med Rep. 2008, 7 (4): 237-41.
Wheeler KB, Garleb KA: Gamma oryzanol-plant sterol supplementation: metabolic, endocrine, and physiologic effects. Int J Sport Nutr. 1991, 1 (2): 170-7.
Fry AC, Bonner E, Lewis DL, Johnson RL, Stone MH, Kraemer WJ: The effects of gamma-oryzanol supplementation during resistance exercise training. Int J Sport Nutr. 1997, 7 (4): 318-29.
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW: Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005, 90 (2): 678-88. 10.1210/jc.2004-1184.
Kuhn CM: Anabolic steroids. Recent Prog Horm Res. 2002, 57: 411-34. 10.1210/rp.57.1.411.
Limbird TJ: Anabolic steroids in the training and treatment of athletes. Compr Ther. 1985, 11 (1): 25-30.
Lukas SE: Current perspectives on anabolic-androgenic steroid abuse. Trends Pharmacol Sci. 1993, 14 (2): 61-8. 10.1016/0165-6147(93)90032-F.
Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP: Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009, 94 (6): 1991-2001. 10.1210/jc.2008-2338.
Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, Bhasin S: Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008, 56 (11): 1991-9. 10.1111/j.1532-5415.2008.01927.x.
Wagner JC: Enhancement of athletic performance with drugs. An overview. Sports Med. 1991, 12 (4): 250-65. 10.2165/00007256-199112040-00004.
Yarasheski KE: Growth hormone effects on metabolism, body composition, muscle mass, and strength. Exerc Sport Sci Rev. 1994, 22: 285-312. 10.1249/00003677-199401000-00013.
Smart T: Other therapies for wasting. GMHC Treat Issues. 1995, 9 (5): 7-8. 12
Casaburi R: Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2001, 33 (7 Suppl): S662-70.
Hayes VY, Urban RJ, Jiang J, Marcell TJ, Helgeson K, Mauras N: Recombinant human growth hormone and recombinant human insulin-like growth factor I diminish the catabolic effects of hypogonadism in man: metabolic and molecular effects. J Clin Endocrinol Metab. 2001, 86 (5): 2211-9. 10.1210/jc.86.5.2211.
Newshan G, Leon W: The use of anabolic agents in HIV disease. Int J STD AIDS. 2001, 12 (3): 141-4. 10.1258/0956462011916893.
Tenover JS: Androgen replacement therapy to reverse and/or prevent age-associated sarcopenia in men. Baillieres Clin Endocrinol Metab. 1998, 12 (3): 419-25. 10.1016/S0950-351X(98)80153-5.
Bross R, Casaburi R, Storer TW, Bhasin S: Androgen effects on body composition and muscle function: implications for the use of androgens as anabolic agents in sarcopenic states. Baillieres Clin Endocrinol Metab. 1998, 12 (3): 365-78. 10.1016/S0950-351X(98)80077-3.
Casaburi R: Rationale for anabolic therapy to facilitate rehabilitation in chronic obstructive pulmonary disease. Baillieres Clin Endocrinol Metab. 1998, 12 (3): 407-18. 10.1016/S0950-351X(98)80134-1.
Johansen KL, Mulligan K, Schambelan M: Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. Jama. 1999, 281 (14): 1275-81. 10.1001/jama.281.14.1275.
Sattler FR, Jaque SV, Schroeder ET, Olson C, Dube MP, Martinez C, Briggs W, Horton R, Azen S: Effects of pharmacological doses of nandrolone decanoate and progressive resistance training in immunodeficient patients infected with human immunodeficiency virus. J Clin Endocrinol Metab. 1999, 84 (4): 1268-76. 10.1210/jc.84.4.1268.
Beiner JM, Jokl P, Cholewicki J, Panjabi MM: The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999, 27 (1): 2-9.
Ferreira IM, Verreschi IT, Nery LE, Goldstein RS, Zamel N, Brooks D, Jardim JR: The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest. 1998, 114 (1): 19-28. 10.1378/chest.114.1.19.
Bhasin S, Bremner WJ: Clinical review 85: Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab. 1997, 82 (1): 3-8. 10.1210/jc.82.1.3.
Hoffman JR, Kraemer WJ, Bhasin S, Storer T, Ratamess NA, Haff GG, Willoughby DS, Rogol AD: Position stand on androgen and human growth hormone use. J Strength Cond Res. 2009, 23 (5 Suppl): S1-S59.
Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ: Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003, 88 (1): 358-62. 10.1210/jc.2002-021041.
Meeuwsen IB, Samson MM, Duursma SA, Verhaar HJ: Muscle strength and tibolone: a randomised, double-blind, placebo-controlled trial. Bjog. 2002, 109 (1): 77-84.
King DS, Sharp RL, Vukovich MD, Brown GA, Reifenrath TA, Uhl NL, Parsons KA: Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men: a randomized controlled trial. Jama. 1999, 281 (21): 2020-8. 10.1001/jama.281.21.2020.
Carter WJ: Effect of anabolic hormones and insulin-like growth factor-I on muscle mass and strength in elderly persons. Clin Geriatr Med. 1995, 11 (4): 735-48.
Soe M, Jensen KL, Gluud C: [The effect of anabolic androgenic steroids on muscle strength, body weight and lean body mass in body-building men]. Ugeskr Laeger. 1989, 151 (10): 610-3.
Griggs RC, Pandya S, Florence JM, Brooke MH, Kingston W, Miller JP, Chutkow J, Herr BE, Moxley RT: Randomized controlled trial of testosterone in myotonic dystrophy. Neurology. 1989, 39 (2 Pt 1): 219-22.
Crist DM, Stackpole PJ, Peake GT: Effects of androgenic-anabolic steroids on neuromuscular power and body composition. J Appl Physiol. 1983, 54 (2): 366-70.
Ward P: The effect of an anabolic steroid on strength and lean body mass. Med Sci Sports. 1973, 5 (4): 277-82.
Varriale P, Mirzai-tehrane M, Sedighi A: Acute myocardial infarction associated with anabolic steroids in a young HIV-infected patient. Pharmacotherapy. 1999, 19 (7): 881-4. 10.1592/phco.19.10.881.31552.
Kibble MW, Ross MB: Adverse effects of anabolic steroids in athletes. Clin Pharm. 1987, 6 (9): 686-92.
Gruber AJ, Pope HG: Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000, 69 (1): 19-26. 10.1159/000012362.
Lamb DR: Anabolic steroids in athletics: how well do they work and how dangerous are they? Am. J Sports Med. 1984, 12 (1): 31-8. 10.1177/036354658401200105.
Salke RC, Rowland TW, Burke EJ: Left ventricular size and function in body builders using anabolic steroids. Med Sci Sports Exerc. 1985, 17 (6): 701-4. 10.1249/00005768-198512000-00014.
Brown GA, Martini ER, Roberts BS, Vukovich MD, King DS: Acute hormonal response to sublingual androstenediol intake in young men. J Appl Physiol. 2002, 92 (1): 142-6.
Brown GA, McKenzie D: Acute resistance exercise does not change the hormonal response to sublingual androstenediol intake. Eur J Appl Physiol. 2006, 97 (4): 404-12. 10.1007/s00421-006-0194-9.
Broeder CE, Quindry J, Brittingham K, Panton L, Thomson J, Appakondu S, Breuel K, Byrd R, Douglas J, Earnest C, Mitchell C, Olson M, Roy T, Yarlagadda C: The Andro Project: physiological and hormonal influences of androstenedione supplementation in men 35 to 65 years old participating in a high-intensity resistance training program. Arch Intern Med. 2000, 160 (20): 3093-104. 10.1001/archinte.160.20.3093.
Ballantyne CS, Phillips SM, MacDonald JR, Tarnopolsky MA, MacDougall JD: The acute effects of androstenedione supplementation in healthy young males. Can J Appl Physiol. 2000, 25 (1): 68-78.
Brown GA, Vukovich MD, Sharp RL, Reifenrath TA, Parsons KA, King DS: Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol. 1999, 87 (6): 2274-83.
van Gammeren D, Falk D, Antonio J: Effects of norandrostenedione and norandrostenediol in resistance-trained men. Nutrition. 2002, 18 (9): 734-7. 10.1016/S0899-9007(02)00834-1.
Van Gammeren D, Falk D, Antonio J: The effects of supplementation with 19-nor-4-androstene-3,17-dione and 19-nor-4-androstene-3,17-diol on body composition and athletic performance in previously weight-trained male athletes. Eur J Appl Physiol. 2001, 84 (5): 426-31. 10.1007/s004210100395.
Pipe A: Effects of testosterone precursor supplementation on intensive weight training. Clin J Sport Med. 2001, 11 (2): 126-10.1097/00042752-200104000-00014.
Mauras N, Lima J, Patel D, Rini A, di Salle E, Kwok A, Lippe B: Pharmacokinetics and dose finding of a potent aromatase inhibitor, aromasin (exemestane), in young males. J Clin Endocrinol Metab. 2003, 88 (12): 5951-6. 10.1210/jc.2003-031279.
Rohle D, Wilborn C, Taylor L, Mulligan C, Kreider R, Willoughby D: Effects of eight weeks of an alleged aromatase inhibiting nutritional supplement 6-OXO (androst-4-ene-3,6,17-trione) on serum hormone profiles and clinical safety markers in resistance-trained, eugonadal males. J Int Soc Sports Nutr. 2007, 4: 13-10.1186/1550-2783-4-13.
Willoughby DS, Wilborn C, Taylor L, Campbell W: Eight weeks of aromatase inhibition using the nutritional supplement Novedex XT: effects in young, eugonadal men. Int J Sport Nutr Exerc Metab. 2007, 17 (1): 92-108.
Antonio J, Uelmen J, Rodriguez R, Earnest C: The effects of Tribulus terrestris on body composition and exercise performance in resistance-trained males. Int J Sport Nutr Exerc Metab. 2000, 10 (2): 208-15.
Brown GA, Vukovich MD, Martini ER, Kohut ML, Franke WD, Jackson DA, King DS: Effects of androstenedione-herbal supplementation on serum sex hormone concentrations in 30- to 59-year-old men. Int J Vitam Nutr Res. 2001, 71 (5): 293-301. 10.1024/0300-9831.71.5.293.
Rogerson S, Riches CJ, Jennings C, Weatherby RP, Meir RA, Marshall-Gradisnik SM: The effect of five weeks of Tribulus terrestris supplementation on muscle strength and body composition during preseason training in elite rugby league players. J Strength Cond Res. 2007, 21 (2): 348-53. 10.1519/R-18395.1.
Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L: Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1995, 95 (6): 2501-9. 10.1172/JCI117951.
Boden G, Chen X, Ruiz J, van Rossum GD, Turco S: Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1996, 45 (9): 1130-5. 10.1016/S0026-0495(96)90013-X.
Halberstam M, Cohen N, Shlimovich P, Rossetti L, Shamoon H: Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes. 1996, 45 (5): 659-66. 10.2337/diabetes.45.5.659.
Fawcett JP, Farquhar SJ, Walker RJ, Thou T, Lowe G, Goulding A: The effect of oral vanadyl sulfate on body composition and performance in weight-training athletes. Int J Sport Nutr. 1996, 6 (4): 382-90.
Fawcett JP, Farquhar SJ, Thou T, Shand BI: Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans. Pharmacol Toxicol. 1997, 80 (4): 202-6. 10.1111/j.1600-0773.1997.tb00397.x.
Kreider R: New weight-control options. Func Foods Nutraceut. 2002, 34-42.
Hoie LH, Bruusgaard D, Thom E: Reduction of body mass and change in body composition on a very low calorie diet. Int J Obes Relat Metab Disord. 1993, 17 (1): 17-20.
Bryner RW, Ullrich IH, Sauers J, Donley D, Hornsby G, Kolar M, Yeater R: Effects of resistance vs. aerobic training combined with an 800 calorie liquid diet on lean body mass and resting metabolic rate. J Am Coll Nutr. 1999, 18 (2): 115-21.
