
- Predictive Analytics Workshops
- Corporate Strategy Workshops
- Advanced Excel for MBA
- Powerpoint Workshops
- Digital Transformation
- Competing on Business Analytics
- Aligning Analytics with Strategy
- Building & Sustaining Competitive Advantages
- Corporate Strategy
- Aligning Strategy & Sales
- Digital Marketing
- Hypothesis Testing
- Time Series Analysis
- Regression Analysis
- Machine Learning
- Marketing Strategy
- Branding & Advertising
- Risk Management
- Hedging Strategies
- Network Plotting
- Bar Charts & Time Series
- Technical Analysis of Stocks MACD
- NPV Worksheet
- ABC Analysis Worksheet
- WACC Worksheet
- Porter 5 Forces
- Porter Value Chain
- Amazing Charts
- Garnett Chart
- HBR Case Solution
- 4P Analysis
- 5C Analysis
- NPV Analysis
- SWOT Analysis
- PESTEL Analysis
- Cost Optimization

Merck & Co.: Evaluating a Drug Licensing Opportunity
- Finance & Accounting / MBA EMBA Resources
Next Case Study Solutions
- Stryker Corp.: In-sourcing PCBs Case Study Solution
- Vyaderm Pharmaceuticals Case Study Solution
- Scripps Research Institute: November 1993 (Abridged) Case Study Solution
- Novartis Pharma: The Business Unit Model, Spanish Version Case Study Solution
- Syntonix Pharmaceuticals Case Study Solution
Previous Case Solutions
- Roche's Acquisition of Genentech Case Study Solution
- Biovail Corporation: Revenue Recognition and FOB Sales Accounting Case Study Solution
- American Home Products Corp. Case Study Solution
- ABRY Partners, LLC: WideOpenWest Case Study Solution
- Walker and Company: Profit Plan Decisions Case Study Solution

Predictive Analytics
August 20, 2024

Popular Tags
Case study solutions.

