- Introduction
- Conclusions
- Article Information
BMI indicates body mass index; SES, socioeconomic status.
a Variables smoking status, SES, drinking pattern, former drinker bias only, occasional drinker bias, median age, and gender were removed.
b Variables race, diet, exercise, BMI, country, follow-up year, publication year, and unhealthy people exclusion were removed.
eAppendix. Methodology of Meta-analysis on All-Cause Mortality and Alcohol Consumption
eReferences
eFigure 1. Flowchart of Systematic Search Process for Studies of Alcohol Consumption and Risk of All-Cause Mortality
eTable 1. Newly Included 20 Studies (194 Risk Estimates) of All-Cause Mortality and Consumption in 2015 to 2022
eFigure 2. Funnel Plot of Log-Relative Risk (In(RR)) of All-Cause Mortality Due to Alcohol Consumption Against Inverse of Standard Error of In(RR)
eFigure 3. Relative Risk (95% CI) of All-Cause Mortality Due to Any Alcohol Consumption Without Any Adjustment for Characteristics of New Studies Published between 2015 and 2022
eFigure 4. Unadjusted, Partially Adjusted, and Fully Adjusted Relative Risk (RR) of All-Cause Mortality for Drinkers (vs Nondrinkers), 1980 to 2022
eTable 2. Statistical Analysis of Unadjusted Mean Relative Risk (RR) of All-Cause Mortality for Different Categories of Drinkers for Testing Publication Bias and Heterogeneity of RR Estimates From Included Studies
eTable 3. Mean Relative Risk (RR) Estimates of All-Cause Mortality Due to Alcohol Consumption up to 2022 for Subgroups (Cohorts Recruited 50 Years of Age or Younger and Followed up to 60 Years of Age)
Data Sharing Statement
- Errors in Figure and Supplement JAMA Network Open Correction May 9, 2023

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zhao J , Stockwell T , Naimi T , Churchill S , Clay J , Sherk A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses . JAMA Netw Open. 2023;6(3):e236185. doi:10.1001/jamanetworkopen.2023.6185
Manage citations:
© 2024
- Permissions
Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses
- 1 Canadian Institute for Substance Use Research, University of Victoria, Victoria, British Columbia, Canada
- 2 Department of Psychology, University of Portsmouth, Portsmouth, Hampshire, United Kingdom
- Correction Errors in Figure and Supplement JAMA Network Open
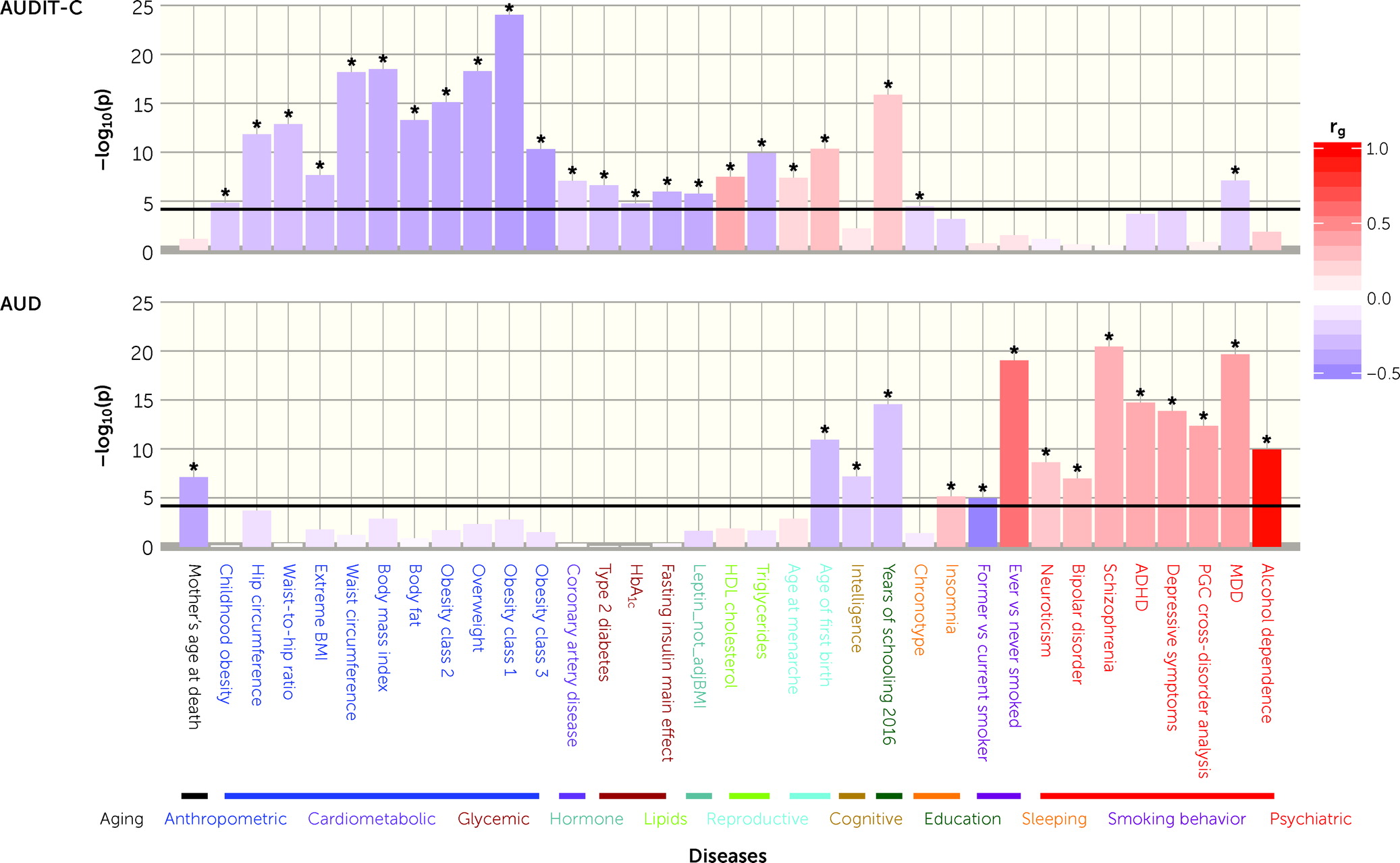
Question What is the association between mean daily alcohol intake and all-cause mortality?
Findings This systematic review and meta-analysis of 107 cohort studies involving more than 4.8 million participants found no significant reductions in risk of all-cause mortality for drinkers who drank less than 25 g of ethanol per day (about 2 Canadian standard drinks compared with lifetime nondrinkers) after adjustment for key study characteristics such as median age and sex of study cohorts. There was a significantly increased risk of all-cause mortality among female drinkers who drank 25 or more grams per day and among male drinkers who drank 45 or more grams per day.