Meckling KA, Sherfey R: A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the Metabolic Syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2007, 32 (4): 743-52. 10.1139/H07-059.
Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T: Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition. 2000, 16 (5): 349-54. 10.1016/S0899-9007(00)00230-6.
Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA: High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 1999, 23 (11): 1202-6. 10.1038/sj.ijo.0801064.
Clifton P: High protein diets and weight control. Nutr Metab Cardiovasc Dis. 2009, 19 (6): 379-82. 10.1016/j.numecd.2009.02.011.
Heymsfield SB, van Mierlo CA, Knaap van der HC, Heo M, Frier HI: Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003, 27 (5): 537-49. 10.1038/sj.ijo.0802258.
Skov AR, Toubro S, Ronn B, Holm L, Astrup A: Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999, 23 (5): 528-36. 10.1038/sj.ijo.0800867.
Toubro S, Astrup AV: [A randomized comparison of two weight-reducing diets. Calorie counting versus low-fat carbohydrate-rich ad libitum diet]. Ugeskr Laeger. 1998, 160 (6): 816-20.
Wal JS, McBurney MI, Cho S, Dhurandhar NV: Ready-to-eat cereal products as meal replacements for weight loss. Int J Food Sci Nutr. 2007, 58 (5): 331-40. 10.1080/09637480701240802.
Reaven GM: Diet and Syndrome X. Curr Atheroscler Rep. 2000, 2 (6): 503-7. 10.1007/s11883-000-0050-z.
Treyzon L, Chen S, Hong K, Yan E, Carpenter CL, Thames G, Bowerman S, Wang HJ, Elashoff R, Li Z: A controlled trial of protein enrichment of meal replacements for weight reduction with retention of lean body mass. Nutr J. 2008, 7: 23-10.1186/1475-2891-7-23.
Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M: A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009, 15 (25): 3073-85. 10.3748/wjg.15.3073.
Greenway FL, De Jonge L, Blanchard D, Frisard M, Smith SR: Effect of a dietary herbal supplement containing caffeine and ephedra on weight, metabolic rate, and body composition. Obes Res. 2004, 12 (7): 1152-7. 10.1038/oby.2004.144.
Coffey CS, Steiner D, Baker BA, Allison DB: A randomized double-blind placebo-controlled clinical trial of a product containing ephedrine, caffeine, and other ingredients from herbal sources for treatment of overweight and obesity in the absence of lifestyle treatment. Int J Obes Relat Metab Disord. 2004, 28 (11): 1411-9. 10.1038/sj.ijo.0802784.
Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA, Strauss R, Meredith T: Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obes Relat Metab Disord. 2002, 26 (5): 593-604. 10.1038/sj.ijo.0802023.
Boozer C, Nasser J, SB H, Wang V, Chen G, Solomon J: An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. Int J Obes Relat Metab Disord. 2001, 25: 316-24. 10.1038/sj.ijo.0801539.
Boozer C, Daly P, Homel P, Solomon J, Blanchard D, Nasser J, Strauss R, Merideth T: Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int J Obesity. 2002, 26: 593-604. 10.1038/sj.ijo.0802023.
Molnar D, Torok K, Erhardt E, Jeges S: Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double-blind placebo-controlled pilot study in adolescents. Int J Obes Relat Metab Disord. 2000, 24 (12): 1573-8. 10.1038/sj.ijo.0801433.
Molnar D: Effects of ephedrine and aminophylline on resting energy expenditure in obese adolescents. Int J Obes Relat Metab Disord. 1993, 17 (Suppl 1): S49-52.
Greenway FL: The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev. 2001, 2 (3): 199-211. 10.1046/j.1467-789x.2001.00038.x.
Greenway F, Raum W, DeLany J: The effect of an herbal dietary supplement containing ephedrine and caffeine on oxygen consumption in humans. J Altern Complement Med. 2000, 6 (6): 553-5. 10.1089/acm.2000.6.553.
Greenway F, Herber D, Raum W, Morales S: Double-blind, randomized, placebo-controlled clinical trials with non-prescription medications for the treatment of obesity. Obes Res. 1999, 7 (4): 370-8.
Greenway FL, Ryan DH, Bray GA, Rood JC, Tucker EW, Smith SR: Pharmaceutical cost savings of treating obesity with weight loss medications. Obes Res. 1999, 7 (6): 523-31.
Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM, Stohs SJ, Stern JS, Keen CL: Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int J Obes (Lond). 2006, 30 (10): 1545-56. 10.1038/sj.ijo.0803283.
Bent S, Tiedt T, Odden M, Shlipak M: The relative safety of ephedra compared with other herbal products. Ann Intern Med. 2003, 138: 468-471.
Fleming GA: The FDA, regulation, and the risk of stroke. N Engl J Med. 2000, 343 (25): 1886-7. 10.1056/NEJM200012213432510.
Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL: Health benefits of dietary fiber. Nutr Rev. 2009, 67 (4): 188-205. 10.1111/j.1753-4887.2009.00189.x.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ: Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008, 359 (3): 229-41. 10.1056/NEJMoa0708681.
Raben A, Jensen ND, Marckmann P, Sandstrom B, Astrup AV: [Spontaneous weight loss in young subjects of normal weight after 11 weeks of unrestricted intake of a low-fat/high-fiber diet]. Ugeskr Laeger. 1997, 159 (10): 1448-53.
Melanson KJ, Angelopoulos TJ, Nguyen VT, Martini M, Zukley L, Lowndes J, Dube TJ, Fiutem JJ, Yount BW, Rippe JM: Consumption of whole-grain cereals during weight loss: effects on dietary quality, dietary fiber, magnesium, vitamin B-6, and obesity. J Am Diet Assoc. 2006, 106 (9): 1380-8. 10.1016/j.jada.2006.06.003. quiz 9-90
Nieman DC, Cayea EJ, Austin MD, Henson DA, McAnulty SR, Jin F: Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr Res. 2009, 29 (6): 414-8. 10.1016/j.nutres.2009.05.011.
Saltzman E, Moriguti JC, Das SK, Corrales A, Fuss P, Greenberg AS, Roberts SB: Effects of a cereal rich in soluble fiber on body composition and dietary compliance during consumption of a hypocaloric diet. J Am Coll Nutr. 2001, 20 (1): 50-7.
Sartorelli DS, Franco LJ, Cardoso MA: High intake of fruits and vegetables predicts weight loss in Brazilian overweight adults. Nutr Res. 2008, 28 (4): 233-8. 10.1016/j.nutres.2008.02.004.
Barr SI: Increased dairy product or calcium intake: is body weight or composition affected in humans?. J Nutr. 2003, 133 (1): 245S-8S.
Lanou AJ, Barnard ND: Dairy and weight loss hypothesis: an evaluation of the clinical trials. Nutr Rev. 2008, 66 (5): 272-9. 10.1111/j.1753-4887.2008.00032.x.
Menon VB, Baxmann AC, Froeder L, Martini LA, Heilberg IP: Effects of calcium supplementation on body weight reduction in overweight calcium stone formers. Urol Res. 2009, 37 (3): 133-9. 10.1007/s00240-009-0187-3.
Shapses SA, Heshka S, Heymsfield SB: Effect of calcium supplementation on weight and fat loss in women. J Clin Endocrinol Metab. 2004, 89 (2): 632-7. 10.1210/jc.2002-021136.
Wagner G, Kindrick S, Hertzler S, DiSilvestro RA: Effects of various forms of calcium on body weight and bone turnover markers in women participating in a weight loss program. J Am Coll Nutr. 2007, 26 (5): 456-61.
Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC, Sebring NG, McHugh T: Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med. 2009, 150 (12): 821-9. W145-6
Zemel M, Thompson W, Zemel P, Nocton A, Milstead A, Morris K, Campbell P: Dietary calcium and dairy products accelerate weight and fat-loss during energy restriction in obese adults. Clin Nutri. 2002, 75-
Zemel MB: Role of dietary calcium and dairy products in modulating adiposity. Lipids. 2003, 38 (2): 139-46. 10.1007/s11745-003-1044-6.
Zemel MB: Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. 2002, 21 (2): 146S-51S.
Zemel MB: Mechanisms of dairy modulation of adiposity. J Nutr. 2003, 133 (1): 252S-6S.
Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC: Regulation of adiposity by dietary calcium. Faseb J. 2000, 14 (9): 1132-8.
Davies KM, Heaney RP, Recker RR, Lappe JM, Barger-Lux MJ, Rafferty K, Hinders S: Calcium intake and body weight. J Clin Endocrinol Metab. 2000, 85 (12): 4635-8. 10.1210/jc.85.12.4635.
Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low Dog T: Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf. 2008, 31 (6): 469-84. 10.2165/00002018-200831060-00003.
Nagle DG, Ferreira D, Zhou YD: Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006, 67 (17): 1849-55. 10.1016/j.phytochem.2006.06.020.
Shixian Q, VanCrey B, Shi J, Kakuda Y, Jiang Y: Green tea extract thermogenesis-induced weight loss by epigallocatechin gallate inhibition of catechol-O-methyltransferase. J Med Food. 2006, 9 (4): 451-8. 10.1089/jmf.2006.9.451.
Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, Yamanaka K, Miyazawa T: Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J Agric Food Chem. 1999, 47 (10): 3967-73. 10.1021/jf981195l.
Dulloo A, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J: Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 2000, 70 (6): 1040-5.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J: Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999, 70 (6): 1040-5.
Di Pierro F, Menghi AB, Barreca A, Lucarelli M, Calandrelli A: Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: a clinical trial. Altern Med Rev. 2009, 14 (2): 154-60.
Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, Jones F, Blumberg JB, Cartwright Y: Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr. 2009, 139 (2): 264-70.
Fallon E, Zhong L, Furne JK, Levitt M: A mixture of extracts of black and green teas and mulberry leaf did not reduce weight gain in rats fed a high-fat diet. Altern Med Rev. 2008, 13 (1): 43-9.
Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P: Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2008, 27 (3): 363-70. 10.1016/j.clnu.2008.03.007.
MacDonald HB: Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr. 2000, 19 (2 Suppl): 111S-8S.
Park Y, Albright KJ, Storkson JM, Liu W, Cook ME, Pariza MW: Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids. 1999, 34 (3): 243-8. 10.1007/s11745-999-0359-7.
Colakoglu S, Colakoglu M, Taneli F, Cetinoz F, Turkmen M: Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J Sports Med Phys Fitness. 2006, 46 (4): 570-7.
Lowery LM, Appicelli PA, PWR L: Conjugated linoleic acid enhances muscle size and strength gains in novice bodybuilders. Med Sci Sports Exerc. 1998, 30 (5): S182-
Riserus U, Arner P, Brismar K, Vessby B: Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002, 25 (9): 1516-21. 10.2337/diacare.25.9.1516.
Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B: Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation. 2002, 106 (15): 1925-9. 10.1161/01.CIR.0000033589.15413.48.
Riserus U, Berglund L, Vessby B: Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: a randomised controlled trial. Int J Obes Relat Metab Disord. 2001, 25 (8): 1129-35. 10.1038/sj.ijo.0801659.
Thom E, Wadstein J, Gudmundsen O: Conjugated linoleic acid reduces body fat in healthy exercising humans. J Int Med Res. 2001, 29 (5): 392-6.
Cornish SM, Candow DG, Jantz NT, Chilibeck PD, Little JP, Forbes S, Abeysekara S, Zello GA: Conjugated linoleic acid combined with creatine monohydrate and whey protein supplementation during strength training. Int J Sport Nutr Exerc Metab. 2009, 19 (1): 79-96.
Beuker F, Haak H, Schwietz H, editors: CLA and body styling. Symposium: Vitamine und Zusatzstoffe; Jena (Thhr.). 1999
Kreider RB, Ferreira MP, Greenwood M, Wilson M, Almada AL: Effects of conjugated linoleic acid supplementation during resistance training on body composition, bone density, strength, and selected hematological markers. J Strength Cond Res. 2002, 16 (3): 325-34. 10.1519/1533-4287(2002)016<0325:EOCLAS>2.0.CO;2.