Case Study Solution | Assignment Help | Case Help
Merck & co.: evaluating a drug licensing opportunity description.
This explores the valuation of an opportunity to license a compound before it enters clinical trials. Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
Case Description Merck & Co.: Evaluating a Drug Licensing Opportunity
Strategic managment tools used in case study analysis of merck & co.: evaluating a drug licensing opportunity, step 1. problem identification in merck & co.: evaluating a drug licensing opportunity case study, step 2. external environment analysis - pestel / pest / step analysis of merck & co.: evaluating a drug licensing opportunity case study, step 3. industry specific / porter five forces analysis of merck & co.: evaluating a drug licensing opportunity case study, step 4. evaluating alternatives / swot analysis of merck & co.: evaluating a drug licensing opportunity case study, step 5. porter value chain analysis / vrio / vrin analysis merck & co.: evaluating a drug licensing opportunity case study, step 6. recommendations merck & co.: evaluating a drug licensing opportunity case study, step 7. basis of recommendations for merck & co.: evaluating a drug licensing opportunity case study, quality & on time delivery.
100% money back guarantee if the quality doesn't match the promise
100% Plagiarism Free
If the work we produce contain plagiarism then we payback 1000 USD
Paypal Secure
All your payments are secure with Paypal security.
300 Words per Page
We provide 300 words per page unlike competitors' 250 or 275
Free Title Page, Citation Page, References, Exhibits, Revision, Charts
Case study solutions are career defining. Order your custom solution now.
Case Analysis of Merck & Co.: Evaluating a Drug Licensing Opportunity
Merck & Co.: Evaluating a Drug Licensing Opportunity is a Harvard Business (HBR) Case Study on Finance & Accounting , Texas Business School provides HBR case study assignment help for just $9. Texas Business School(TBS) case study solution is based on HBR Case Study Method framework, TBS expertise & global insights. Merck & Co.: Evaluating a Drug Licensing Opportunity is designed and drafted in a manner to allow the HBR case study reader to analyze a real-world problem by putting reader into the position of the decision maker. Merck & Co.: Evaluating a Drug Licensing Opportunity case study will help professionals, MBA, EMBA, and leaders to develop a broad and clear understanding of casecategory challenges. Merck & Co.: Evaluating a Drug Licensing Opportunity will also provide insight into areas such as – wordlist , strategy, leadership, sales and marketing, and negotiations.
Case Study Solutions Background Work
Merck & Co.: Evaluating a Drug Licensing Opportunity case study solution is focused on solving the strategic and operational challenges the protagonist of the case is facing. The challenges involve – evaluation of strategic options, key role of Finance & Accounting, leadership qualities of the protagonist, and dynamics of the external environment. The challenge in front of the protagonist, of Merck & Co.: Evaluating a Drug Licensing Opportunity, is to not only build a competitive position of the organization but also to sustain it over a period of time.
Strategic Management Tools Used in Case Study Solution
The Merck & Co.: Evaluating a Drug Licensing Opportunity case study solution requires the MBA, EMBA, executive, professional to have a deep understanding of various strategic management tools such as SWOT Analysis, PESTEL Analysis / PEST Analysis / STEP Analysis, Porter Five Forces Analysis, Go To Market Strategy, BCG Matrix Analysis, Porter Value Chain Analysis, Ansoff Matrix Analysis, VRIO / VRIN and Marketing Mix Analysis.
Texas Business School Approach to Finance & Accounting Solutions
In the Texas Business School, Merck & Co.: Evaluating a Drug Licensing Opportunity case study solution – following strategic tools are used - SWOT Analysis, PESTEL Analysis / PEST Analysis / STEP Analysis, Porter Five Forces Analysis, Go To Market Strategy, BCG Matrix Analysis, Porter Value Chain Analysis, Ansoff Matrix Analysis, VRIO / VRIN and Marketing Mix Analysis. We have additionally used the concept of supply chain management and leadership framework to build a comprehensive case study solution for the case – Merck & Co.: Evaluating a Drug Licensing Opportunity
Step 1 – Problem Identification of Merck & Co.: Evaluating a Drug Licensing Opportunity - Harvard Business School Case Study
The first step to solve HBR Merck & Co.: Evaluating a Drug Licensing Opportunity case study solution is to identify the problem present in the case. The problem statement of the case is provided in the beginning of the case where the protagonist is contemplating various options in the face of numerous challenges that Tree Licensing is facing right now. Even though the problem statement is essentially – “Finance & Accounting” challenge but it has impacted by others factors such as communication in the organization, uncertainty in the external environment, leadership in Tree Licensing, style of leadership and organization structure, marketing and sales, organizational behavior, strategy, internal politics, stakeholders priorities and more.
Step 2 – External Environment Analysis
Texas Business School approach of case study analysis – Conclusion, Reasons, Evidences - provides a framework to analyze every HBR case study. It requires conducting robust external environmental analysis to decipher evidences for the reasons presented in the Merck & Co.: Evaluating a Drug Licensing Opportunity. The external environment analysis of Merck & Co.: Evaluating a Drug Licensing Opportunity will ensure that we are keeping a tab on the macro-environment factors that are directly and indirectly impacting the business of the firm.
What is PESTEL Analysis? Briefly Explained
PESTEL stands for political, economic, social, technological, environmental and legal factors that impact the external environment of firm in Merck & Co.: Evaluating a Drug Licensing Opportunity case study. PESTEL analysis of " Merck & Co.: Evaluating a Drug Licensing Opportunity" can help us understand why the organization is performing badly, what are the factors in the external environment that are impacting the performance of the organization, and how the organization can either manage or mitigate the impact of these external factors.