Meaning Low-volume alcohol drinking was not associated with protection against death from all causes.
Importance A previous meta-analysis of the association between alcohol use and all-cause mortality found no statistically significant reductions in mortality risk at low levels of consumption compared with lifetime nondrinkers. However, the risk estimates may have been affected by the number and quality of studies then available, especially those for women and younger cohorts.
Objective To investigate the association between alcohol use and all-cause mortality, and how sources of bias may change results.
Data Sources A systematic search of PubMed and Web of Science was performed to identify studies published between January 1980 and July 2021.
Study Selection Cohort studies were identified by systematic review to facilitate comparisons of studies with and without some degree of controls for biases affecting distinctions between abstainers and drinkers. The review identified 107 studies of alcohol use and all-cause mortality published from 1980 to July 2021.
Data Extraction and Synthesis Mixed linear regression models were used to model relative risks, first pooled for all studies and then stratified by cohort median age (<56 vs ≥56 years) and sex (male vs female). Data were analyzed from September 2021 to August 2022.
Main Outcomes and Measures Relative risk estimates for the association between mean daily alcohol intake and all-cause mortality.
Results There were 724 risk estimates of all-cause mortality due to alcohol intake from the 107 cohort studies (4 838 825 participants and 425 564 deaths available) for the analysis. In models adjusting for potential confounding effects of sampling variation, former drinker bias, and other prespecified study-level quality criteria, the meta-analysis of all 107 included studies found no significantly reduced risk of all-cause mortality among occasional (>0 to <1.3 g of ethanol per day; relative risk [RR], 0.96; 95% CI, 0.86-1.06; P = .41) or low-volume drinkers (1.3-24.0 g per day; RR, 0.93; P = .07) compared with lifetime nondrinkers. In the fully adjusted model, there was a nonsignificantly increased risk of all-cause mortality among drinkers who drank 25 to 44 g per day (RR, 1.05; P = .28) and significantly increased risk for drinkers who drank 45 to 64 and 65 or more grams per day (RR, 1.19 and 1.35; P < .001). There were significantly larger risks of mortality among female drinkers compared with female lifetime nondrinkers (RR, 1.22; P = .03).
Conclusions and Relevance In this updated systematic review and meta-analysis, daily low or moderate alcohol intake was not significantly associated with all-cause mortality risk, while increased risk was evident at higher consumption levels, starting at lower levels for women than men.
The proposition that low-dose alcohol use protects against all-cause mortality in general populations continues to be controversial. 1 Observational studies tend to show that people classified as “moderate drinkers” have longer life expectancy and are less likely to die from heart disease than those classified as abstainers. 2 Systematic reviews and meta-analyses of this literature 3 confirm J-shaped risk curves (protective associations at low doses with increasing risk at higher doses). However, mounting evidence suggests these associations might be due to systematic biases that affect many studies. For example, light and moderate drinkers are systematically healthier than current abstainers on a range of health indicators unlikely to be associated with alcohol use eg, dental hygiene, exercise routines, diet, weight, income 4 ; lifetime abstainers may be systematically biased toward poorer health 5 ; studies fail to control for biases in the abstainer reference group, in particular failing to remove “sick quitters” or former drinkers, many of whom cut down or stop for health reasons 2 ; and most studies have nonrepresentative samples leading to an overrepresentation of older White men. Adjustment of cohort samples to make them more representative has been shown to eliminate apparent protective associations. 6 Mendelian randomization studies that control for the confounding effects of sociodemographic and environmental factors find no evidence of cardioprotection. 7
We published 2 previous systematic reviews and meta-analyses that investigated these hypotheses. The first of these focused on all-cause mortality, 8 finding negligible reductions in mortality risk with low-volume alcohol use when study-level controls were introduced for potential bias and confounding, such as the widespread practice of misclassifying former drinkers and/or current occasional drinkers as abstainers (ie, not restricting reference groups to lifetime abstainers). 8 Our alcohol and coronary heart disease (CHD) mortality meta-analysis of 45 cohort studies 9 found that CHD mortality risk differed widely by age ranges and sex of study populations. In particular, young cohorts followed up to old age did not show significant cardio-protection for low-volume use. Cardio-protection was only apparent among older cohorts that are more exposed to lifetime selection biases (ie, increasing numbers of “sick-quitters” in the abstainer reference groups and the disproportionate elimination of drinkers from the study sample who had died or were unwell).
The present study updates our earlier systematic review and meta-analysis for all-cause mortality and alcohol use, 8 including studies published up to July 2021 (ie, 6.5 years of additional publications). The study also investigated the risk of all-cause mortality for alcohol consumption according to (1) median ages of the study populations (younger than 56 years or 56 years and older), replicating the methods of Zhao et al 9 ; (2) the sex distribution of the study populations, and (3) studies of cohorts recruited before a median age of 51 years of age and followed up in health records until a median age of at least 60 years (ie, with stricter rules to further minimize lifetime selection biases). Because younger cohorts followed up to an age at which they may experience heart disease are less likely to be affected by lifetime selection biases, 9 we hypothesized that such studies would be less likely to show reduced mortality risks for low-volume drinkers. Finally, we reran the analyses using occasional drinkers (<1 drink per week) as the reference, for whom physiological health benefits are unlikely. Occasional drinkers are a more appropriate reference group, given evidence demonstrating that lifetime abstainers may be biased toward ill health. 10
The present study updates the systematic reviews and meta-analyses described above 8 by including studies published up to July 2021 to investigate whether the risk differed for subgroups. The study protocol was preregistered on the Open Science Framework. 11 Inclusion criteria, search strategy, study selection, data extraction, and statistical analytical methods of the study are summarized in later sections (see eAppendix in Supplement 1 for more details).