Malpuech-Brugere C, Verboeket-van de Venne WP, Mensink RP, Arnal MA, Morio B, Brandolini M, Saebo A, Lassel TS, Chardigny JM, Sebedio JL, Beaufrere B: Effects of two conjugated linoleic Acid isomers on body fat mass in overweight humans. Obes Res. 2004, 12 (4): 591-8. 10.1038/oby.2004.68.
Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, Nelson GJ, Erickson KL: Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Lipids. 2000, 35 (7): 783-8. 10.1007/s11745-000-0586-y.
Salas-Salvado J, Marquez-Sandoval F, Bullo M: Conjugated linoleic acid intake in humans: a systematic review focusing on its effect on body composition, glucose, and lipid metabolism. Crit Rev Food Sci Nutr. 2006, 46 (6): 479-88. 10.1080/10408390600723953.
Von Loeffelholz C: Influence of conjugated linoleic acid (CLA) supplementation on body composition and strength in bodybuilders. Jena (Thnr). 1999, 7: 238-43.
Wang Y, Jones PJ: Dietary conjugated linoleic acid and body composition. Am J Clin Nutr. 2004, 79 (6 Suppl): 1153S-8S.
Wang YW, Jones PJ: Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord. 2004, 28 (8): 941-55. 10.1038/sj.ijo.0802641.
Zambell KL, Keim NL, Van Loan MD, Gale B, Benito P, Kelley DS, Nelson GJ: Conjugated linoleic acid supplementation in humans: effects on body composition and energy expenditure. Lipids. 2000, 35 (7): 777-82. 10.1007/s11745-000-0585-z.
Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, Winzell MS, Wahle KW, Ahren B, Williams LM: Effect of a conjugated linoleic acid and omega-3 fatty acid mixture on body composition and adiponectin. Obesity (Silver Spring). 2008, 16 (5): 1019-24. 10.1038/oby.2008.41.
Shigematsu N, Asano R, Shimosaka M, Okazaki M: Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol Pharm Bull. 2001, 24 (6): 713-7. 10.1248/bpb.24.713.
Shigematsu N, Asano R, Shimosaka M, Okazaki M: Effect of long term-administration with Gymnema sylvestre R. BR on plasma and liver lipid in rats. Biol Pharm Bull. 2001, 24 (6): 643-9. 10.1248/bpb.24.643.
Luo H, Kashiwagi A, Shibahara T, Yamada K: Decreased bodyweight without rebound and regulated lipoprotein metabolism by gymnemate in genetic multifactor syndrome animal. Mol Cell Biochem. 2007, 299 (1-2): 93-8. 10.1007/s11010-005-9049-7.
Preuss HG, Rao CV, Garis R, Bramble JD, Ohia SE, Bagchi M, Bagchi D: An overview of the safety and efficacy of a novel, natural(-)-hydroxycitric acid extract (HCA-SX) for weight management. J Med. 2004, 35 (1-6): 33-48.
Garcia Neto M, Pesti GM, Bakalli RI: Influence of dietary protein level on the broiler chicken's response to methionine and betaine supplements. Poult Sci. 2000, 79 (10): 1478-84.
Schwab U, Torronen A, Toppinen L, Alfthan G, Saarinen M, Aro A, Uusitupa M: Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002, 76 (5): 961-7.
Hoffman JR, Ratamess NA, Kang J, Rashti SL, Faigenbaum AD: Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr. 2009, 6: 7-10.1186/1550-2783-6-7.
Ammon HP, Muller AB: Forskolin: from an ayurvedic remedy to a modern agent. Planta Med. 1985, 473-7. 10.1055/s-2007-969566. 6
Ammon HP, Muller AB: Effect of forskolin on islet cyclic AMP, insulin secretion, blood glucose and intravenous glucose tolerance in rats. Naunyn Schmiedebergs Arch Pharmacol. 1984, 326 (4): 364-7. 10.1007/BF00501444.
de Souza NJ, Dohadwalla AN, Reden J: Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev. 1983, 3 (2): 201-19. 10.1002/med.2610030205.
Litosch I, Hudson TH, Mills I, Li SY, Fain JN: Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol Pharmacol. 1982, 22 (1): 109-15.
Litosch I, Saito Y, Fain JN: Forskolin as an activator of cyclic AMP accumulation and secretion in blowfly salivary glands. Biochem J. 1982, 204 (1): 147-51.
Seamon KB, Padgett W, Daly JW: Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA. 1981, 78 (6): 3363-7. 10.1073/pnas.78.6.3363.
Henderson S, Magu B, Rasmussen C, Lancaster S, Kerksick C, Smith P, Melton C, Cowan P, Greenwood M, Earnest C, Almada A, Milnor P, Magrans T, Bowden R, Ounpraseuth S, Thomas A, Kreider RB: Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J Int Soc Sports Nutr. 2005, 2: 54-62. 10.1186/1550-2783-2-2-54.
Godard MP, Johnson BA, Richmond SR: Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes Res. 2005, 13 (8): 1335-43. 10.1038/oby.2005.162.
Kreider RB, Henderson S, Magu B, Rasmussen C, Lancaster S, Kerksick C, Smith P, Melton C, Cowan P, Greenwood M, Earnest C, Almada A, Milnor P: Effects of coleus forskohlii supplementation on body composition and markers of health in sedentary overweight females. FASEB J. 2002, LB59-
Ebeling P, Koivisto VA: Physiological importance of dehydroepiandrosterone. Lancet. 1994, 343 (8911): 1479-81. 10.1016/S0140-6736(94)92587-9.
Denti L, Pasolini G, Sanfelici L, Ablondi F, Freddi M, Benedetti R, Valenti G: Effects of aging on dehydroepiandrosterone sulfate in relation to fasting insulin levels and body composition assessed by bioimpedance analysis. Metabolism. 1997, 46 (7): 826-32. 10.1016/S0026-0495(97)90130-X.
De Pergola G, Zamboni M, Sciaraffia M, Turcato E, Pannacciulli N, Armellini F, Giorgino F, Perrini S, Bosello O, Giorgino R: Body fat accumulation is possibly responsible for lower dehydroepiandrosterone circulating levels in premenopausal obese women. Int J Obes Relat Metab Disord. 1996, 20 (12): 1105-10.
Nestler JE, Barlascini CO, Clore JN, Blackard WG: Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988, 66 (1): 57-61. 10.1210/jcem-66-1-57.
Vogiatzi MG, Boeck MA, Vlachopapadopoulou E, el-Rashid R, New MI: Dehydroepiandrosterone in morbidly obese adolescents: effects on weight, body composition, lipids, and insulin resistance. Metabolism. 1996, 45 (8): 1011-5. 10.1016/S0026-0495(96)90272-3.
von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R: Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008, 19 (5): 699-707. 10.1007/s00198-007-0520-z.
Kalman DS, Colker CM, Swain MA, Torina GC, Shi Q: A randomized double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy overweight adults. Curr Thera. 2000, 61: 435-42. 10.1016/S0011-393X(00)80026-0.
Zenk JL, Frestedt JL, Kuskowski MA: HUM5007, a novel combination of thermogenic compounds, and 3-acetyl-7-oxo-dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults. J Nutr Biochem. 2007, 18 (9): 629-34. 10.1016/j.jnutbio.2006.11.008.
Zenk JL, Leikam SA, Kassen LJ, Kuskowski MA: Effect of lean system 7 on metabolic rate and body composition. Nutrition. 2005, 21 (2): 179-85. 10.1016/j.nut.2004.05.025.
Stanko RT, Arch JE: Inhibition of regain in body weight and fat with addition of 3-carbon compounds to the diet with hyperenergetic refeeding after weight reduction. Int J Obes Relat Metab Disord. 1996, 20 (10): 925-30.
Stanko RT, Tietze DL, Arch JE: Body composition, energy utilization, and nitrogen metabolism with a severely restricted diet supplemented with dihydroxyacetone and pyruvate. Am J Clin Nutr. 1992, 55 (4): 771-6.
Stanko RT, Reynolds HR, Hoyson R, Janosky JE, Wolf R: Pyruvate supplementation of a low-cholesterol, low-fat diet: effects on plasma lipid concentrations and body composition in hyperlipidemic patients. Am J Clin Nutr. 1994, 59 (2): 423-7.
Kalman D, Colker CM, Wilets I, Roufs JB, Antonio J: The effects of pyruvate supplementation on body composition in overweight individuals. Nutrition. 1999, 15 (5): 337-40. 10.1016/S0899-9007(99)00034-9.
Stone MH, Sanborn K, Smith LL, O'Bryant HS, Hoke T, Utter AC, Johnson RL, Boros R, Hruby J, Pierce KC, Stone ME, Garner B: Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football players. Int J Sport Nutr. 1999, 9 (2): 146-65.
Koh-Banerjee PK, Ferreira MP, Greenwood M, Bowden RG, Cowan PN, Almada AL, Kreider RB: Effects of calcium pyruvate supplementation during training on body composition, exercise capacity, and metabolic responses to exercise. Nutrition. 2005, 21 (3): 312-9. 10.1016/j.nut.2004.06.026.
Gallaher DD, Gallaher CM, Mahrt GJ, Carr TP, Hollingshead CH, Hesslink R, Wise J: A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J Am Coll Nutr. 2002, 21 (5): 428-33.
Gallaher CM, Munion J, Hesslink R, Wise J, Gallaher DD: Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J Nutr. 2000, 130 (11): 2753-9.
Chiang MT, Yao HT, Chen HC: Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci Biotechnol Biochem. 2000, 64 (5): 965-71. 10.1271/bbb.64.965.
Tai TS, Sheu WH, Lee WJ, Yao HT, Chiang MT: Effect of chitosan on plasma lipoprotein concentrations in type 2 diabetic subjects with hypercholesterolemia. Diabetes Care. 2000, 23 (11): 1703-4. 10.2337/diacare.23.11.1703a.
Wuolijoki E, Hirvela T, Ylitalo P: Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find Exp Clin Pharmacol. 1999, 21 (5): 357-61. 10.1358/mf.1999.21.5.793477.
Gades MD, Stern JS: Chitosan supplementation and fecal fat excretion in men. Obes Res. 2003, 11 (5): 683-8. 10.1038/oby.2003.97.
Guerciolini R, Radu-Radulescu L, Boldrin M, Dallas J, Moore R: Comparative evaluation of fecal fat excretion induced by orlistat and chitosan. Obes Res. 2001, 9 (6): 364-7. 10.1038/oby.2001.47.
Gades MD, Stern JS: Chitosan supplementation and fat absorption in men and women. J Am Diet Assoc. 2005, 105 (1): 72-7. 10.1016/j.jada.2004.10.004.
Pittler MH, Abbot NC, Harkness EF, Ernst E: Randomized, double-blind trial of chitosan for body weight reduction. Eur J Clin Nutr. 1999, 53 (5): 379-81. 10.1038/sj.ejcn.1600733.
Ho SC, Tai ES, Eng PH, Tan CE, Fok AC: In the absence of dietary surveillance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese Asian subjects. Singapore Med J. 2001, 42 (1): 006-10.
Vincent J: The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003, 33 (3): 213-30. 10.2165/00007256-200333030-00004.
Lukaski HC, Siders WA, Penland JG: Chromium picolinate supplementation in women: effects on body weight, composition, and iron status. Nutrition. 2007, 23 (3): 187-95. 10.1016/j.nut.2006.12.001.
Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK: Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J Agric Food Chem. 2002, 50 (1): 10-22. 10.1021/jf010753k.
Ishihara K, Oyaizu S, Onuki K, Lim K, Fushiki T: Chronic (-)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J Nutr. 2000, 130 (12): 2990-5.