How to do PESTEL / PEST / STEP Analysis? What are the components of PESTEL Analysis?
As mentioned above PESTEL Analysis has six elements – political, economic, social, technological, environmental, and legal. All the six elements are explained in context with Merck & Co.: Evaluating a Drug Licensing Opportunity macro-environment and how it impacts the businesses of the firm.
How to do PESTEL Analysis for Merck & Co.: Evaluating a Drug Licensing Opportunity
To do comprehensive PESTEL analysis of case study – Merck & Co.: Evaluating a Drug Licensing Opportunity , we have researched numerous components under the six factors of PESTEL analysis.
Political Factors that Impact Merck & Co.: Evaluating a Drug Licensing Opportunity
Political factors impact seven key decision making areas – economic environment, socio-cultural environment, rate of innovation & investment in research & development, environmental laws, legal requirements, and acceptance of new technologies.
Government policies have significant impact on the business environment of any country. The firm in “ Merck & Co.: Evaluating a Drug Licensing Opportunity ” needs to navigate these policy decisions to create either an edge for itself or reduce the negative impact of the policy as far as possible.
Data safety laws – The countries in which Tree Licensing is operating, firms are required to store customer data within the premises of the country. Tree Licensing needs to restructure its IT policies to accommodate these changes. In the EU countries, firms are required to make special provision for privacy issues and other laws.
Competition Regulations – Numerous countries have strong competition laws both regarding the monopoly conditions and day to day fair business practices. Merck & Co.: Evaluating a Drug Licensing Opportunity has numerous instances where the competition regulations aspects can be scrutinized.
Import restrictions on products – Before entering the new market, Tree Licensing in case study Merck & Co.: Evaluating a Drug Licensing Opportunity" should look into the import restrictions that may be present in the prospective market.
Export restrictions on products – Apart from direct product export restrictions in field of technology and agriculture, a number of countries also have capital controls. Tree Licensing in case study “ Merck & Co.: Evaluating a Drug Licensing Opportunity ” should look into these export restrictions policies.
Foreign Direct Investment Policies – Government policies favors local companies over international policies, Tree Licensing in case study “ Merck & Co.: Evaluating a Drug Licensing Opportunity ” should understand in minute details regarding the Foreign Direct Investment policies of the prospective market.
Corporate Taxes – The rate of taxes is often used by governments to lure foreign direct investments or increase domestic investment in a certain sector. Corporate taxation can be divided into two categories – taxes on profits and taxes on operations. Taxes on profits number is important for companies that already have a sustainable business model, while taxes on operations is far more significant for companies that are looking to set up new plants or operations.
Tariffs – Chekout how much tariffs the firm needs to pay in the “ Merck & Co.: Evaluating a Drug Licensing Opportunity ” case study. The level of tariffs will determine the viability of the business model that the firm is contemplating. If the tariffs are high then it will be extremely difficult to compete with the local competitors. But if the tariffs are between 5-10% then Tree Licensing can compete against other competitors.
Research and Development Subsidies and Policies – Governments often provide tax breaks and other incentives for companies to innovate in various sectors of priority. Managers at Merck & Co.: Evaluating a Drug Licensing Opportunity case study have to assess whether their business can benefit from such government assistance and subsidies.
Consumer protection – Different countries have different consumer protection laws. Managers need to clarify not only the consumer protection laws in advance but also legal implications if the firm fails to meet any of them.
Political System and Its Implications – Different political systems have different approach to free market and entrepreneurship. Managers need to assess these factors even before entering the market.
Freedom of Press is critical for fair trade and transparency. Countries where freedom of press is not prevalent there are high chances of both political and commercial corruption.
Corruption level – Tree Licensing needs to assess the level of corruptions both at the official level and at the market level, even before entering a new market. To tackle the menace of corruption – a firm should have a clear SOP that provides managers at each level what to do when they encounter instances of either systematic corruption or bureaucrats looking to take bribes from the firm.
Independence of judiciary – It is critical for fair business practices. If a country doesn’t have independent judiciary then there is no point entry into such a country for business.
Government attitude towards trade unions – Different political systems and government have different attitude towards trade unions and collective bargaining. The firm needs to assess – its comfort dealing with the unions and regulations regarding unions in a given market or industry. If both are on the same page then it makes sense to enter, otherwise it doesn’t.
Economic Factors that Impact Merck & Co.: Evaluating a Drug Licensing Opportunity
Social factors that impact merck & co.: evaluating a drug licensing opportunity, technological factors that impact merck & co.: evaluating a drug licensing opportunity, environmental factors that impact merck & co.: evaluating a drug licensing opportunity, legal factors that impact merck & co.: evaluating a drug licensing opportunity, step 3 – industry specific analysis, what is porter five forces analysis, step 4 – swot analysis / internal environment analysis, step 5 – porter value chain / vrio / vrin analysis, step 6 – evaluating alternatives & recommendations, step 7 – basis for recommendations, references :: merck & co.: evaluating a drug licensing opportunity case study solution.
- sales & marketing ,
- leadership ,
- corporate governance ,
- Advertising & Branding ,
- Corporate Social Responsibility (CSR) ,
Amanda Watson
Leave your thought here