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. 12 The review sought cohort studies of all-cause mortality and alcohol consumption. We identified all potentially relevant articles published up to July 31, 2021, regardless of language, by searching PubMed and Web of Science, through reference list cross-checking of previous meta-analyses (eFigure 1 in Supplement 1 ). There were 87 studies identified by Stockwell et al. 8 After inclusion of 20 new studies meeting inclusion criteria, there were a total of 107 cohort studies (eTable 1 in Supplement 1 ). 13 - 32
Three coders (J. Z., F. A., and J. C.) reviewed all eligible studies to extract and code data independently from all studies fulfilling the inclusion criteria. Data extracted included (1) outcome, all-cause mortality; (2) measures of alcohol consumption; (3) study characteristics, including cohort ages at recruitment and follow-up; (4) types of misclassification error of alcohol consumers and abstainers; (5) controlled variables in individual studies. Alcoholic drinks were converted into grams per day according to country-specific definitions if not otherwise defined. 33 , 34
We also assessed publication bias, heterogeneity, and confounding of covariates that might potentially affect the association of interest using several statistical approaches. 35 - 41 Relative risk (RR), including hazard ratios or rate ratios, were converted to natural log-transformed formats to deal with skewness. Publication bias was assessed through visual inspection of the funnel plot of log-RR of all-cause mortality due to alcohol consumption against the inverse standard error of log-RR 42 and Egger’s linear regression method. 36 We also plotted forest graphs of log-RR of all-cause mortality for any level of drinking to assess heterogeneity among studies. 42 The between-study heterogeneity of RRs were assessed using Cochran Q 37 and the I 2 statistic. 38 If heterogeneity was detected, mixed-effects models were used to obtain the summarized RR estimates. Mixed-effects regression analyses were performed in which drinking groups and control variables were treated as fixed-effects with a random study effect because of significant heterogeneity. 43
All analyses were weighted by the inverse of the estimated variance of the natural log relative risk. Variance was estimated from reported standard errors, confidence intervals, or number of deaths. The weights for each individual study were created using the inverse variance weight scheme and used in mixed regression analysis to get maximum precision for the main results of the meta-analysis. 42 In comparison with lifetime abstainers, the study estimated the mean RR of all-cause mortality for former drinkers (ie, now completely abstaining), current occasional (<9.1 g per week), low-volume (1.3-24.0 g per day), medium-volume (25.0-44.0 g per day), high-volume (45.0-64.0 g) and highest-volume drinkers (≥65.0 grams per day). The analyses adjusted for the potential confounding effects of study characteristics including the median age and sex distribution of study samples, drinker biases, country where a study was conducted, follow-up years and presence or absence of confounders. Analyses were also repeated using occasional drinkers as the reference group. We used t tests to calculate P values, and significance was set at .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute) and the SAS MIXED procedure was used to model the log-transformed RR. 44 Data were analyzed from September 2021 to August 2022.
There were 724 estimates of the risk relationship between level of alcohol consumption and all-cause mortality from 107 unique studies 13 - 32 , 45 - 131 , including 4 838 825 participants and 425 564 deaths available for the analysis. Table 1 describes the sample characteristics of the metadata. Of 39 studies 13 , 15 , 18 , 21 , 23 - 26 , 29 , 31 , 45 - 47 , 49 , 50 , 52 - 54 , 57 - 59 , 62 , 64 , 70 , 80 , 81 , 85 , 87 , 91 , 94 , 96 , 100 , 104 , 107 , 118 , 124 , 125 , 127 , 130 reporting RR estimates for men and women separately, 33 14 , 17 , 48 , 51 , 61 , 63 , 66 , 68 , 69 , 72 , 76 , 79 , 83 , 84 , 86 , 88 , 90 , 92 , 93 , 97 , 98 , 101 , 103 , 105 , 109 - 111 , 113 - 115 , 119 , 120 , 128 were for males only, 8 16 , 65 , 73 , 99 , 102 , 108 , 112 , 123 for females only, and 30 13 , 19 - 22 , 26 - 30 , 32 , 55 , 56 , 67 , 71 , 74 , 75 , 77 , 78 , 82 , 84 , 89 , 95 , 106 , 116 , 117 , 121 , 122 , 126 , 129 for both sexes. Twenty-one studies 13 , 17 , 19 , 21 , 22 , 26 , 27 , 45 - 58 (220 risk estimates) were free from abstainer bias (ie, had a reference group of strictly defined lifetime abstainers). There were 50 studies 14 - 16 , 18 , 20 , 23 - 25 , 29 , 59 - 99 (265 risk estimates) with both former and occasional drinker bias; 28 studies 28 , 30 - 32 , 100 - 122 , 130 (177 risk estimates) with only former drinker bias; and 8 studies 123 - 129 , 131 (62 risk estimates) with only occasional drinker bias.
Unadjusted mean RR estimates for most study subgroups categorized by methods/sample characteristics showed markedly or significantly higher RRs for alcohol consumers as a group vs abstainers. Exceptions were for studies with less than 10 years of follow-up and those with some form of abstainer bias ( Table 1 ). Bivariable analyses showed that mortality risks for alcohol consumers varied considerably according to other study characteristics, such as quality of the alcohol consumption measure, whether unhealthy individuals were excluded at baseline, and whether socioeconomic status was controlled for ( Table 1 ).
No evidence of publication bias was detected either by inspection of symmetry in the funnel plot of log-RR estimates and their inverse standard errors (eFigure 2 in Supplement 1 ) or by Egger linear regression analysis (eTable 2 in Supplement 1 , all P > .05 for each study group). Significant heterogeneity was observed across studies for all drinking categories confirmed by both the Q statistic ( Q 723 = 5314.80; P < .001) and I 2 estimates (all >85.87%). (See eFigure 3 in Supplement 1 for forest plot of unadjusted risk estimates of mortality risks for the 20 newly identified studies).
Pooled unadjusted estimates (724 observations) showed significantly higher risk for former drinkers (RR, 1.22; 95% CI, 1.11-1.33; P = .001) and significantly lower risk for low-volume drinkers (RR, 0.85; 95% CI, 0.81-0.88; P = .001) compared with abstainers as defined in the included studies ( Table 2 ; eFigure 4 in Supplement 1 ). In the fully adjusted model, mortality RR estimates increased for all drinking categories, becoming nonsignificant for low-volume drinkers (RR, 0.93; 95% CI, 0.85-1.01; P = .07), occasional drinkers (>0 to <1.3 g of ethanol per day; RR, 0.96; 95% CI, 0.86-1.06; P = .41), and drinkers who drank 25 to 44 g per day (RR, 1.05; 95% CI, 0.96-1.14; P = .28). There was a significantly increased risk among drinkers who drank 45 to 64 g per day (RR, 1.19; 95% CI, 1.07-1.32; P < .001) and 65 or more grams (RR, 1.35; 95% CI, 1.23-1.47; P < .001). The Figure shows the changes in RR estimates for low-volume drinkers when removing each covariate from the fully adjusted model. In most cases, removing study-level covariates tended to yield lower risk estimates from alcohol use.
Table 2 presents the RR estimates when occasional drinkers were the reference group. In fully adjusted models, higher though nonsignificant mortality risks were observed for both abstainers and medium-volume drinkers (RR, 1.04; 95% CI, 0.94-1.16; P = .44 and RR, 1.09; 95% CI, 0.96-1.25; P = .19, respectively). There were significantly elevated risks for both high and higher volume drinkers (RR, 1.24; 95% CI, 1.07-1.44; P = .004 and RR, 1.41; 95% CI, 1.23-1.61; . P = 001, respectively).