Kriketos AD, Thompson HR, Greene H, Hill JO: (-)-Hydroxycitric acid does not affect energy expenditure and substrate oxidation in adult males in a post-absorptive state. Int J Obes Relat Metab Disord. 1999, 23 (8): 867-73. 10.1038/sj.ijo.0800965.
Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C: Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. Jama. 1998, 280 (18): 1596-600. 10.1001/jama.280.18.1596.
Mattes RD, Bormann L: Effects of (-)-hydroxycitric acid on appetitive variables. Physiol Behav. 2000, 71 (1-2): 87-94. 10.1016/S0031-9384(00)00321-8.
Kraemer WJ, Volek JS, Dunn-Lewis C: L-carnitine supplementation: influence upon physiological function. Curr Sports Med Rep. 2008, 7 (4): 218-23.
Smith WA, Fry AC, Tschume LC, Bloomer RJ: Effect of glycine propionyl-L-carnitine on aerobic and anaerobic exercise performance. Int J Sport Nutr Exerc Metab. 2008, 18 (1): 19-36.
Brass EP: Supplemental carnitine and exercise. Am J Clin Nutr. 2000, 72 (2 Suppl): 618S-23S.
Villani RG, Gannon J, Self M, Rich PA: L-Carnitine supplementation combined with aerobic training does not promote weight loss in moderately obese women. Int J Sport Nutr Exerc Metab. 2000, 10 (2): 199-207.
Bloomer RJ, Smith WA: Oxidative stress in response to aerobic and anaerobic power testing: influence of exercise training and carnitine supplementation. Res Sports Med. 2009, 17 (1): 1-16. 10.1080/15438620802678289.
Volek JS, Kraemer WJ, Rubin MR, Gomez AL, Ratamess NA, Gaynor P: L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am J Physiol Endocrinol Metab. 2002, 282 (2): E474-82.
Kaciuba-Uscilko H, Nazar K, Chwalbinska-Moneta J, Ziemba A, Kruk B, Szczepanik J, Titow-Stupnicka E, Bicz B: Effect of phosphate supplementation on metabolic and neuroendocrine responses to exercise and oral glucose load in obese women during weight reduction. J Physiol Pharmacol. 1993, 44 (4): 425-40.
Nazar K, Kaciuba-Uscilko H, Szczepanik J, Zemba AW, Kruk B, Chwalbinska-Moneta J, Titow-Stupnicka E, Bicz B, Krotkiewski M: Phosphate supplementation prevents a decrease of triiodothyronine and increases resting metabolic rate during low energy diet. J Physiol Pharmacol. 1996, 47 (2): 373-83.
Grases F, Llompart I, Conte A, Coll R, March JG: Glycosaminoglycans and oxalocalcic urolithiasis. Nephron. 1994, 68 (4): 449-53. 10.1159/000188306.
Grases F, Melero G, Costa-Bauza A, Prieto R, March JG: Urolithiasis and phytotherapy. Int Urol Nephrol. 1994, 26 (5): 507-11. 10.1007/BF02767650.
Dolan RL, Crosby EC, Leutkemeir MJ, Barton RG, Askew EW: The effects of diuretics on resting metabolic rate and subsequent shifts in respiratory exchange ratios. Med Sci Sports Exerc. 2001, 33: S163-10.1097/00005768-200105001-00921.
Crosby EC, Dolan RL, Benson JE, Leutkemeir MJ, Barton RG, Askew EW: Herbal diuretic induced dehydration and resting metabolic rate. Med Sci Sports Exerc. 2001, 33: S163-10.1097/00005768-200105001-00920.
Von Duvillard SP, Braun WA, Markofski M, Beneke R, Leithauser R: Fluids and hydration in prolonged endurance performance. Nutrition. 2004, 20 (7-8): 651-6. 10.1016/j.nut.2004.04.011.
von Duvillard SP, Arciero PJ, Tietjen-Smith T, Alford K: Sports drinks, exercise training, and competition. Curr Sports Med Rep. 2008, 7 (4): 202-8.
Winnick JJ, Davis JM, Welsh RS, Carmichael MD, Murphy EA, Blackmon JA: Carbohydrate feedings during team sport exercise preserve physical and CNS function. Med Sci Sports Exerc. 2005, 37 (2): 306-15. 10.1249/01.MSS.0000152803.35130.A4.
Kendall RW, Jacquemin G, Frost R, Burns SP: Creatine supplementation for weak muscles in persons with chronic tetraplegia: a randomized double-blind placebo-controlled crossover trial. J Spinal Cord Med. 2005, 28 (3): 208-13.
Kendall KL, Smith AE, Graef JL, Fukuda DH, Moon JR, Beck TW, Cramer JT, Stout JR: Effects of four weeks of high-intensity interval training and creatine supplementation on critical power and anaerobic working capacity in college-aged men. J Strength Cond Res. 2009, 23 (6): 1663-9.
Kreider RB, Ferreira M, Wilson M, Grindstaff P, Plisk S, Reinardy J, Cantler E, Almada AL: Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998, 30 (1): 73-82.
Derave W, Op'T Eijinde B, Richter EA, Hespel P: Combined creatine and protein supplementation improves glucose tolerance and muscle glycogen accumulation in humans. Abstracts of 6th Internationl Conference on Guanidino Compounds in Biology and Medicine. 2001
Nelson AG, Arnall DA, Kokkonen J, Day R, Evans J: Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med Sci Sports Exerc. 2001, 33 (7): 1096-100.
Op 't Eijnde B, Richter EA, Henquin JC, Kiens B, Hespel P: Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol Scand. 2001, 171 (2): 169-76. 10.1046/j.1365-201x.2001.00786.x.
Chwalbinska-Moneta J: Effect of creatine supplementation on aerobic performance and anaerobic capacity in elite rowers in the course of endurance training. Int J Sport Nutr Exerc Metab. 2003, 13 (2): 173-83.
Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff P: Carbohydrate feeding augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol. 1996, 271: E821-E6.
Nelson AG, Day R, Glickman-Weiss EL, Hegsted M, Kokkonen J, Sampson B: Creatine supplementation alters the response to a graded cycle ergometer test. Eur J Appl Physiol. 2000, 83 (1): 89-94. 10.1007/s004210000244.
Nelson AG, Day R, Glickman-Weiss EL, Hegsted M, Sampson B: Creatine supplementation raises anaerobic threshold. FASEB Journal. 1997, 11: A589-
Kreider RB, Miller GW, Williams MH, Somma CT, Nasser TA: Effects of phosphate loading on oxygen uptake, ventilatory anaerobic threshold, and run performance. Med Sci Sports Exerc. 1990, 22 (2): 250-6.
Cade R, Conte M, Zauner C, Mars D, Peterson J, Lunne D, Hommen N, Packer D: Effects of phosphate loading on 2,3 diphosphoglycerate and maximal oxygen uptake. Med Sci Sports Exerc. 1984, 16: 263-8.
Kreider RB, Miller GW, Schenck D, Cortes CW, Miriel V, Somma CT, Rowland P, Turner C, Hill D: Effects of phosphate loading on metabolic and myocardial responses to maximal and endurance exercise. Int J Sport Nutr. 1992, 2 (1): 20-47.
Stewart I, McNaughton L, Davies P, Tristram S: Phosphate loading and the effects of VO2max in trained cyclists. Res Quart. 1990, 61: 80-4.
Folland JP, Stern R, Brickley G: Sodium phosphate loading improves laboratory cycling time-trial performance in trained cyclists. J Sci Med Sport. 2008, 11 (5): 464-8. 10.1016/j.jsams.2007.04.004.
McNaughton L, Backx K, Palmer G, Strange N: Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol. 1999, 80 (4): 333-6. 10.1007/s004210050600.
Applegate E: Effective nutritional ergogenic aids. Int J Sport Nutr. 1999, 9 (2): 229-39.
Kronfeld DS, Ferrante PL, Grandjean D: Optimal nutrition for athletic performance, with emphasis on fat adaptation in dogs and horses. J Nutr. 1994, 124 (12 Suppl): 2745S-53S.
Kraemer WJ, Gordon SE, Lynch JM, Pop ME, Clark KL: Effects of multibuffer supplementation on acid-base balance and 2,3-diphosphoglycerate following repetitive anaerobic exercise. Int J Sport Nutr. 1995, 5 (4): 300-14.
Matson LG, Tran ZV: Effects of sodium bicarbonate ingestion on anaerobic performance: a meta-analytic review. Int J Sport Nutr. 1993, 3 (1): 2-28.
Lindh AM, Peyrebrune MC, Ingham SA, Bailey DM, Folland JP: Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008, 29 (6): 519-23. 10.1055/s-2007-989228.
Wiles JD, Coleman D, Tegerdine M, Swaine IL: The effects of caffeine ingestion on performance time, speed and power during a laboratory-based 1 km cycling time-trial. J Sports Sci. 2006, 24 (11): 1165-71. 10.1080/02640410500457687.
Ivy JL, Kammer L, Ding Z, Wang B, Bernard JR, Liao YH, Hwang J: Improved cycling time-trial performance after ingestion of a caffeine energy drink. Int J Sport Nutr Exerc Metab. 2009, 19 (1): 61-78.
McNaughton LR, Lovell RJ, Siegler J, Midgley AW, Moore L, Bentley DJ: The effects of caffeine ingestion on time trial cycling performance. Int J Sports Physiol Perform. 2008, 3 (2): 157-63.
Graham TE: Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001, 31 (11): 785-807. 10.2165/00007256-200131110-00002.
Carr A, Dawson B, Schneiker K, Goodman C, Lay B: Effect of caffeine supplementation on repeated sprint running performance. J Sports Med Phys Fitness. 2008, 48 (4): 472-8.
Glaister M, Howatson G, Abraham CS, Lockey RA, Goodwin JE, Foley P, McInnes G: Caffeine supplementation and multiple sprint running performance. Med Sci Sports Exerc. 2008, 40 (10): 1835-40. 10.1249/MSS.0b013e31817a8ad2.
Tarnopolsky MA, Atkinson SA, MacDougall JD, Sale DG, Sutton JR: Physiological responses to caffeine during endurance running in habitual caffeine users. Med Sci Sports Exerc. 1989, 21 (4): 418-24.
Armstrong LE: Caffeine, body fluid-electrolyte balance, and exercise performance. Int J Sport Nutr Exerc Metab. 2002, 12 (2): 189-206.
Falk B, Burstein R, Rosenblum J, Shapiro Y, Zylber-Katz E, Bashan N: Effects of caffeine ingestion on body fluid balance and thermoregulation during exercise. Can J Physiol Pharmacol. 1990, 68 (7): 889-92.
Harris R, Dunnett M, Greenhaf P: Carnosine and Taurine contents in individual fibres of human vastus lateralis muscle. J Sport Sci. 1998, 16: 639-43. 10.1080/026404198366443.
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA: The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006, 30 (3): 279-89. 10.1007/s00726-006-0299-9.
Stout JR, Cramer JT, Mielke M, O'Kroy J, Torok DJ, Zoeller RF: Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. J Strength Cond Res. 2006, 20 (4): 928-31. 10.1519/R-19655.1.
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA: Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 2007, 32 (2): 225-33. 10.1007/s00726-006-0364-4.
Hoffman J, Ratamess NA, Ross R, Kang J, Magrelli J, Neese K, Faigenbaum AD, Wise JA: beta-Alanine and the Hormonal Response to Exercise. Int J Sports Med. 2008, 29 (12): 952-8. 10.1055/s-2008-1038678.
Smith AE, Walter AA, Graef JL, Kendall KL, Moon JR, Lockwood CM, Fakuda DH, Beck TW, Cramer JT, Stout JR: Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J Int Soc Sports Nutr. 2009, 6 (1): 5. 10.1186/1550-2783-6-5.
Derave W, Ozdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E: beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol. 2007, 103 (5): 1736-43. 10.1152/japplphysiol.00397.2007.