© 2019 Texas Business School. All Rights Reserved
USEFUL LINKS
Follow us on.
Subscribe to our newsletter to receive news on update.

Dark Brown Leather Watch
$200.00 $180.00

Dining Chair
$300.00 $220.00

Creative Wooden Stand
$100.00 $80.00
2 x $180.00
2 x $220.00
Subtotal: $200.00
Free Shipping on All Orders Over $100!

Wooden round table
$360.00 $300.00
Hurley Dry-Fit Chino Short. Men's chino short. Outseam Length: 19 Dri-FIT Technology helps keep you dry and comfortable. Made with sweat-wicking fabric. Fitted waist with belt loops. Button waist with zip fly provides a classic look and feel .
| SKU: | 12345 |
| Categories: | , , |
| Tags: | , |
| Share on: |
The marketplace for case solutions.
Merck & Co.: Evaluating a Drug Licensing Opportunity – Case Solution
This case study discusses the event where Merck & Co. was approached by LAB Pharmaceuticals for a prospective licensing deal for a newly developed drug compound called Davanrik. It looks into the value of the proposed deal.
Richard S. Ruback and David Krieger Harvard Business Review ( 201023-PDF-ENG ) October 30, 2000
Case questions answered:
- How has Merck & Co. been able to achieve substantial returns to capital given the high costs and lengthy time to develop a drug?
- Build a decision tree that shows the cash flows and probabilities at all stages of the FDA approval process.
- Should Merck bid to license Davanrik? How much should they pay?
- What is the expected value of the licensing arrangement to LAB? Assume a 5% royalty fee on any cash flows that Merck receives from Davanrik after a successful launch.
Not the questions you were looking for? Submit your own questions & get answers .
Merck & Co.: Evaluating a Drug Licensing Opportunity Case Answers
This case solution includes an Excel file with calculations.
Introduction – Merck & Co.
Merck & Co. Inc. (Merck) is an international, researched-based pharmaceutical company that has been in the business for a while and has been running successfully. At the time, patents for the company’s most popular drugs, such as Pepcid, Prinivil, Vasotec, and Mevacor, were due to expire in 2002.
Since generic substitutions would essentially replace these compounds, Merck could stand to lose almost 50% of its sales revenue, equating to a $5.7 billion loss, if it did not come up with a new drug to bring to the market.
The company refreshes its product portfolio periodically to have stable cash flows through its internal research as well as through joint ventures with other biotechnology companies.
In 2000, Merck & Co. was approached by LAB Pharmaceuticals for a prospective licensing deal for a newly developed drug compound called Davanrik.
It was initially developed to treat depression, but the preclinical development research revealed that the drug has influential effects on blocking antidepressant receptors as well as blocking the receptor that causes hunger, allowing the compound to be used in depression and obesity treatment.
The company lacked commercialization and marketing experience since none of its drugs had passed the FDA approval process.
With a recent denial from the FDA for another compound, the company was hesitant to issue underpriced equity to finance the three-stage clinical trial process since the stock price dropped by over 30%. In these pharmaceutical licensing deals, the various tasks are divided between the licensor and the licensee.
Merck (the licensor) would be responsible for the approval of Davanrik, its manufacture, and its marketing. They would pay LAB (the licensee) an initial licensing fee, a royalty on all sales, and make additional payments as Davanrik completed each stage of the approval process.
Rich Kender, the Vice President of Financial Evaluation and Analysis at Merck, was working with a team to decide whether the company should license Davanrik.
Merck must also decide and conduct an analysis of how much to bid for the license and the potential of failure/success of the drug through the seven-year FDA approval process. The drug would have ten years of commercial life, after which the drug would have little to no value.
The paper is prepared to explain the business model of Merck and the reasons for its success in the pharmaceutical industry. Moreover, it also evaluates the financial potential of Davanrik using decision trees for both Merck and LAB Pharmaceuticals with recommendations on whether to pursue the licensing deal or not.
Merck & Co.’s Business Model
Merck & Co. is in the business of developing compounds for pharmaceutical drugs. The required research and development efforts preceding the launch of a successful blockbuster drug is an extensive and lengthy process and is, therefore, a very expensive one. Nevertheless, Merck & Co. has proven perfectly capable of achieving high returns on capital. The return on assets (ROA) has averaged about 16.5% over the last two years. This is a result of numerous factors.
First of all, Merck has been able to generate tremendous amounts of sales. Since 1995, Merck has launched 15 new products, resulting in sales of $32.7 billion in 1999, which includes $15.2 billion in pharmaceutical benefit management services (PBM) sales.
Since Merck has a well-diversified portfolio of drugs, its four most popular drugs only account for 17% of sales. Some of these products were developed through joint ventures, allowing for a division of costs but also future revenues. This is evident through the moderate share of costs of goods sold in the sales structure (around 50%).
Furthermore, these new products are protected by law under patents, which gives Merck exclusive rights to production and sales. The company utilizes this opportunity to maximize the revenues, after which other companies can produce substitutes that push the profit margins down.
The company, therefore, is always on the lookout for additions to its product portfolio. Merck is also diversified in terms of business lines since it discovers, develops, licenses, and manufactures drugs for commercial use for humans and pets.
The valuation of a pharmaceutical licensing deal varies from that of a generic valuation technique. The differences are due to the fact that the drugs that are in the initial stages of clinical trials would have significantly negative cash flows prior to the approval of a drug.
In other cases, there is historical information about revenues that can then be used to forecast future cash flows. Since pharmaceutical cash flows are risky, the risk can be characterized based on the stage of development.
Risk-Adjusted NPV (rNPV) is a risk-weighted NPV that is widely used in assessing risky projects. It involves forecasting the revenues, costs, and their respective timing but additionally requires the relevant success rate for each stage of development. To account for risk, the expected net cash flow for a given time period is multiplied by the probability of it occurring.
The appropriate probability of success depends on the drug’s therapeutic area and stage of development. Once the net cash flow of each time period has been correctly risk-adjusted, these cash flows are then discounted using an appropriate discount rate. As most of the pharmaceutical revenue forecasts are real, the appropriate discount rate is the real discount rate.
In order to visualize the different stages of the FDA approval process with its likelihood and expected cash flows for Merck, a decision tree has been constructed. It is a decision support tool mostly used in decision analysis.
Merck’s Decision Tree
The decision tree for Merck & Co. can be found in Appendix I. As can be seen, the tree starts with the decision to license and begin Phase I with an initial cost of $30 million (including a $5 million licensing fee) with a 60% probability of success.
The alternative decision is to not license the drug. If Phase I is successful, Phase II trials start. This phase has a cost of $40 million with different probabilities of success. The probability of success as a depression medication is 10%.
For the drug to have a positive impact as a weight loss medication, the chances are 15%, and the probability of the drug successfully treating both depression and weight loss is 5%. The probability of passing Phase II is significantly low since it is administered to many people as compared to the Phase I trial.
If any of these outcomes are successful, testing begins in Phase III. Depending on the result of Phase II, each of Phase III possible outcomes has its own cost and probability of success. If phase III is successful, Merck will spend more money to commercialize the drug so that it can be marketed. In this case, the total cost of the whole phase will consist not only of the Phase III cost but also of the product launching cost.
All of the cash flows and probabilities have been taken into account in order to estimate the expected value of each possible outcome. For example, in order to calculate the expected value of the first possible outcome where the drug is only found to treat depression and is launched commercially, the cash flow was calculated as a commercialization present value minus total costs incurred.
Also, the total probability was calculated as a multiple of probabilities in each phase. Similar calculations were done for each branch, including those with failure as an outcome.
The decision tree provides a compelling argument for Merck & Co. to act upon it and move forward to acquire the license of Davanrik. With an NPV of $13.98 million, the benefits outweigh the associated risks of licensing the drug. The product portfolio, with the addition of Davanrik, would provide the company with the likelihood of higher future revenues.
Currently, Merck & Co. is considering paying the licensee $5 million to acquire the license since the initial payment upon the initiation of phase I is considered the amount paid out for the acquisition of the license.
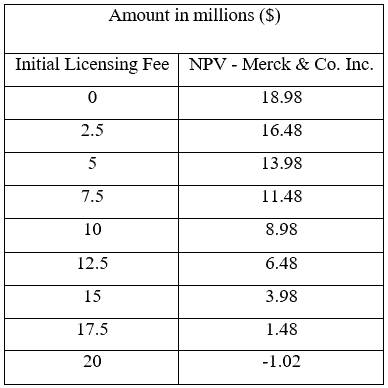
A sensitivity analysis was conducted to look into the different payment amounts and the respective value it would have for Merck & Co. to recommend Kinder and his team about the price they should pay for the license. The sensitivity analysis is provided in Table 1.
Table 1 – Sensitivity analysis
Unlock Case Solution Now!
Get instant access to this case solution with a simple, one-time payment ($24.90).
After purchase:
- You'll be redirected to the full case solution.
- You will receive an access link to the solution via email.
Best decision to get my homework done faster! Michael MBA student, Boston
How do I get access?
Upon purchase, you are forwarded to the full solution and also receive access via email.
Is it safe to pay?
Yes! We use Paypal and Stripe as our secure payment providers of choice.
What is Casehero?
We are the marketplace for case solutions - created by students, for students.
- Harvard Business School →
- Faculty & Research →
- October 2000 (Revised March 2003)
- HBS Case Collection
Merck & Company: Evaluating a Drug Licensing Opportunity
- Format: Print