As hypothesized, there was a significant interaction between cohort age and mortality risk ( P = .02; F 601 = 2.93) and so RR estimates for drinkers were estimated in analyses stratified by median age of the study populations at enrollment ( Table 3 ). In unadjusted and partially adjusted analyses, older cohorts displayed larger reductions in mortality risk associated with low-volume consumption than younger cohorts. However, in fully adjusted analyses with multiple covariates included for study characteristics, these differences disappeared. Younger cohorts also displayed greater mortality risks than older cohorts at higher consumption levels. Among studies in which participants were recruited at age 50 years or younger and followed up to age 60 years (ie, there was likely reduced risk of lifetime selection bias) higher RR estimates were observed for all drinking groups vs lifetime abstainers. These differences were significant in all drinking groups except low-volume drinkers (eTable 3 in Supplement 1 ).
Across all levels of alcohol consumption, female drinkers had a higher RR of all-cause mortality than males ( P for interaction = .001). As can be seen in Table 4 , all female drinkers had a significantly increased mortality risk compared with female lifetime nondrinkers (RR, 1.22; 95% CI, 1.02-1.46; P = .03). Compared with lifetime abstainers, there was significantly increased risk of all-cause mortality among male drinkers who drank 45 to 64 g per day (RR, 1.15; 95% CI, 1.03-1.28; P = .01) and drank 65 or more (RR, 1.34; 95% CI, 1.23-1.47; P < .001), and among female drinkers who drank 25 to 44 g per day (RR, 1.21; 95% CI, 1.08-1.36; P < .01), 45 to 64 g (RR, 1.34; 95% CI, 1.11-1.63; P < .01) and 65 or more grams (RR, 1.61; 95% CI, 1.44-1.80; P = .001).
In fully adjusted, prespecified models that accounted for effects of sampling, between-study variation, and potential confounding from former drinker bias and other study-level covariates, our meta-analysis of 107 studies found (1) no significant protective associations of occasional or low-volume drinking (moderate drinking) with all-cause mortality; and (2) an increased risk of all-cause mortality for drinkers who drank 25 g or more and a significantly increased risk when drinking 45 g or more per day.
Several meta-analytic strategies were used to explore the role of abstainer reference group biases caused by drinker misclassification errors and also the potential confounding effects of other study-level quality covariates in studies. 2 Drinker misclassification errors were common. Of 107 studies identified, 86 included former drinkers and/or occasional drinkers in the abstainer reference group, and only 21 were free of both these abstainer biases. The importance of controlling for former drinker bias/misclassification is highlighted once more in our results which are consistent with prior studies showing that former drinkers have significantly elevated mortality risks compared with lifetime abstainers.
In addition to presenting our fully adjusted models, a strength of the study was the examination of the differences in relative risks according to unadjusted and partially adjusted models, including the effect of removing individual covariates from the fully adjusted model. We found evidence that abstainer biases and other study characteristics changed the shape of the risk relationship between mortality and rising alcohol consumption, and that most study-level controls increased the observed risks from alcohol, or attenuated protective associations at low levels of consumption such that they were no longer significant. The reduced RR estimates for occasional or moderate drinkers observed without adjustment may be due to the misclassification of former and occasional drinkers into the reference group, a possibility which is more likely to have occurred in studies of older cohorts which use current abstainers as the reference group. This study also demonstrates the degree to which observed associations between consumption and mortality are highly dependent on the modeling strategy used and the degree to which efforts are made to minimize confounding and other threats to validity.
It also examined risk estimates when using occasional drinkers rather than lifetime abstainers as the reference group. The occasional drinker reference group avoids the issue of former drinker misclassification that can affect the abstainer reference group, and may reduce confounding to the extent that occasional drinkers are more like low-volume drinkers than are lifetime abstainers. 2 , 8 , 132 In the unadjusted and partially adjusted analyses, using occasional drinkers as the reference group resulted in nonsignificant protective associations and lower point estimates for low-volume drinkers compared with significant protective associations and higher point estimates when using lifetime nondrinkers as the reference group. In the fully adjusted models, there were nonsignificant protective associations for low-volume drinkers whether using lifetime abstainers or occasional drinkers as the reference group, though this was only a RR of 0.97 for the latter.
Across all studies, there were few differences in risk for studies when stratified by median age of enrollment above or below age 56 years in the fully adjusted analyses. However, in the subset of studies who enrolled participants aged 50 years or younger who were followed for at least 10 years, occasional drinkers and medium-volume drinkers had significantly increased risk of mortality and substantially higher risk estimates for high- and higher-volume consumption compared with results from all studies. This is consistent with our previous meta-analysis for CHD, 9 in which younger cohorts followed up to older age did not show a significantly beneficial association of low-volume consumption, while older cohorts, with more opportunity for lifetime selection bias, showed marked, significant protective associations.
Our study also found sex differences in the risk of all-cause mortality. A larger risk of all-cause mortality for women than men was observed when drinking 25 or more grams per day, including a significant increase in risk for medium-level consumption for women that was not observed for men. However, mortality risk for mean consumption up to 25 g per day were very similar for both sexes.
A number of limitations need to be acknowledged. A major limitation involves imperfect measurement of alcohol consumption in most included studies, and the fact that consumption in many studies was assessed at only 1 point in time. Self-reported alcohol consumption is underreported in most epidemiological studies 133 , 134 and even the classification of drinkers as lifetime abstainers can be unreliable, with several studies in developed countries finding that the majority of self-reported lifetime abstainers are in fact former drinkers. 135 , 136 If this is the case, the risks of various levels of alcohol consumption relative to presumed lifetime abstainers are underestimates. Merely removing former drinkers from analyses may bias studies in favor of drinkers, since former drinkers may be unhealthy, and should rightly be reallocated to drinking groups according to their history. However, this has only been explored in very few studies. Our study found that mortality risk differed significantly by cohort age and sex. It might be that the risk is also higher for other subgroups, such as people living with HIV, 137 a possibility future research should investigate.
The number of available studies in some stratified analyses was small, so there may be limited power to control for potential study level confounders. However, the required number of estimates per variable for linear regression can be much smaller than in logistic regression, and a minimum of at least 2 estimates per variable is recommended for linear regression analysis, 138 suggesting the sample sizes were adequate in all models presented. It has been demonstrated that a pattern of binge (ie, heavy episodic) drinking removes the appearance of reduced health risks even when mean daily volume is low. 139 Too few studies adequately controlled for this variable to investigate its association with different outcomes across studies. Additionally, our findings only apply to the net effect of alcohol at different doses on all-cause mortality, and different risk associations likely apply for specific disease categories. The biases identified here likely apply to estimates of risk for alcohol and all diseases. It is likely that correcting for these biases will raise risk estimates for many types of outcome compared with most existing estimates.