Hoffman JR, Ratamess NA, Faigenbaum AD, Ross R, Kang J, Stout JR, Wise JA: Short-duration beta-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr Res. 2008, 28 (1): 31-5. 10.1016/j.nutres.2007.11.004.
Hoffman J, Ratamess N, Kang J, Mangine G, Faigenbaum A, Stout J: Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int J Sport Nutr Exerc Metab. 2006, 16 (4): 430-46.
Kendrick IP, Harris RC, Kim HJ, Kim CK, Dang VH, Lam TQ, Bui TT, Smith M, Wise JA: The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids. 2008, 34 (4): 547-54. 10.1007/s00726-007-0008-3.
Tarnopolsky MA, Parise G, Yardley NJ, Ballantyne CS, Olatinji S, Phillips SM: Creatine-dextrose and protein-dextrose induce similar strength gains during training. Med Sci Sports Exerc. 2001, 33 (12): 2044-52. 10.1097/00005768-200112000-00011.
Kreider RB, Earnest CP, Lundberg J, Rasmussen C, Greenwood M, Cowan P, Almada AL: Effects of ingesting protein with various forms of carbohydrate following resistance-exercise on substrate availability and markers of anabolism, catabolism, and immunity. J Int Soc Sports Nutr. 2007, 4: 18-10.1186/1550-2783-4-18.
Cribb PJ, Hayes A: Effects of supplement timing and resistance exercise on skeletal muscle hypertrophy. Med Sci Sports Exerc. 2006, 38 (11): 1918-25. 10.1249/01.mss.0000233790.08788.3e.
Kerksick CM, Rasmussen CJ, Lancaster SL, Magu B, Smith P, Melton C, Greenwood M, Almada AL, Earnest CP, Kreider RB: The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. J Strength Cond Res. 2006, 20 (3): 643-53. 10.1519/R-17695.1.
Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR: Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003, 284: E76-E89.
Hoffman JR, Cooper J, Wendell M, Im J, Kang J: Effects of beta-hydroxy beta-methylbutyrate on power performance and indices of muscle damage and stress during high-intensity training. J Strength Cond Res. 2004, 18 (4): 747-52. 10.1519/13973.1.
Thomson JS, Watson PE, Rowlands DS: Effects of nine weeks of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in resistance trained men. J Strength Cond Res. 2009, 23 (3): 827-35.
Wagner DR: Hyperhydrating with glycerol: implications for athletic performance. J Am Diet Assoc. 1999, 99 (2): 207-12. 10.1016/S0002-8223(99)00049-8.
Inder WJ, Swanney MP, Donald RA, Prickett TC, Hellemans J: The effect of glycerol and desmopressin on exercise performance and hydration in triathletes. Med Sci Sports Exerc. 1998, 30 (8): 1263-9. 10.1097/00005768-199808000-00013.
Montner P, Stark DM, Riedesel ML, Murata G, Robergs R, Timms M, Chick TW: Pre-exercise glycerol hydration improves cycling endurance time. Int J Sports Med. 1996, 17 (1): 27-33. 10.1055/s-2007-972804.
Boulay MR, Song TM, Serresse O, Theriault G, Simoneau JA, Bouchard C: Changes in plasma electrolytes and muscle substrates during short-term maximal exercise in humans. Can J Appl Physiol. 1995, 20 (1): 89-101.
Tikuisis P, Ducharme MB, Moroz D, Jacobs I: Physiological responses of exercised-fatigued individuals exposed to wet-cold conditions. J Appl Physiol. 1999, 86 (4): 1319-28.
Jimenez C, Melin B, Koulmann N, Allevard AM, Launay JC, Savourey G: Plasma volume changes during and after acute variations of body hydration level in humans. Eur J Appl Physiol Occup Physiol. 1999, 80 (1): 1-8. 10.1007/s004210050550.
Magal M, Webster MJ, Sistrunk LE, Whitehead MT, Evans RK, Boyd JC: Comparison of glycerol and water hydration regimens on tennis-related performance. Med Sci Sports Exerc. 2003, 35 (1): 150-6. 10.1097/00005768-200301000-00023.
Kavouras SA, Armstrong LE, Maresh CM, Casa DJ, Herrera-Soto JA, Scheett TP, Stoppani J, Mack GW, Kraemer WJ: Rehydration with glycerol: endocrine, cardiovascular, and thermoregulatory responses during exercise in the heat. J Appl Physiol. 2006, 100 (2): 442-50. 10.1152/japplphysiol.00187.2005.
Jeukendrup AE, Thielen JJ, Wagenmakers AJ, Brouns F, Saris WH: Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am J Clin Nutr. 1998, 67 (3): 397-404.
Goedecke JH, Elmer-English R, Dennis SC, Schloss I, Noakes TD, Lambert EV: Effects of medium-chain triaclyglycerol ingested with carbohydrate on metabolism and exercise performance. Int J Sport Nutr. 1999, 9 (1): 35-47.
Calabrese C, Myer S, Munson S, Turet P, Birdsall TC: A cross-over study of the effect of a single oral feeding of medium chain triglyceride oil vs. canola oil on post-ingestion plasma triglyceride levels in healthy men. Altern Med Rev. 1999, 4 (1): 23-8.
Angus DJ, Hargreaves M, Dancey J, Febbraio MA: Effect of carbohydrate or carbohydrate plus medium-chain triglyceride ingestion on cycling time trial performance. J Appl Physiol. 2000, 88 (1): 113-9.
Van Zyl CG, Lambert EV, Hawley JA, Noakes TD, Dennis SC: Effects of medium-chain triglyceride ingestion on fuel metabolism and cycling performance. J Appl Physiol. 1996, 80 (6): 2217-25.
Misell LM, Lagomarcino ND, Schuster V, Kern M: Chronic medium-chain triacylglycerol consumption and endurance performance in trained runners. J Sports Med Phys Fitness. 2001, 41 (2): 210-5.
Goedecke JH, Clark VR, Noakes TD, Lambert EV: The effects of medium-chain triacylglycerol and carbohydrate ingestion on ultra-endurance exercise performance. Int J Sport Nutr Exerc Metab. 2005, 15 (1): 15-27.
Burke LM, Kiens B, Ivy JL: Carbohydrates and fat for training and recovery. J Sports Sci. 2004, 22 (1): 15-30. 10.1080/0264041031000140527.
Thorburn MS, Vistisen B, Thorp RM, Rockell MJ, Jeukendrup AE, Xu X, Rowlands DS: Attenuated gastric distress but no benefit to performance with adaptation to octanoate-rich esterified oils in well-trained male cyclists. J Appl Physiol. 2006, 101 (6): 1733-43. 10.1152/japplphysiol.00393.2006.
Nosaka N, Suzuki Y, Nagatoishi A, Kasai M, Wu J, Taguchi M: Effect of ingestion of medium-chain triacylglycerols on moderate- and high-intensity exercise in recreational athletes. J Nutr Sci Vitaminol (Tokyo). 2009, 55 (2): 120-5. 10.3177/jnsv.55.120.
Tullson PC, Terjung RL: Adenine nucleotide synthesis in exercising and endurance-trained skeletal muscle. Am J Physiol. 1991, 261 (2 Pt 1): C342-7.
Gross M, Kormann B, Zollner N: Ribose administration during exercise: effects on substrates and products of energy metabolism in healthy subjects and a patient with myoadenylate deaminase deficiency. Klin Wochenschr. 1991, 69 (4): 151-5. 10.1007/BF01665856.
Wagner DR, Gresser U, Kamilli I, Gross M, Zollner N: Effects of oral ribose on muscle metabolism during bicycle ergometer in patients with AMP-deaminase-deficiency. Adv Exp Med Biol. 1991, 383-5.
Pliml W, von Arnim T, Stablein A, Hofmann H, Zimmer HG, Erdmann E: Effects of ribose on exercise-induced ischaemia in stable coronary artery disease. Lancet. 1992, 340 (8818): 507-10. 10.1016/0140-6736(92)91709-H.
Pauly DF, Pepine CJ: D-Ribose as a supplement for cardiac energy metabolism. J Cardiovasc Pharmacol Ther. 2000, 5 (4): 249-58. 10.1054/JCPT.2000.18011.
Op 't Eijnde B, Van Leemputte M, Brouns F, Vusse Van Der GJ, Labarque V, Ramaekers M, Van Schuylenberg R, Verbessem P, Wijnen H, Hespel P: No effects of oral ribose supplementation on repeated maximal exercise and de novo ATP resynthesis. J Appl Physiol. 2001, 91 (5): 2275-81.
Berardi JM, Ziegenfuss TN: Effects of ribose supplementation on repeated sprint performance in men. J Strength Cond Res. 2003, 17 (1): 47-52. 10.1519/1533-4287(2003)017<0047:EORSOR>2.0.CO;2.
Kreider RB, Melton C, Greenwood M, Rasmussen C, Lundberg J, Earnest C, Almada A: Effects of oral D-ribose supplementation on anaerobic capacity and selected metabolic markers in healthy males. Int J Sport Nutr Exerc Metab. 2003, 13 (1): 76-86.
Dunne L, Worley S, Macknin M: Ribose versus dextrose supplementation, association with rowing performance: a double-blind study. Clin J Sport Med. 2006, 16 (1): 68-71. 10.1097/01.jsm.0000180022.44889.94.
Kerksick C, Rasmussen C, Bowden R, Leutholtz B, Harvey T, Earnest C, Greenwood M, Almada A, Kreider R: Effects of ribose supplementation prior to and during intense exercise on anaerobic capacity and metabolic markers. Int J Sport Nutr Exerc Metab. 2005, 15 (6): 653-64.
Hargreaves M, McKenna MJ, Jenkins DG, Warmington SA, Li JL, Snow RJ, Febbraio MA: Muscle metabolites and performance during high-intensity, intermittent exercise. J Appl Physiol. 1998, 84 (5): 1687-91.
Starling RD, Trappe TA, Short KR, Sheffield-Moore M, Jozsi AC, Fink WJ, Costill DL: Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med Sci Sports Exerc. 1996, 28 (9): 1193-8.
Williams MH, Kreider RB, Hunter DW, Somma CT, Shall LM, Woodhouse ML, Rokitski L: Effect of inosine supplementation on 3-mile treadmill run performance and VO2 peak. Med Sci Sports Exerc. 1990, 22 (4): 517-22.
McNaughton L, Dalton B, Tarr J: Inosine supplementation has no effect on aerobic or anaerobic cycling performance. Int J Sport Nutr. 1999, 9 (4): 333-44.
Braham R, Dawson B, Goodman C: The effect of glucosamine supplementation on people experiencing regular knee pain. Br J Sports Med. 2003, 37 (1): 45-9. 10.1136/bjsm.37.1.45. discussion 9
Vad V, Hong HM, Zazzali M, Agi N, Basrai D: Exercise recommendations in athletes with early osteoarthritis of the knee. Sports Med. 2002, 32 (11): 729-39. 10.2165/00007256-200232110-00004.
Nieman DC: Exercise immunology: nutritional countermeasures. Can J Appl Physiol. 2001, 26 (Suppl): S45-55.
Gleeson M, Lancaster GI, Bishop NC: Nutritional strategies to minimise exercise-induced immunosuppression in athletes. Can J Appl Physiol. 2001, 26 (Suppl): S23-35.
Gleeson M, Bishop NC: Elite athlete immunology: importance of nutrition. Int J Sports Med. 2000, 21 (Suppl 1): S44-50. 10.1055/s-2000-1451.
Nieman DC, Pedersen BK: Exercise and immune function. Recent developments. Sports Med. 1999, 27 (2): 73-80. 10.2165/00007256-199927020-00001.
Lowery L, Berardi JM, Ziegenfuss Antioxidants: Sports Supplements. Edited by: Antonio J, Stout J. 2001, Baltimore, MD: Lippincott, Williams & Wilkins, 260-78.