About The Author
Richard S. Ruback
More from the authors.
- August 2024
- Faculty Research
Lucky Ones Coffee: Employing People with Disabilities
Fail safe testing, inc. (b), fail safe testing, inc. (a).
- Lucky Ones Coffee: Employing People with Disabilities By: Richard S. Ruback, Robin Greenwood, Joe Higgins, Matthew Preble and Dave Habeeb
- Fail Safe Testing, Inc. (B) By: Richard S. Ruback and Royce Yudkoff
- Fail Safe Testing, Inc. (A) By: Richard S. Ruback and Royce Yudkoff
Merck & Co.: Evaluating a Drug Licensing Opportunity
HBS Publishing Case No.: 9-201-023; Teaching Note No.: 5-201-001
Posted: 11 Jan 2006
Richard S. Ruback
Harvard Business School
Date Written: March 25, 2003
SUBJECT AREAS: Capital budgeting, Decision trees, Investments, Present value, Valuation CASE SETTINGS: Pharmaceutical industry; Fortune 500; $33 billion revenues; 1999 This explores the valuation of an opportunity to license a compound before it enters clinical trials. Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
Note: Teaching Note available.
Suggested Citation: Suggested Citation
Richard S. Ruback (Contact Author)
Harvard business school ( email ).
Boston, MA 02163 United States 617-495-6422 (Phone) 617-496-8443 (Fax)
Do you have a job opening that you would like to promote on SSRN?
Paper statistics, related ejournals, social sciences education ejournal.
Subscribe to this fee journal for more curated articles on this topic
Finance Education eJournal
- Corpus ID: 166569394
Merck & Co.: Evaluating a Drug Licensing Opportunity
- Richard S. Ruback
- Published 25 March 2003
- Business, Medicine
10 Citations
1 r & d licensing in the biopharmaceutical industry : how to structure a good deal, ip licensing : how to structure a good deal, ip licensing: how to structure a good deal, the outsourcing of r&d through acquisitions in the pharmaceutical industry, valuations using royalty data in the life sciences area—focused on anticancer and cardiovascular therapies, research and development project valuation and licensing negotiations at phytopharm plc, development of technology portfolio analysis method for technology-outsourcing of pharmaceutical cooperations, process modeling proposition in biopharmaceutical manufacturing, innovations, real options, risk and return: evidence from the pharmaceutical and biotechnology industries, the allocation of control rights in pharmaceutical alliances, related papers.
Showing 1 through 3 of 0 Related Papers

- Case Studies
Finance & Accounting

Merck & Co.: Evaluating a Drug Licensing Opportunity

Merck & Co.: Evaluating a Drug Licensing Opportunity ^ 201023
Are you an educator.
Register as a Premium Educator at hbsp.harvard.edu , plan a course, and save your students up to 50% with your academic discount.
Product Description
Publication Date: October 30, 2000
Source: Harvard Business School
This explores the valuation of an opportunity to license a compound before it enters clinical trials. Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.