This updated meta-analysis did not find significantly reduced risk of all-cause mortality associated with low-volume alcohol consumption after adjusting for potential confounding effects of influential study characteristics. Future longitudinal studies in this field should attempt to minimize lifetime selection biases by not including former and occasional drinkers in the reference group, and by using younger cohorts (ie, age distributions that are more representative of drinkers in the general population) at baseline.
Accepted for Publication: February 17, 2023.
Published: March 31, 2023. doi:10.1001/jamanetworkopen.2023.6185
Correction: This article was corrected on May 9, 2023, to fix errors in the Figure and Supplement.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zhao J et al. JAMA Network Open .
Corresponding Author: Jinhui Zhao, PhD, Canadian Institute for Substance Use Research, University of Victoria, PO Box 1700 STN CSC, Victoria, BC V8Y 2E4, Canada ( [email protected] ).
Author Contributions: Drs Zhao and Stockwell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhao, Stockwell, Naimi, Churchill, Sherk.
Acquisition, analysis, or interpretation of data: Zhao, Stockwell, Naimi, Clay.
Drafting of the manuscript: Zhao, Stockwell, Clay.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Zhao, Churchill.
Obtained funding: Zhao, Stockwell, Sherk.
Administrative, technical, or material support: Zhao, Stockwell, Naimi.
Supervision: Zhao, Stockwell, Naimi.
Conflict of Interest Disclosures: Dr Stockwell reported receiving personal fees from Ontario Public Servants Employees Union for expert witness testimony and personal fees from Alko outside the submitted work. Dr Sherk reported receiving grants from Canadian Centre on Substance Use and Addiction (CCSA) during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was partly funded by the CCSA as a subcontract for a Health Canada grant to develop guidance for Canadians on alcohol and health.
Role of the Funder/Sponsor: Health Canada had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. CCSA staff conducted a preliminary search to identify potentially relevant articles but did not participate in decisions about inclusion/exclusion of studies, coding, analysis, interpretation of results or approving the final manuscript.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We gratefully acknowledge contributions by Christine Levesque, PhD (CCSA), and Nitika Sanger, PhD (CCSA), who conducted a preliminary literature search for potentially relevant articles. We also acknowledge the leadership of Drs Catherine Paradis, PhD (CCSA), and Peter Butt, MD (University of Saskatchewan), who cochaired the process of developing Canada’s new guidance on alcohol and health, a larger project which contributed some funds for the work undertaken for this study. We are grateful to Fariha Alam, MPH (Canadian Institute for Substance Use and Research), for her help coding the studies used in this study. None of them received any compensation beyond their normal salaries for this work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The risks associated with alcohol use and alcoholism
Affiliation.
- 1 University of Toronto, Toronto, Canada.
- PMID: 22330211
- PMCID: PMC3307043
Alcohol consumption, particularly heavier drinking, is an important risk factor for many health problems and, thus, is a major contributor to the global burden of disease. In fact, alcohol is a necessary underlying cause for more than 30 conditions and a contributing factor to many more. The most common disease categories that are entirely or partly caused by alcohol consumption include infectious diseases, cancer, diabetes, neuropsychiatric diseases (including alcohol use disorders), cardiovascular disease, liver and pancreas disease, and unintentional and intentional injury. Knowledge of these disease risks has helped in the development of low-risk drinking guidelines. In addition to these disease risks that affect the drinker, alcohol consumption also can affect the health of others and cause social harm both to the drinker and to others, adding to the overall cost associated with alcohol consumption. These findings underscore the need to develop effective prevention efforts to reduce the pain and suffering, and the associated costs, resulting from excessive alcohol use.
PubMed Disclaimer
Publication types
- Search in MeSH
Related information
- Cited in Books
- PubChem Compound
- PubChem Substance
Grants and funding
- HHSN267200700041C/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full text sources.
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Overview of Alcohol Use Disorder
Information & authors, metrics & citations, view options, current u.s. rates of alcohol consumption, binge drinking, heavy drinking, and aud, adverse consequences of aud, neurobiology of aud, etiology of aud.
Current Treatments for AUD
Psychosocial treatments, pharmacological treatments, fda-approved medications, disulfiram., naltrexone., acamprosate., off-label medications, topiramate., gabapentin., promising medications that require further study, psychedelic drugs., phosphodiesterase-4 inhibitors., precision treatments, conclusions, information, published in.

- Substance-Related and Addictive Disorders
- Psychopharmacology
Competing Interests
Funding information, export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Purchase Options
Purchase this article to access the full text.
PPV Articles - American Journal of Psychiatry
Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
PREVIOUS ARTICLE
Next article, request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
As described within the American Psychiatric Association (APA)'s Privacy Policy and Terms of Use , this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences. Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 25 August 2022
Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review
- Lauren Kuhns ORCID: orcid.org/0000-0002-3156-8905 1 , 2 ,
- Emese Kroon ORCID: orcid.org/0000-0003-1803-9336 1 , 2 ,
- Heidi Lesscher 3 ,
- Gabry Mies 1 &
- Janna Cousijn 1 , 2 , 4
Translational Psychiatry volume 12 , Article number: 345 ( 2022 ) Cite this article
5123 Accesses
8 Citations
3 Altmetric
Metrics details
- Human behaviour
Adolescence is an important developmental period associated with increased risk for excessive alcohol use, but also high rates of recovery from alcohol use-related problems, suggesting potential resilience to long-term effects compared to adults. The aim of this systematic review is to evaluate the current evidence for a moderating role of age on the impact of chronic alcohol exposure on the brain and cognition. We searched Medline, PsycInfo, and Cochrane Library databases up to February 3, 2021. All human and animal studies that directly tested whether the relationship between chronic alcohol exposure and neurocognitive outcomes differs between adolescents and adults were included. Study characteristics and results of age-related analyses were extracted into reference tables and results were separately narratively synthesized for each cognitive and brain-related outcome. The evidence strength for age-related differences varies across outcomes. Human evidence is largely missing, but animal research provides limited but consistent evidence of heightened adolescent sensitivity to chronic alcohol’s effects on several outcomes, including conditioned aversion, dopaminergic transmission in reward-related regions, neurodegeneration, and neurogenesis. At the same time, there is limited evidence for adolescent resilience to chronic alcohol-induced impairments in the domain of cognitive flexibility, warranting future studies investigating the potential mechanisms underlying adolescent risk and resilience to the effects of alcohol. The available evidence from mostly animal studies indicates adolescents are both more vulnerable and potentially more resilient to chronic alcohol effects on specific brain and cognitive outcomes. More human research directly comparing adolescents and adults is needed despite the methodological constraints. Parallel translational animal models can aid in the causal interpretation of observed effects. To improve their translational value, future animal studies should aim to use voluntary self-administration paradigms and incorporate individual differences and environmental context to better model human drinking behavior.