Gomez AL, Volek JS, Ratamess NA, Rubin MR, Wickham RB, Mazzetti SA, Doan BK, Newton RU, Kraemer WJ: Creatine supplementation enhances body composition during short-term reisstance training overreaching. Journal of Strength and Conditioning Research. 2000, 14 (3):
French DN, Volek JS, Ratamess NA, Mazzetti SA, Rubin MR, Gomez AL, Wickham RB, Doan BK, McGuigan MR, Scheett TP, Newton RU, Dorofeyeva E, Kraemer WJ: The effects of creatine supplementation on resting serum hormonal concentrations during short-term resistance training overreaching. Med Sci Sports & Exerc. 2001, 33 (5): S203-10.1097/00005768-200105001-01142.
Mero A: Leucine supplementation and intensive training. Sports Med. 1999, 27 (6): 347-58. 10.2165/00007256-199927060-00001.
Harris WS, Appel LJ: New guidelines focus on fish, fish oil, omega-3 fatty acids. American Heart Association, 2002(November 11), [ http://www.americanheart.org/presenter.jhtml?identifier=3065754 ]
Williams MH: Vitamin supplementation and athletic performance. Int J Vitam Nutr Res Suppl. 1989, 30: 163-91.
Reid IR: Therapy of osteoporosis: calcium, vitamin D, and exercise. Am J Med Sci. 1996, 312 (6): 278-86. 10.1097/00000441-199612000-00006.
Goldfarb AH: Antioxidants: role of supplementation to prevent exercise-induced oxidative stress. Med Sci Sports Exerc. 1993, 25 (2): 232-6.
Goldfarb AH: Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999, 24 (3): 249-66.
Appell HJ, Duarte JA, Soares JM: Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997, 18 (3): 157-60. 10.1055/s-2007-972612.
Tiidus PM, Houston ME: Vitamin E status and response to exercise training. Sports Med. 1995, 20 (1): 12-23. 10.2165/00007256-199520010-00002.
Craciun AM, Wolf J, Knapen MH, Brouns F, Vermeer C: Improved bone metabolism in female elite athletes after vitamin K supplementation. Int J Sports Med. 1998, 19 (7): 479-84. 10.1055/s-2007-971948.
Fogelholm M, Ruokonen I, Laakso JT, Vuorimaa T, Himberg JJ: Lack of association between indices of vitamin B1, B2, and B6 status and exercise-induced blood lactate in young adults. Int J Sport Nutr. 1993, 3 (2): 165-76.
Garg R, Malinow M, Pettinger M, Upson B, Hunninghake D: Niacin treatment increases plasma homocyst(e)ine levels. Am Heart J. 1999, 138 (6 Pt 1): 1082-7. 10.1016/S0002-8703(99)70073-6.
Alaswad K, O'Keefe JH, Moe RM: Combination drug therapy for dyslipidemia. Curr Atheroscler Rep. 1999, 1 (1): 44-9. 10.1007/s11883-999-0049-z.
Murray R, Bartoli WP, Eddy DE, Horn MK: Physiological and performance responses to nicotinic-acid ingestion during exercise. Med Sci Sports Exerc. 1995, 27 (7): 1057-62. 10.1249/00005768-199507000-00015.
Bonke D: Influence of vitamin B1, B6, and B12 on the control of fine motoric movements. Bibl Nutr Dieta. 1986 (38): 104-9.
Bonke D, Nickel B: Improvement of fine motoric movement control by elevated dosages of vitamin B1, B6, and B12 in target shooting. Int J Vitam Nutr Res Suppl. 1989, 30: 198-204.
Van Dyke DC, Stumbo PJ, Mary JB, Niebyl JR: Folic acid and prevention of birth defects. Dev Med Child Neurol. 2002, 44 (6): 426-9. 10.1017/S0012162201002316.
Mattson MP, Kruman II, Duan W: Folic acid and homocysteine in age-related disease. Ageing Res Rev. 2002, 1 (1): 95-111. 10.1016/S0047-6374(01)00365-7.
Weston PM, King RF, Goode AW, Williams NS: Diet-induced thermogenesis in patients with gastrointestinal cancer cachexia. Clin Sci (Lond). 1989, 77 (2): 133-8.
Webster MJ: Physiological and performance responses to supplementation with thiamin and pantothenic acid derivatives. Eur J Appl Physiol Occup Physiol. 1998, 77 (6): 486-91. 10.1007/s004210050364.
Beek van der EJ, Lowik MR, Hulshof KF, Kistemaker C: Combinations of low thiamin, riboflavin, vitamin B6 and vitamin C intake among Dutch adults. (Dutch Nutrition Surveillance System). J Am Coll Nutr. 1994, 13 (4): 383-91.
Beek van der EJ: Vitamin supplementation and physical exercise performance. J Sports Sci. 1991, 77-90. Spec No
Pedersen BK, Bruunsgaard H, Jensen M, Krzywkowski K, Ostrowski K: Exercise and immune function: effect of ageing and nutrition. Proc Nutr Soc. 1999, 58 (3): 733-42.
Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J, Pedersen BK: Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol Cell Physiol. 2001, 280 (6): C1570-5.
Grados F, Brazier M, Kamel S, Duver S, Heurtebize N, Maamer M, Mathieu M, Garabedian M, Sebert JL, Fardellone P: Effects on bone mineral density of calcium and vitamin D supplementation in elderly women with vitamin D deficiency. Joint Bone Spine. 2003, 70 (3): 203-8. 10.1016/S1297-319X(03)00046-0.
Brutsaert TD, Hernandez-Cordero S, Rivera J, Viola T, Hughes G, Haas JD: Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr. 2003, 77 (2): 441-8.
Bohl CH, Volpe SL: Magnesium and exercise. Crit Rev Food Sci Nutr. 2002, 42 (6): 533-63. 10.1080/20024091054247.
Lukaski HC: Magnesium, zinc, and chromium nutrition and athletic performance. Can J Appl Physiol. 2001, 26 (Suppl): S13-22.
Morton DP, Callister R: Characteristics and etiology of exercise-related transient abdominal pain. Med Sci Sports Exerc. 2000, 32 (2): 432-8. 10.1097/00005768-200002000-00026.
Noakes TD: Fluid and electrolyte disturbances in heat illness. Int J Sports Med. 1998, 19 (Suppl 2): S146-9. 10.1055/s-2007-971982.
Margaritis I, Tessier F, Prou E, Marconnet P, Marini JF: Effects of endurance training on skeletal muscle oxidative capacities with and without selenium supplementation. J Trace Elem Med Biol. 1997, 11 (1): 37-43.
Tessier F, Margaritis I, Richard MJ, Moynot C, Marconnet P: Selenium and training effects on the glutathione system and aerobic performance. Med Sci Sports Exerc. 1995, 27 (3): 390-6.
McCutcheon LJ, Geor RJ: Sweating. Fluid and ion losses and replacement. Vet Clin North Am Equine Pract. 1998, 14 (1): 75-95.
Gibson RS, Heath AL, Ferguson EL: Risk of suboptimal iron and zinc nutriture among adolescent girls in Australia and New Zealand: causes, consequences, and solutions. Asia Pac J Clin Nutr. 2002, 11 (Suppl 3): S543-52. 10.1046/j.1440-6047.11.supp3.10.x.
Singh A, Failla ML, Deuster PA: Exercise-induced changes in immune function: effects of zinc supplementation. J Appl Physiol. 1994, 76 (6): 2298-303.
Download references
Acknowledgements
The authors would like to thank all of the research participants, graduate students, and researchers that contributed to the body of research cited in this comprehensive review. The authors would like to thank Mr. Chris Noonan for reviewing definition and regulation of dietary supplement section. This article was reviewed and approved by the Research Committee of the ISSN and therefore can be viewed as the official position of the ISSN. Individuals interested in trying some of these nutritional recommendations should do so only after consulting with their personal physician.
Author information
Authors and affiliations.
Exercise & Sports Nutrition Lab, Texas A&M University, College Station, TX, USA
Richard B Kreider
Exercise & Sport Science Department, University of Mary-Hardin Baylor, Belton, TX, USA
Colin D Wilborn & Lem Taylor
School of Physical Education & Exercise Science, University of South Florida, Tampa, FL, USA
Bill Campbell
GENr8, Inc, Dana Point, CA, USA
Anthony L Almada
Collins, McDonald & Gann, PC, Mineola, NY, USA
Rick Collins
Schools of Medicine & Health Movement Studies, The University of Queensland, Herston, Queensland, AU
Mathew Cooke
Pennington Biomedical Reseach Center, Baton Rouge, LA, USA
Conrad P Earnest
Department of Health, Human Performance, and Recreation, Baylor University, Box 97313, Waco, TX, USA
Mike Greenwood, Brian Leutholtz & Darryn S Willoughby
Miami Research Associates, Miami, FL, USA
Douglas S Kalman
Department of Health and Exercise Science, University of Oklahoma, Norman, OK, USA
Chad M Kerksick & Abbie Smith
High Performance Nutrition LLC, Mercer Island, WA, USA
Susan M Kleiner
Northwestern University Feinberg School of Medicine, Department of Physical Medicine and Rehabilitation, Rehabilitation Institute of Chicago, Chicago, IL, USA
Hector Lopez
Nutrition Assessment Laboratory, Nutrition Center, University of Akron, Akron, Ohio, USA
Lonnie M Lowery
Department of Human Performance & Sport Management, Mount Union College, Alliance, Ohio, USA
Marie Spano Nutrition Consulting, Atlanta, GA, USA
Marie Spano
Department of Human Nutrition, Kansas State University, Manhattan, KS, USA
Robert Wildman
Division of Sports Nutrition and Exercise Science, the Center for Applied Health Sciences, Fairlawn, OH, USA
Tim N Ziegenfuss
Farquhar College of Arts and Sciences, Nova Southeastern University, Fort Lauderdale, FL, USA
Jose Antonio
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Richard B Kreider .
Additional information
Competing interests.