This Product Also Appears In
Buy together, related products.

Merck & Co., Inc.

Merck & Co., Inc. (A)

Pilgrim Drug Co.
Copyright permissions.
If you'd like to share this PDF, you can purchase copyright permissions by increasing the quantity.
Order for your team and save!
To read this content please select one of the options below:
Please note you do not have access to teaching notes, risk analysis for merck and company: product kl-798.
Publication date: 20 January 2017
Teaching notes
This case builds on the case "Merck & Company: Product KL-798" (UVA-QA-0582) by providing market uncertainties for the drug (drug quality, the presence of a competitor, market growth, and the time to the drug's release). Student and faculty spreadsheets are provided for the calculation of net present values for the scenarios. There is an additional challenge of how to treat the several downstream decisions (using OptQuest, for example) and how to value the license opportunity. A teaching note is also available to registered faculty members.
- Decision analysis
- Monte Carlo simulation
Bodily, S.E. and Faulk, J. (2017), "Risk Analysis for Merck and Company: Product KL-798", . https://doi.org/10.1108/case.darden.2016.000262
University of Virginia Darden School Foundation
Copyright © 2003 by the University of Virginia Darden School Foundation, Charlottesville, VA. All rights reserved.
You do not currently have access to these teaching notes. Teaching notes are available for teaching faculty at subscribing institutions. Teaching notes accompany case studies with suggested learning objectives, classroom methods and potential assignment questions. They support dynamic classroom discussion to help develop student's analytical skills.
Related articles
All feedback is valuable.
Please share your general feedback
Report an issue or find answers to frequently asked questions
Contact Customer Support
Marketing Process Analysis
Segmentation, targeting, positioning, marketing strategic planning, marketing 5 concepts analysis, swot analysis & matrix, porter five forces analysis, pestel / pest / step analysis, cage distance analysis international marketing analysis leadership, organizational resilience analysis, bcg matrix / growth share matrix analysis, block chain supply chain management, paei management roles, leadership with empathy & compassion, triple bottom line analysis, mckinsey 7s analysis, smart analysis, vuca analysis ai ethics analysis analytics, merck & co.: evaluating a drug licensing opportunity case memo, recommendation memo / mba resources.
- Merck & Co.: Evaluating a Drug Licensing Opportunity
- Finance & Accounting / MBA Resources
Introduction to Case Memo & Recommendation Memo
EMBA Pro Case Memo for Merck & Co.: Evaluating a Drug Licensing Opportunity case study
This explores the valuation of an opportunity to license a compound before it enters clinical trials. Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
Case Authors : Richard S. Ruback, David Krieger
Topic : finance & accounting, related areas : decision making, financial analysis, emba pro case memo & recommendation memo approach for merck & co.: evaluating a drug licensing opportunity.
At EMBA PRO , we provide corporate level professional Marketing Mix and Marketing Strategy solutions. Merck & Co.: Evaluating a Drug Licensing Opportunity case study is a Harvard Business School (HBR) case study written by Richard S. Ruback, David Krieger. The Merck & Co.: Evaluating a Drug Licensing Opportunity (referred as “Tree Licensing” from here on) case study provides evaluation & decision scenario in field of Finance & Accounting. It also touches upon business topics such as - Marketing Mix, Product, Price, Place, Promotion, 4P, Decision making, Financial analysis. Our immersive learning methodology from – case study discussions to simulations tools help MBA and EMBA professionals to - gain new insight, deepen their knowledge of the Finance & Accounting field, company, context, collaborators, competitors, customers, Marketing Mix factors, Products related decisions, pricing strategies and more.
Urgent - 12Hr
- 100% Plagiarism Free
- On Time Delivery | 27x7
- PayPal Secure
- 300 Words / Page
Case Study Recommendation Memo Overview
At EMBA Pro, we write Merck & Co.: Evaluating a Drug Licensing Opportunity case study recommendation memo as per the Harvard Business Review (HBR) Communication case memo framework. If you are looking for MBA, Executive MBA or Corporate / Professional level recommendation memo then feel free to connect with us. Our Merck & Co.: Evaluating a Drug Licensing Opportunity recommendation memos are concisely written and exhibit and illustrate the clear strategic thought process of the protagonist in the case study.
What is Merck & Co.: Evaluating a Drug Licensing Opportunity Case Study Recommendation Memo
The Merck & Co.: Evaluating a Drug Licensing Opportunity case study recommendation memo is one page or at max two page document (not including the exhibits) that recommends the course of action and provide its rationale in brief. The popularity of the format is because of the limited time available to most leaders and managers in today’s corporations. According to survey by Harvard Business Review, recommendation memo is the most used document in fortune 500 firms and extensively preferred by CEOs of transnational organizations. The greatest advantage of recommendation memo is that it cuts the slab out of communication and makes the writer focus on the most critical aspects. Like all other forms of communication, writing case study recommendation memo requires practice and clear insight into what is required.
Merck & Co.: Evaluating a Drug Licensing Opportunity Case Memo Structure
First paragraph ....
The first paragraph of the Merck & Co.: Evaluating a Drug Licensing Opportunity recommendation memo includes recommended course of action for Tree Licensing. It will clearly state your intent or course of action you like the organization to pursue. It usually starts with – “This recommends .... ” Topic of the Merck & Co.: Evaluating a Drug Licensing Opportunity – This section encapsulates the “what” of the core issue rather than how and when. Caveats for writing first paragraph yourself - Don’t focus on the how and when of events in the case, as they take away the precision of the intent or course of action. There should be clear purpose and objective to the first paragraph.
Background of Merck & Co.: Evaluating a Drug Licensing Opportunity