Similar content being viewed by others
Systematic review and meta-analysis on the effects of chronic peri-adolescent cannabinoid exposure on schizophrenia-like behaviour in rodents
Yohimbine as a pharmacological probe for alcohol research: a systematic review of rodent and human studies

Consequences of adolescent drug use
Introduction.
Alcohol use disorder (AUD) is the most prevalent substance use disorder worldwide [ 1 ]. Most AUDs remain untreated [ 2 ] and for those seeking treatment, relapse rates are high [ 3 ]. Adolescence marks a rapid increase in AUD and an earlier onset of AUD is associated with worse long-term outcomes, including greater problem severity and more relapses [ 4 , 5 ]. Loss of control over alcohol use is a core aspect of AUD [ 6 ] and the developmentally normative difficulty to control motivational urges in tempting and arousing situations is thought to put adolescents at risk for developing addictive behaviors [ 7 ]. Moreover, neurotoxic consequences of alcohol use may be more severe for a developing brain [ 8 ]. Paradoxically, adolescence is also a period of remarkable behavioral flexibility and neural plasticity [ 9 , 10 , 11 ], allowing adolescents to adapt their goals and behavior to changing situations [ 12 ] and to recover from brain trauma more easily than adults [ 10 ]. In line with this, the transition from adolescence to adulthood is associated with high rates of AUD recovery without formal intervention [ 13 ]. While the adolescent brain may be a vulnerability for the development of addiction, it may also be more resilient to long-term effects compared to adults. Increased neural plasticity during this period could help protect adolescents from longer-term alcohol use-related cognitive impairments across multiple domains, from learning and memory to decision-making and cognitive flexibility. Therefore, the goal of this systematic review was to examine the evidence of age-related differences in the effect of alcohol on the brain and cognitive outcomes, evaluating evidence from both human and animal studies.
In humans, the salience and reinforcement learning network as well as the central executive network are involved in the development and maintenance of AUD [ 7 , 14 ]. The central executive network encompasses fronto-parietal regions and is the main network involved in cognitive control [ 15 ]. The salience network encompasses fronto-limbic regions crucial for emotion regulation, salience attribution, and integration of affective information into decision-making [ 15 , 16 ], which overlaps with fronto-limbic areas of the reinforcement learning network (Fig. 1 ). Relatively early maturation of salience and reinforcement learning networks compared to the central executive network is believed to put adolescents at heightened risk for escalation of alcohol use compared to adults [ 7 ]. Rodent models are regularly used for AUD research and allow in-depth neurobehavioral analyses of the effects of ethanol exposure during different developmental periods while controlling for experimental conditions such as cumulative ethanol exposure in a way that is not possible using human subjects because exposure is inherently confounded with age. For example, animal models allow for detailed neurobiological investigation of the effects of alcohol exposure in a specific age range on neural activation, protein expression, gene expression, epigenetic changes, and neurotransmission in brain regions that are homologous to those that have been implicated in AUD in humans.
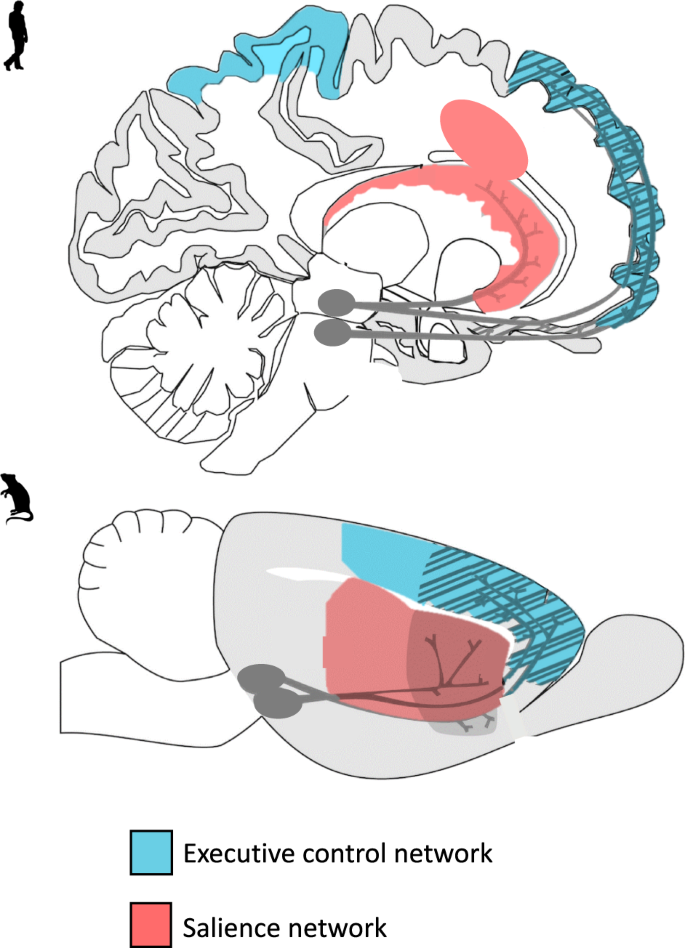
A visual representation of the translational model of the executive control and salience networks in humans and rodents. The executive control and salience are key networks believed to play a part in adolescent vulnerability to alcohol-related problems.
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain and cognition in adolescents and young adults specifically [ 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 ]. Heavy or binge drinking has been associated with reduced gray and white matter. Also, altered task-related brain activity [ 20 ], structural abnormalities [ 25 ], and overlapping behavioral impairment in executive functioning have been identified in adolescent and young adult alcohol users [ 19 ]. While some of the observed neurocognitive differences between drinkers and non-drinkers may be predisposing factors, they may be further exacerbated by heavy and binge drinking [ 21 , 23 ]. Furthermore, reviews of longitudinal studies concluded that adolescent alcohol use is associated with neural and cognitive alterations in a dose-dependent manner [ 17 , 22 ].