Authors of this paper have not received any financial remuneration for preparing or reviewing this paper. However, in an interest of full-disclosure as recommended in this paper, authors report the following competing interests. RBK has received university-funded grants to conduct research on several nutrients discussed in this paper and currently receives research funding from Curves International, General Mills Bell Institute for Human Nutrition; and, the National Institutes of Health. In addition, he has served as a paid consultant for industry; is currently serving as a product development consultant for Supreme Protein, has received honoraria for speaking at conferences and writing lay articles about topics discussed in this paper; receives royalties from the sale of several exercise and nutrition-related books; and, has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements. CW has received academic and industry funding related to dietary supplements and honoraria from speaking engagements on the topic. LT has received academic and industry funding related to dietary supplements and honoraria from speaking engagements on the topic. BC has received university and private sector funded grants to conduct research on several nutrients discussed in this paper and has received compensation for speaking at conferences and writing lay articles/books about topics discussed in this paper. ALA has received consulting fees from AquaGenus, Bergstrom Nutrition, Bioiberica, Curves International, Indena, Indfrag, Miami Research Associates, Omniactives, Sabinsa, and Yor Health; received dietary ingredient materials from Alzchem, Glanbia, and Lonza; sits on the board of New Era; has executive positions in Fein Innovations, Fierce Foods, and GENr8; has equity in AquaGenus, Fein Innovations, Fierce Foods, and GENr8; has stock options in New Era Nutrition and Scientific Food Solutions; has received royalties from Isatori; is a lead inventor on a patent pending related to vitamin K and MSM; has received travel and lodging reimbursement from Bergstrom, Danisco, Indfrag, and New Era Nutrition; has received in-kind compensation from Advanced Research Press; and is on the editorial advisory board of Nutrition Business Journal , and is a columnist for Nutraceuticals World and Muscular Development . RC is the attorney for numerous companies in the dietary supplement industry and has received payment for consultancy and the writing of lay articles discussing nutritional supplements. MC has served as a consultant for industry and received honoraria for speaking about topics discussed in this paper. CPE received honoraria from scientific and lay audience speaking engagements; has served as an expert witness for several patent litigations involving dietary supplements on the behalf of the plaintiff and defense; and, currently has a grant from the Gatorade Sports Science Institute involving the examination of a dietary supplement and its effect on athletic performance. MG has received academic and industry funding to conduct sport/exercise nutritional supplement research; has served as a paid consultant for the sports nutrition industry; and, has received honoraria for speaking engagements and publishing articles in lay sport nutrition venues. DSK has received grants and contracts to conduct research on several nutrients discussed in this paper; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about topics discussed in this paper; receives royalties from the sale of several exercise and nutrition-related books; and, has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements. CMK has received academic and industry funding related to dietary supplements and honoraria from speaking engagements on the topic. In addition, he has received payment for writing of lay articles discussing nutritional supplements. SMK has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about topics discussed in this paper; receives royalties from the sale of several exercise and nutrition related books; and, receives commission and has stock in companies that sell products produced from several ingredients discussed in this paper. HL reports having received honoraria for lectures from scientific, educational and community groups; serving as a consultant and scientific advisory board member for Nordic Naturals, Inc.; payment for scientific and technical writing for Optimal Aging and Aesthetic Medicine, LLC.; payment for commercial writing for Essentials of Healthy Living; consultancy fees as owner of Physicians Pioneering Performance, LLC.; owner and medical director of Performance Spine and Sports Medicine, LLC.; and, owner and medical director of Northeast Spine and Sports Medicine, PC. LML has received academic and industry funding related to dietary supplements and honoraria from speaking engagements on the topic and has received payment for consultancy and the writing of lay articles discussing nutritional supplements. RM has received industry funding and stock options related to dietary supplement research. RM has also received honoraria for speaking and payment for consultancy and the writing of lay articles on nutritional supplements. AS reports no competing interests. MS has received honoraria from academic organizations for speaking at conferences and writing lay articles on various sports nutrition topics. TNZ has received university and contract research organization-funded grants to conduct research on several ingredients discussed in this paper; has served as a paid consultant for the sports nutrition industry; has received honoraria for speaking at conferences and writing lay articles about topics discussed in this paper; has received royalties from the sale of dietary supplements; has stock in a company that sells several ingredients discussed in this paper; and, has served as an expert witness in cases involving dietary supplements. RW has received industry funds for consultancy and employment related to dietary supplement development and marketing. DSW has received university and contract research organization-funded grants to conduct research on several ingredients discussed in this paper. He has previously served as a paid consultant for the nutraceutical and sports nutrition industry with the companies, Amino Vital and Transformation Enzyme, and is presently a paid consultant for VPX. He has received honoraria for speaking at conferences and writing lay articles about topics discussed in this paper. JA is the CEO of the ISSN and has received academic and industry (i.e. VPX/Redline) funding related to dietary supplement consultation, speaking engagements and writing on the topic.
Authors' contributions
RBK contributed most of the content and served as senior editor of the paper. CDW, LT, and BC updated references, updated several sections of the paper, and assisted in editing content. ALA, RC, MC, CPE, MG, DSK, CMK, SMK, BL, HL, LML, RM, AS, MS, RW, DSW, TNZ, and JA reviewed and edited the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Kreider, R.B., Wilborn, C.D., Taylor, L. et al. ISSN exercise & sport nutrition review: research & recommendations. J Int Soc Sports Nutr 7 , 7 (2010). https://doi.org/10.1186/1550-2783-7-7
Download citation
Received : 18 January 2010
Accepted : 02 February 2010
Published : 02 February 2010
DOI : https://doi.org/10.1186/1550-2783-7-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sports Nutrition
- Ergogenic Value
- Dietary Supplement Health And Education Act (DSHEA)
- Creatine Monohydrate
Journal of the International Society of Sports Nutrition
ISSN: 1550-2783
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

3L Annabelle Lincoln Presents Pioneering Paper at SportsLand Summit

Third-year law student Annabelle Lincoln recently presented her research at the inaugural SportsLand Summit, held at the Cleveland Browns Stadium. The summit gathered prominent figures in sports, healthcare, technology and human performance.
Lincoln's presentation focused on a research paper she co-authored with fellow CWRU Law students Nathaniel Arnholt and Trey Quillin. The research began in the fall of 2023, with the students exploring varying sports law topics. Ultimately, Lincoln, Arnholt and Quillin were encouraged to further explore their research in the spring semester, culminating in a research paper that was finalized over the summer.
Their paper explores the critical issue of collegiate athletes’ control over their personal data within college athletics. With rapid advancements in wearable technology, the ability to collect vast amounts of data on athletes both on and off the field has grown exponentially. This data is increasingly valuable to organizations, raising important questions about ownership of that information.
The legal frameworks governing athlete data rights are complex and vary depending on the athlete's status—whether professional, collegiate or amateur. While the research of Lincoln and her team primarily focused on collegiate athletes, they believe their findings may have broader implications for athletes at all levels. Those interested in the topic can find the full paper on the CWRU Law Athlete Data Lab website .
Reflecting on her experience, Lincoln expressed her gratitude for the opportunity to speak at such a prestigious event, which featured influential leaders such as David Jenkins, COO of the Cleveland Browns, and the CEO of the Rock Entertainment Group, Nic Barlage, along with top team physicians and HealthTech experts. She also thanked Sports Data Labs founders Stan Mimoto and Mark Gorski, and commended Professor Craig Nard for his guidance throughout her research journey.
Lincoln concluded by emphasizing the importance of their research, stating, "Nathan, Trey and I researched this topic extensively and hope our paper will be helpful to players and industry leaders moving forward."
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Paediatr Child Health
- v.18(4); 2013 Apr

Language: English | French
Sport nutrition for young athletes
Nutrition is an important part of sport performance for young athletes, in addition to allowing for optimal growth and development. Macronutrients, micronutrients and fluids in the proper amounts are essential to provide energy for growth and activity. To optimize performance, young athletes need to learn what, when and how to eat and drink before, during and after activity.
La nutrition est une partie importante de la performance sportive des jeunes athlètes, sans compter qu’elle favorise une croissance et un développement optimaux. Il est essentiel de consommer la bonne quantité de macronutriments, de micronutriments et de liquides pour fournir l’énergie nécessaire à la croissance et aux activités. Pour optimiser leur performance, les jeunes athlètes doivent apprendre quoi, quand et comment manger et boire avant, pendant et après l’activité.
Français en page 203
Proper nutrition is vital for child and adolescent athletes to attain proper growth and perform optimally in sports. Young athletes need to learn what foods are good for energy, when to eat certain foods, how to eat during an event, and when and what to eat to replenish after activity. A well-balanced diet containing appropriate amounts of macronutrients (protein, carbohydrates and fat) and micronutrients (vitamins and minerals) is essential to provide enough energy for growth and activity. Fluids are also essential for hydration to support growth and athletic performance.
ENERGY REQUIREMENTS
Basic nutrition is important for growth, achieving good health and scholastic achievement, and providing energy ( 1 ). Sports nutrition enhances athletic performance by decreasing fatigue and the risk of disease and injury; it also enables athletes to optimize training and recover faster ( 1 ). Balancing energy intake with energy expenditure is crucial to prevent an energy deficit or excess. Energy deficits can cause short stature, delayed puberty, menstrual dysfunction, loss of muscle mass and increased susceptibility for fatigue, injury or illness ( 2 , 3 ). Energy excess can result in overweight and obesity ( 4 ).
Before puberty, minimum nutritional and energy requirements (caloric needs) are similar for boys and girls. Energy requirements for adolescents are more variable, depending on age, activity level, growth rate and stage of physical maturity ( Table 1 ) ( 1 ). These recommended energy allowances are the minimum necessary to ensure proper growth and bodily functions. Extra calories are needed during growth spurts and to replenish energy expended during athletic endeavours ( 1 , 5 , 6 ). For example, a 30 kg girl playing soccer for 60 min would expend an average of 270 calories, or a 60 kg boy playing ice hockey for 60 min would expend an average of 936 calories ( 6 ).
Recommended energy requirements, Kcal/day
| 4–6 | 1800 | 1800 |
| 7–10 | 2000 | 2000 |
| 11–14 | 2500 | 2200 |
| 15–18 | 3000 | 2200 |
Adapted with permission from reference 1
MACRONUTRIENTS
Macronutrients, such as carbohydrates, protein and fats, provide the fuel for physical activity and sports participation.
Carbohydrates
Carbohydrates are the most important fuel source for athletes because they provide the glucose used for energy. One gram of carbohydrate contains approximately four kilocalories of energy. Glucose is stored as glycogen in muscles and liver. Muscle glycogen is the most readily available energy source for working muscle and can be released more quickly than other energy sources ( 1 ). Carbohydrates should comprise 45% to 65% of total caloric intake for four- to 18-year-olds ( 1 , 7 ). Good sources of carbohydrates include whole grains, vegetables, fruits, milk and yogurt.
Proteins build and repair muscle, hair, nails and skin. For mild exercise and exercise of short duration, proteins do not act as a primary source of energy. However, as exercise duration increases, proteins help to maintain blood glucose through liver gluconeogenesis ( 2 ). One gram of protein provides four kilocalories of energy. Protein should comprise approximately 10% to 30% of total energy intake for four- to 18-year-olds (7). Good sources of protein include lean meat and poultry, fish, eggs, dairy products, beans and nuts, including peanuts.
Fat is necessary to absorb fat-soluble vitamins (A, D, E, K), to provide essential fatty acids, protect vital organs and provide insulation. Fat also provides the feeling of satiety. It is a calorie-dense source of energy (one gram provides nine kilocalories) but is more difficult to use. Fats should comprise 25% to 35% of total energy intake for four- to 18-year-olds ( 7 ). Saturated fats should comprise no more than 10% of total energy intake ( 1 , 3 ). Good sources of fat include lean meat and poultry, fish, nuts, seeds, dairy products, and olive and canola oils. Fat from chips, candy, fried foods and baked goods should be minimized.
MICRONUTRIENTS
Although there are many vitamins and minerals required for good health, particular attention should be devoted to ensuring that athletes consume proper amounts of calcium, vitamin D and iron. Calcium is important for bone health, normal enzyme activity and muscle contraction. The daily recommended intake of calcium is 1000 mg/day for four- to eight-year-olds and 1300 mg/day for nine- to 18-year-olds ( 7 , 8 ). Calcium is contained in a variety of foods and beverages, including milk, yogurt, cheese, broccoli, spinach and fortified grain products.
Vitamin D is necessary for bone health and is involved in the absorption and regulation of calcium. Current recommendations suggest 600 IU/day for four- to 18-year-olds ( 8 ). Normal values of vitamin D also vary depending on geographical location and race. Athletes living in northern latitudes or who train indoors (eg, figure skaters, gymnasts, dancers) are more likely to be vitamin D deficient ( 2 ). Sources of vitamin D include fortified foods, such as milk, and sun exposure. Dairy products other than milk, such as yogurt, do not contain vitamin D.
Iron is important for oxygen delivery to body tissues. During adolescence, more iron is required to support growth as well as increases in blood volume and lean muscle mass ( 1 ). Boys and girls nine to 13 years of age should ingest 8 mg/day to avoid depletion of iron stores and iron-deficiency anemia ( 7 ). Adolescents 14 to 18 years of age require more iron, up to 11 mg/day for males and 15 mg/day for females ( 7 ). Iron depletion is common in athletes because of diets poor in meat, fish and poultry, or increased iron losses in urine, feces, sweat or menstrual blood ( 2 ). Therefore, athletes, particularly female athletes, vegetarians and distance runners should be screened periodically for iron status ( 2 ). Iron-rich foods include eggs, leafy green vegetables, fortified whole grains and lean meat.