The background paragraph of briefly states – the historical context, illustrate the moment that brought the protagonist into the present situation and why she needs to make a decision. • How the technology challenges will impact the operations of companyname? • How companyname inability to attract best talent is impacting its capacity to innovate. What it needs to do to improve the conditions. • How Tree Licensing's big budget and small budget financial performance differs and what is preferred way going forward. It is a good practice to state the constraints such as – technology, capacity, budget, and people etc in the background section as it will help in building the analysis part plus provide the reader to relate to the recommendations part in the first paragraph. The section needs to be both brief and factual. Only use data that is relevant to explain bigger picture. Detailed charts and tables can go into exhibits. Checklist for writing an excellent background section –
- Does the background section of recommendation memo explain why action is needed now?
- Is the background clear, concise, and easy to follow?
- After reading the background section can the reader come across the appropriate sense of urgency for why action is needed now?
- Are only the pertinent facts from the case study outlined?
Issues and Analysis
List 3-4 most pressing issues the protagonist in Merck & Co.: Evaluating a Drug Licensing Opportunity case study is facing. How these issues can impact the Tree Licensing. Conduct a situational analysis using strategic tools such as – PESTEL analysis, Porter Five Forces analysis, SWOT analysis.
Recommendations
In this section you should present the details of – What to do, how to do it and when to do it. The recommendations should pass the criteria of – Suitability, Acceptability, and Feasibility. Are the recommendations suitable for the Tree Licensing given the scenario and issues discussed in the Merck & Co.: Evaluating a Drug Licensing Opportunity. Are the recommendations acceptable given the culture of the Tree Licensing. Often consulting companies make this error that they strive to provide best in class solution even though implementing it may run counter to the culture of the organization. The recommendations should be consistent with the culture of Tree Licensing. Finally recommendations should meet the feasibility criteria based on the facts and details provided in the casename. You can conduct a VRIO analysis of Tree Licensing to assess whether the recommended course of action is feasible under the present – resources, skills, technological know how, and financial ability of the organization.
Basis for recommendations
Providing supporting argument and evidences on why each recommendation is unique and need to implemented to change the present situation. The supporting evidences can include – financial statements, growth trajectory, organization culture and leadership style in the organization. For greater transparency and integrity of Merck & Co.: Evaluating a Drug Licensing Opportunity case study recommendation memo – always explicitly mention the assumptions. The assumptions are often your business judgment based on industry knowledge and facts provided in case study analysis.
Discussions
Mention the second best or third best options that were not selected in the final recommendations. This will provide a reader an ability to look beyond the recommended solution. Always discuss the risks and key assumptions. If you prefer you can make a full disclosure grid of Tree Licensing based on the description provided in the case study. Risk associated with the recommendations should also be clearly addressed based on thorough analysis and structured line of reasoning.
This step require a detail road map for the execution of the recommendations. It may involve what are the resources required, how much time it will take. What is the desired sequence of activities and key milestones in the course of implementation of the recommendations.
If you have used extensive analysis, statistical models and other strategic tools to come to the conclusions then add those in the exhibit section. This will help in explaining your work process and how you reached the conclusions you have reached and what assumptions underpin those conclusions. Make sure to clearly lay out – references, books details, and tables that are used for the purpose of analysis.
5C Marketing Analysis of Merck & Co.: Evaluating a Drug Licensing Opportunity
4p marketing analysis of merck & co.: evaluating a drug licensing opportunity, porter five forces analysis and solution of merck & co.: evaluating a drug licensing opportunity, porter value chain analysis and solution of merck & co.: evaluating a drug licensing opportunity, case memo & recommendation memo of merck & co.: evaluating a drug licensing opportunity, blue ocean analysis and solution of merck & co.: evaluating a drug licensing opportunity, marketing strategy and analysis merck & co.: evaluating a drug licensing opportunity, vrio /vrin analysis & solution of merck & co.: evaluating a drug licensing opportunity, pestel / step / pest analysis of merck & co.: evaluating a drug licensing opportunity, case study solution of merck & co.: evaluating a drug licensing opportunity, swot analysis and solution of merck & co.: evaluating a drug licensing opportunity, references & further readings.
Richard S. Ruback, David Krieger (2018) , "Merck & Co.: Evaluating a Drug Licensing Opportunity Harvard Business Review Case Study. Published by HBR Publications.
Kotler & Armstrong (2017) "Principles of Marketing Management Management", Published by Pearson Publications.
M. E. Porter , Competitive Strategy(New York: Free Press, 1980)
Case Memo & Recommendation Memo
- Stryker Corp.: In-sourcing PCBs Case Memo & Recommendation Memo
- Vyaderm Pharmaceuticals Case Memo & Recommendation Memo
- Scripps Research Institute: November 1993 (Abridged) Case Memo & Recommendation Memo
- Novartis Pharma: The Business Unit Model, Spanish Version Case Memo & Recommendation Memo
- Syntonix Pharmaceuticals Case Memo & Recommendation Memo
- Roche's Acquisition of Genentech Case Memo & Recommendation Memo
- Biovail Corporation: Revenue Recognition and FOB Sales Accounting Case Memo & Recommendation Memo
- American Home Products Corp. Case Memo & Recommendation Memo
- ABRY Partners, LLC: WideOpenWest Case Memo & Recommendation Memo
- Walker and Company: Profit Plan Decisions Case Memo & Recommendation Memo
Explore More
Feel free to connect with us if you need business research.
You can download Excel Template of Case Memo & Recommendation Memo of Merck & Co.: Evaluating a Drug Licensing Opportunity