Although previous reviews underscore the potential negative consequences of heavy alcohol use on the brain and cognition in adolescence, they do not typically address the question of whether adolescents are differentially vulnerable compared to adults to the effects of alcohol on these outcomes. Explicit comparisons between adolescents and adults are crucial to identify potential risk and resilience factors. In the current review, we aimed to extend previous work by systematically examining this critical question: does the relationship between chronic alcohol use and neurocognitive outcomes differ between adolescents and adults? To address this question, we systematically reviewed human and animal studies that included both age groups and used a factorial design that would allow for the comparison of the effects of chronic alcohol use on cognitive and brain-related outcomes across age groups. We specifically highlight outcomes from voluntary self-administration paradigms when available and discuss the translational quality of the animal evidence base. We conclude with a discussion of prominent knowledge gaps, future research directions, and clinical implications.
Study inclusion criteria and search strategy
We followed the PRISMA guidelines for the current systematic review (The PRIMSA Group, 2009). An initial MedLine, Cochrane Library, and PsycInfo search was conducted during September of 2018 with terms related to alcohol, cognition, adolescence/adulthood, and study type (see Appendix for full search strategy and syntax). Two search updates using the same search strategy were conducted on 31 March 2020 and 3 February 2021. For all searches, the identified citations were split into batches and at least two of the following assessors (GM, LK, JC, or CG) conducted a blinded review to determine whether articles met the inclusion criteria. In the first phase of screening, only titles and abstracts were screened and articles that clearly did not meet the inclusion criteria were excluded. In the second phase, the remaining articles received a full-text review and those that did not meet all inclusion criteria were excluded. The first inclusion criterion that was not adhered to was recorded as the reason for excluding. If there was a discrepancy between authors after initial and full-text screening process, the reviewing authors discussed the article and a consensus was reached.
The inclusion criteria were: (1) Human samples including both adolescents younger than 18 and adults older than 18 and animal samples including adolescent (Post Natal Day (PND) 25–42 for rodents) and adult [ 8 ] animals (greater than PND 65 for rodents); (2) Exploration of alcohol as the independent variable and cognitive, reward-related, or brain outcomes as the dependent variables; (3) Alcohol and cognitive outcomes must meet our operationalization defined below; (4) Study design comparing adults and adolescents on outcome measures; (5) Administering or measuring alcohol use during adolescence or adulthood, not retrospectively (e.g., no age of onset work in humans using retrospective self-reports of alcohol consumption); (6) Primary quantitative data collection (no case studies, or review papers); (7) Solely looking at alcohol-related factors as the independent variables (e.g., cannot explore alcohol-related factors in individuals with psychosis); (8) Written in English; (9) Published in a peer-reviewed journal before February 3, 2021 (see Fig. 2 for a detailed screening process).
The definitions for adolescence are variable, hampering the direct comparison of human and rodent research. In rodents, the end of early-mid adolescence is considered to be approximately PND 42 when rats reach sexual puberty. By contrast, the boundaries for the onset of early adolescence are less clear. Based on the notion that most age-typical physiological changes that are characteristic of adolescence emerge from PND 28 [ 26 ], the conservative boundary for adolescence has been set at PND 28 (e.g., seminal review on adolescence [ 27 ]). The preceding week (PND 21-PND 28) has been described as the juvenile period (e.g., [ 28 , 29 ]) but these same reports consider PND 21-PND 23 as the lower boundary for early adolescence [ 28 , 29 ], further emphasizing that the boundary of PND28 may be too conservative. Indeed, multiple studies (e.g., [ 30 , 31 ]), have chosen to take PND25 as the boundary for early adolescence. Hence, we have decided to also follow this less conservative approach and include all studies where alcohol was administered between PND 25 and PND 42.
The exact boundaries of human adolescence are similarly nebulous. From a neurodevelopmental perspective, adolescence is now often thought of as continuing until approximately age 25 because of the continuing maturation of the brain [ 32 ]. However, the delineation of adolescence and adulthood is also dependent on societal norms, and is commonly defined as the transitional period between puberty and legal adulthood and independence which typically begins around age eighteen. In light of this, we chose a relatively liberal inclusion criteria for the human studies; studies needed to include at least some adolescents below eighteen, the age at which drinking typically begins, as well as ‘adult’ participants over the age of eighteen. We are careful to interpret the results of human studies within the neurodevelopmental framework of adolescence, such that 18–25-year-olds are considered late adolescents to young adults who are still undergoing cognitive and brain maturation.
Notably, we excluded studies that assessed alcohol exposure retrospectively (primarily early onset alcohol studies) because age of onset variables are often inaccurate, with reported age of alcohol onset increasing with both historical age [ 33 ] and current alcohol use patterns [ 34 ]. In addition, we excluded work that has not undergone peer-review to ensure high-quality papers.
In humans, we defined cognition as any construct that typically falls within the umbrella of neuropsychological testing, as well as brain-based studies. We also included more distal constructs of cognition, like craving and impulsivity, because they play a prominent role in addictive behaviors [ 35 , 36 ]. In rodents, we defined cognition as attention, learning, and memory in line with a seminal review paper [ 37 ]. Given the importance of social cognition in patterns of alcohol use particularly in adolescence [ 38 ] and its proposed role in adolescent risk and resilience to addiction [ 39 ], we included social behavior as an outcome. Furthermore, because many rodent studies assessed anxiety-related behaviors and the high degree of comorbidity between anxiety disorders and alcohol addiction [ 40 ], we also included anxiety as a secondary outcome. On the other hand, locomotor activity was excluded as an outcome because even though behavioral sensitization is considered to reflect neurobiological changes that may underlie certain aspects of addictive behavior [ 36 ], the translational relevance for addictive behavior and human addiction in particular remains unclear [ 41 , 42 ]. Across both rodents and humans, general alcohol metabolization and ethanol withdrawal studies were not included except if they included brain-related outcomes. The relevant reported findings (i.e., the results of an analysis of comparing age groups on the effect of alcohol on an included outcome) were extracted by a one reviewer and then confirmed by at least one other reviewer. In addition, the characteristics of the sample, details of alcohol exposure, and study design were extracted by a single reviewer and then confirmed by at least one other reviewer. No automation tools were used for extraction. Within the included studies, peripheral findings that did not relate to cognition were excluded from review and not extracted. The protocol for this systematic review was not registered and no review protocol can be accessed.
Study search
Our searches identified 7229 studies once duplicates were removed. A total of 6791 studies were excluded after initial review of abstracts. Then, 434 studies received a full-text review and 371 were excluded for failing to meet all inclusion criteria. See Fig. 2 for a flow diagram of the full screening process. At the end of the inclusion process, 59 rodent studies and 4 human studies were included. The characteristics and findings of the final studies are detailed in Table 1 (rodents) and Table 2 (humans). Due to the heterogeneity of outcomes, meta-regression was not suitable for synthesizing results. Results are narratively synthesized and grouped based on forced or voluntary ethanol exposure and by outcome within the tables and by outcome only in text. Two authors independently rated the quality of evidence for human studies (Table 2 ) based on criteria used in a similar systematic review [ 43 ]: (1) strong level of causality: longitudinal design comparing adolescent and adults while adjusting for relevant covariates; (2) moderate level of causality: longitudinal design comparing adolescents and adults without adjusting for relevant covariates or cross-sectional designs with matched groups that considered relevant covariates; (3) weak level of causality: cross-sectional design without matched adolescent and adult groups and/or did not adjust for relevant covariates. A methodological quality assessment was not conducted for the animal studies due to a lack of empirically validated risk of bias tools and lack of standardized reporting requirements in the animal literature.