Fluids, particularly water, are important nutrients for athletes. Athletic performance can be affected by what, how much and when an athlete drinks. Fluids help to regulate body temperature and replace sweat losses during exercise ( 8 , 9 ). Environmental temperature and humidity can affect how much an athlete sweats and how much fluid intake is required ( 1 , 9 , 10 ). Hotter temperatures and higher humidity make a person sweat more, and more fluid is needed to maintain hydration. Dehydration can decrease performance and put athletes at risk for heat exhaustion or heat stroke ( 1 , 9 , 10 ).
Proper hydration requires fluid intake before, during and after exercise or activity. The amount of fluid required depends on many factors, including age and body size ( 9 , 10 ) ( Table 2 ). Before activity, athletes should consume 400 mL to 600 mL of cold water 2 h to 3 h before their event ( 1 , 2 , 10 ). During sporting activities, athletes should consume 150 mL to 300 mL of fluid every 15 min to 20 min ( 1 , 2 , 10 ). For events lasting less than 1 h, water is sufficient ( 4 ). For events lasting longer than 60 min, and/or taking place in hot, humid weather, sports drinks containing 6% carbohydrates and 20 mEq/L to 30 mEq/L of sodium chloride are recommended to replace energy stores and fluid/electrolyte losses ( 3 , 4 , 5 , 9 ). Following activity, athletes should drink enough fluid to replace sweat losses ( Table 2 ). This usually requires consuming approximately 1.5 L of fluid/kg of body weight lost ( 1 , 10 ). The consumption of sodium-containing fluids and snacks after exercise helps with rehydration by stimulating thirst and fluid retention ( 1 , 2 , 10 ). For non-athletes, routine ingestion of carbohydrate-containing sports drinks can result in consumption of excessive calories, increasing the risks of overweight and obesity, as well as dental caries and, therefore, should be avoided ( 4 ).
Recommended minimal fluid intake during and after exercise in child athletes, based on the calculation of 13 mL/kg during exercise and 4 mL/kg after exercise
| 25 | 325 | 100 |
| 30 | 390 | 120 |
| 35 | 455 | 140 |
| 40 | 520 | 160 |
| 45 | 585 | 180 |
| 50 | 650 | 200 |
| 55 | 715 | 220 |
| 60 | 780 | 240 |
Adapted with permission from reference 9
RECOVERY FOODS
Recovery foods should be consumed within 30 min of exercise, and again within 1 h to 2 h of exercise, to help reload muscles with glycogen and allow for proper recovery. These foods should include protein and carbohydrates ( 2 , 6 ). Examples include graham crackers with peanut butter and juice, yogurt with fruit, or a sports drink with fruit and cheese ( 6 ).
MEAL PLANNING
One of the trickiest things to manage is meal planning around athletic events. The timing of meals is very important and needs to be individualized. It is important for athletes to discover which foods they like that also help to maximize performance. They should not experiment with new foods or new routines on the day of competition.
General guidelines include eating meals a minimum of 3 h before an event to allow for proper digestion and to minimize incidence of gastrointestinal upset during exercise. Meals should include carbohydrates, protein and fat. Fibre should be limited. High-fat meals should be avoided before exercise because they can delay gastric-emptying, make athletes feel sluggish and thereby adversely affect performance ( 2 , 6 ). For early morning practices or events, having a snack or liquid meal 1 h to 2 h before exercise, followed by a full breakfast after the event, will help ensure sufficient energy to maximize performance ( 2 , 6 ).
Pre-game snacks or liquid meals should be ingested 1 h to 2 h before an event to allow for digestion before start of exercise ( 2 , 6 ). Snacks can include fresh fruit, dried fruit, a bowl of cereal with milk, juice or fruit-based smoothies. During an event, sports drinks, fruit or granola bars can be ingested to help refuel and keep energy levels high.
REACHING THE FINISH LINE
A well-balanced diet is essential for growing athletes to maintain proper growth and optimize performance in athletic endeavours. An ideal diet comprises 45% to 65% carbohydrates, 10% to 30% protein and 25% to 35% fat. Fluids are very important for maintaining hydration and should be consumed before, during and after athletic events to prevent dehydration. Timing of food consumption is important to optimize performance. Meals should be eaten a minimum of 3 h before exercise and snacks should be eaten 1 h to 2 h before activity. Recovery foods should be consumed within 30 min of exercise and again within 1 h to 2 h of activity to allow muscles to rebuild and ensure proper recovery.
USEFUL WEBSITES
- American Society for Nutrition: www.nutrition.org
- Australian Sport Institute: www.ausport.gov.au/ais/nutrition
- The Canadian Nutrient File is a searchable database containing average values for nutrients in foods: www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/user_guide_d_utilisation01-eng.php
- Coaching Association of Canada: http://coach.ca/fueling-the-young-athlete-p140142 ; http://coach.ca/sport-nutrition-tips-s13426
- Dietitians of Canada has a table of iron-rich foods: www.dietitians.ca/Nutrition-Resources-A-Z/Factsheets/Minerals/Food-Sources-of-Iron . aspx
- Gatorade Sport Science Institute: http://gssiweb.com
- KidsHealth (Nemours), Feeding your child athlete: www.kidshealth.org/parent/nutrition_center/dietary_needs/feed_child_athlete.html
Acknowledgments
This practice point has been reviewed by the Canadian Paediatric Society’s Nutrition and Gastroenterology Committee.
CPS PAEDIATRIC SPORTS AND EXERCISE MEDICINE EXECUTIVE
Neil Cooper MD (Member at large); David Fecteau MD (Secretary-treasurer); Erika Persson MD (Member at large); John F Philpott MD (President-elect); Laura K Purcell MD (President); Eric Koelink MD (Liaison, CPS Residents Section); David W Warren MD (former Liaison, CPS Emergency Medicine Section)
Principal author: Laura K Purcell MD
The recommendations in this document do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. All Canadian Paediatric Society position statements and practice points are reviewed on a regular basis. Retired statements are removed from the website. Please consult the Position Statements section of the CPS website ( www.cps.ca ) for the full-text, current version.

IMAGES
VIDEO
COMMENTS
The purpose of this study was to determine... Lee M. Margolis, Marques A. Wilson, Claire C. Whitney, Christopher T. Carrigan, Nancy E. Murphy, Adrienne Hatch-McChesney and Stefan M. Pasiakos. Journal of the International Society of Sports Nutrition 2021 18:56. Research article Published on: 10 July 2021. Full Text.
Among athletes, nutrition plays an important role since the regimen and composition of the diet are associated with success in sports [23,24].Concerns about weight and body shape strongly influence food choices for the general population [] and have a similar effect on athletes, where attempts to achieve their goals are associated with external data on physique, weight, and performance [].
14 Exercise & Sports Nutrition Lab, Human Clinical Research Facility, Texas A&M University, College Station, TX, USA. [email protected]. ... This paper is an ongoing update of the sports nutrition review article originally published as the lead paper to launch the Journal of the International Society of Sports Nutrition in 2004 and updated in ...
Introduction to the importance and influence of nutrition on exercise. Nutrition is increasingly recognized as a key component of optimal sporting performance, with both the science and practice of sports nutrition developing rapidly.1 Recent studies have found that a planned scientific nutritional strategy (consisting of fluid, carbohydrate, sodium, and caffeine) compared with a self-chosen ...
A major emphasis in all of the papers was a focus on strengths and weaknesses for various sports nutrition strategies, and insights for future research. Kerksick et al. [ 11 ] defined the role that proper doses of plant proteins can have in supporting health, the environment, and exercise training adaptations.
ISSN exercise & sports nutrition review update: research & ...
The effects of nicotine withdrawal on exercise-related physical ability and sports performance in nicotine addicts: a systematic review and meta-analysis. Kangzhe Bao, Kai Zheng, Xianxian Zhou, Baichao Chen, Zerui He & Danyang Zhu. Article: 2302383. Published online: 11 Jan 2024.
Aims and scope. Journal metrics Editorial board. Journal of the International Society of Sports Nutrition (JISSN) focuses on the acute and chronic effects of sports nutrition and supplementation strategies on body composition, physical performance and metabolism. JISSN is aimed at researchers and sport enthusiasts focused on delivering ...
Abstract. Diet can significantly influence athletic performance, but recent research developments have substantially changed our understanding of sport and exercise nutrition. Athletes adopt various nutritional strategies in training and competition in the pursuit of success. The aim of training is to promote changes in the structure and ...
nutrition, the ISSN Exercise & Sports Nutrition Review: Research & Recommendations has been up-dated. The initial version of this paper was the first publication used to help launch the Journal of the International Society of Sports Nutrition (JISSN, ori-ginally called the Sports Nutrition Review Journal). This paper provides a definition of ...
This paper is an ongoing update of the sports nutrition review article originally published as the lead paper to launch the Journal of the International Society of Sports Nutrition in 2004 and updated in 2010. It presents a well-referenced overview of the current state of the science related to optimization of training and performance ...
The first paper [] addresses the intriguing topic of translating sports performance nutrition research into the real world and ultimately the chances of it helping an athlete maximize their performance to reach the "podium."The authors present a framework they call the "Paper-2-Podium Matrix" which provides several criteria to critically evaluate performance nutrition-related research ...
Abstract and Figures. Nutrition plays an essential role on sports performance. Following an adequate nutrition pattern determines winning the gold medal or failing in the attempt. That is why it ...
Proper nutrition is a key component in the preparation and training of the competitive athlete. The dietary recommendations for sports nutrition are surprisingly conventional, similar to that for the prevention of chronic disease (such as cancer, diabetes, stroke, hypertension, and cardiovascular disease). Few specialized supplements are required above a well-balanced diet of sufficient ...
Position statement The International Society of Sports Nutrition (ISSN) provides an objective and critical review related to the intake of protein for healthy, exercising individuals. Based on the current available literature, the position of the Society is as follows: 1) An acute exercise stimulus, particularly resistance exercise, and protein ingestion both stimulate muscle protein synthesis ...
The authors present a framework they call the "Paper-2-Podium Matrix" which provides several criteria to critically evaluate performance nutrition-related research papers. In this manner, the sports nutrition practitioner can decide whether the research in question can be translated into something useful for the athletes they advise.
luids, timing of intake, and supplement choices for optimal health and exercise performance. This updated position paper couples a rigorous, systematic, evidence-based analysis of nutrition and performance-specific literature with current scientific data related to energy needs, assessment of body composition, strategies for weight change, nutrient and fluid needs, special nutrient needs ...
Although, we primarily think of innovation in sports nutrition as directed at athlete performance outcomes, we also need to be innovative in our methods of synthesizing and dispersing research. The sports nutrition research data generated to date, have been compiled in several consensus documents on general sports (Thomas D. T. et al., 2016 ...
The paper on nutrition and athlete bone health also stresses the need for more athlete-specific research, especially as it relates to longer-term bone health (e.g., risk of osteopenia and osteoporosis) and shorter-term risk of bony injuries. Bone is a nutritionally modified tissue and generally benefits from weight-bearing activities, although ...
Research has also shown that athletes involved in high volume intense training (e.g., 3-6 hours per day of intense training in 1-2 workouts for 5-6 days per week) may need to consume 8-10 grams/day of carbohydrate (i.e., 400 - 1,500 grams/day for 50 - 150 kg athletes) in order to maintain muscle glycogen levels [1, 6].
Sports nutrition is a constantly evolving field with hundreds of research papers published annually. For this reason, keeping up to date with the literature is often difficult. This paper is a five year update of the sports nutrition review article published as the lead paper to launch the JISSN in 2004 and presents a well-referenced overview of the current state of the science related to how ...
The summit gathered prominent figures in sports, healthcare, technology and human performance. Lincoln's presentation focused on a research paper she co-authored with fellow CWRU Law students Nathaniel Arnholt and Trey Quillin. The research began in the fall of 2023, with the students exploring varying sports law topics.
Sport nutrition for young athletes - PMC