IMAGES
COMMENTS
Background of Merck Leveraged. Merck & Company Decision Tree (Davanrik for Weight Loss Launching Cost: $225 million) Phase III 87.5 Succeed Gain: 60 85% 27.5 Phase I LAB Decision Tree Failure Decision Node: Bid for the license 15% 34.75 Enter Phase III Succeed 78.5 60% 75% Gain:
Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. ... Tree Licensing in case study Merck & Co.: Evaluating a Drug ...
This explores the valuation of an opportunity to license a compound before it enters clinical trials. Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an ...
The decision tree for Merck & Co. can be found in Appendix I. As can be seen, the tree starts with the decision to license and begin Phase I with an initial cost of $30 million (including a $5 million licensing fee) with a 60% probability of success. The alternative decision is to not license the drug. If Phase I is successful, Phase II trials ...
Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
The valuation of an opportunity to license a compound before it enters clinical trials is explored, including an introduction to decision tree analysis and valuation. SUBJECT AREAS: Capital budgeting, Decision trees, Investments, Present value, Valuation CASE SETTINGS: Pharmaceutical industry; Fortune 500; $33 billion revenues; 1999 This explores the valuation of an opportunity to license a ...
Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the ...
Describes Merck's decision tree evaluation process is presented. Information required to evaluate a specific licensing opportunity is provided, including the costs of the three phases of the review process, the revenues if approved, and the probability of various outcomes. It includes an introduction to decision tree analysis and valuation.
Abstract. This case builds on the case "Merck & Company: Product KL-798" (UVA-QA-0582) by providing market uncertainties for the drug (drug quality, the presence of a competitor, market growth, and the time to the drug's release). Student and faculty spreadsheets are provided for the calculation of net present values for the scenarios.
M3 Case Study Discussion: Merck & Company: Evaluating a Drug Licensing Opportunity Overview Individually, read the Merck & Company case study found in your Harvard Business Review Course Pack. Then work in small groups to answer the case study questions. Case Study Question Use a decision tree and the information contained in the case study to ...
The Merck & Co.: Evaluating a Drug Licensing Opportunity (referred as "Tree Licensing" from here on) case study provides evaluation & decision scenario in field of Finance & Accounting. It also touches upon business topics such as - Marketing Mix, Product, Price, Place, Promotion, 4P, Decision making, Financial analysis.
Pre-clinical development: Ready to enter 3 phases clinical approval process. Potential Cash flow: 17yrs - 7yrs = 10yrs of exclusivity. According to the decision tree we calculate the total NPV to be $13,980,000 and the black scholes value to be $50,895,693. Since both values are positives we conclude that we should license the drug - Davanrik.
A decision tree was created to display the various stages of the FDA approval process and the expected cash flows for Merck. It is a decision support tool that is mainly used for decision analysis. Merck's Decision Tree. The Merck decision tree is in an Excel spreadsheet. We see that this tree begins with the permitting decision and begins at ...