PRIMSA flow diagram detailing the screening process.
Animal studies
Cognitive outcomes, learning and memory.
Human evidence clearly suggests that alcohol is related to learning and memory impairments, both during intoxication [ 44 ] and after sustained heavy use and dependence [ 45 , 46 ]. Paradigms that assess learning and memory provide insight into the negative consequences of alcohol consumption on brain functioning, as well as the processes underlying the development and maintenance of learned addictive behaviors.
Conditioned alcohol aversion or preference: Lower sensitivity to alcohol’s aversive effects (e.g., nausea, drowsiness, motor incoordination) but higher sensitivity to alcohol’s rewarding effects has been hypothesized to underlie the higher levels of alcohol use, especially binge-like behavior, in adolescents compared to adults [ 47 ]. Several conditioning paradigms have been developed to assess the aversive and motivational effects of alcohol exposure.
The conditioned taste aversion (CTA) paradigm is widely used to measure perceived aversiveness of alcohol in animals. Repeated high-dose ethanol injections are paired with a conditioned stimulus (CS, e.g., a saccharin or NaCL solution). The reduction in CS consumption after conditioning is used as an index of alcohol aversion. Two studies examined CTA in mice [ 48 , 49 ] and two in rats [ 50 , 51 ]. Three of the four studies found age-related differences. In all three studies using a standard CTA paradigm, adolescents required a higher ethanol dosage to develop aversion compared to adults [ 48 , 49 , 50 ]. Using a similar second-order conditioning (SOC) paradigm pairing high doses of ethanol (3.0 g/kg) with sucrose (CS), both adolescent and adult rats developed equal aversion to the testing compartment paired with ethanol [ 51 ].
Overall, three studies found support for lower sensitivity to alcohol’s aversive effects in adolescents, whereas one observed no differences. Future research should employ intragastric as opposed intraperitoneal exposure to better mimic human binge-like drinking in order to increase the translational value of the findings.
To measure differences in alcohol’s motivational value, conditioned place preference (CPP) paradigms have been used. This involves repeated pairings of ethanol injections with one compartment and saline injections with another compartment of the testing apparatus. On test days, CPP is assessed by measuring how long the animal stays in the compartment paired with ethanol relative to saline injections. Four studies examined CPP, with two studies observing age-related differences [ 52 , 53 , 54 , 55 ]. In the only mouse study, history of chronic ethanol exposure during adolescence (2.0 g/kg for 15 days) but not adulthood [ 52 ] led to increased CPP after brief abstinence (5 days) before the conditioning procedure (2.0 g/kg, four doses over 8 days). This suggests that early ethanol exposure increases alcohol’s rewarding properties later on. However, two rat studies did not observe either preference or aversion in either age when using lower ethanol doses and a shorter exposure period (0.5 and 1.0 g/kg for 8 days) [ 53 ], nor when using higher doses and intermittent exposure (3.0 g/kg, 2 days on, 2 days off schedule) [ 55 ]. Next to species and exposure-specific factors, environmental factors also play a role [ 54 ], with adolescents raised in environmentally enriched conditions demonstrating CPP (2 g/kg) while adolescents raised in standard conditions did not. In contrast, CPP was insensitive to rearing conditions in adults with both enriched and standard-housed rats showing similar levels of CPP.
Overall, there is inconsistent evidence for age-related differences in the motivational value of ethanol. One study found support for increased sensitivity to the rewarding effects of ethanol in adolescents, whereas one found support for adults being more sensitive and two observed no differences.
Fear conditioning and retention: Pavlovian fear conditioning paradigms are used to investigate associative learning and memory in animals. These paradigms are relevant for addiction because fear and drug-seeking behavior are considered conditioned responses with overlapping neural mechanisms [ 56 ]. Rodents are administered an unconditioned stimulus (US; e.g., foot shock) in the presence of a conditioned stimulus (CS; unique context or cue). Conditioned responses (CR; e.g., freezing behavior) are then measured in the presence of the CS without the US as a measure of fear retention. Contextual fear conditioning is linked to hippocampus and amygdala functioning and discrete cue-based (e.g., tone) fear is linked to amygdala functioning. [ 57 , 58 , 59 ], and fear extinction involves medial PFC functioning [ 60 ]. Five studies investigated fear conditioning, four in rats [ 61 , 62 , 63 , 64 ] and one in mice [ 65 ].
Only one of the four studies observed age-related differences in tone fear conditioning. Bergstrom et al. [ 61 ] found evidence for impaired tone fear conditioning in male and female alcohol-exposed (18d) adolescent compared to adult rats after extended abstinence (30d). However, adolescent rats consumed more ethanol during the one-hour access period than adults, which may explain the observed age differences in fear tone conditioning. Small but significant sex differences in consumption also emerged in the adolescent group, with males showing more persistent impairment across the test sessions compared to females, despite adolescent females consuming more ethanol than males. In contrast, three studies found no evidence of impaired tone fear conditioning in either age group after chronic alcohol exposure (4 g/kg, every other day for 20d) and extended abstinence [ 62 , 63 ] (22d), [ 64 ].
Two of the three studies observed age-related differences in contextual fear conditioning [ 62 , 63 , 64 ]. In two studies with similar exposure paradigms, only adolescents exposed to chronic high dosages of ethanol (4 g/kg) showed disrupted contextual fear conditioning after extended abstinence (22d) [ 62 , 63 ]. Importantly, differences disappeared when the context was also paired with a tone, which is suggestive of a potential disruption in hippocampal-linked contextual fear conditioning specifically [ 64 ]. Furthermore, there may be distinct vulnerability periods during adolescence as contextual fear retention was disrupted after chronic alcohol exposure (4 g/kg, every other day for 20d) during early-mid adolescence but not late adolescence [ 62 ]. In the only study to combine chronic exposure and acute ethanol challenges, contextual conditioning was impaired by the acute challenge (1 g/kg) but there was no effect of pre-exposure history in either age group (4 g/kg, every other day for 20d) [ 63 ].
Only one study examined fear extinction, and found no effect of ethanol exposure (4/kg, every other day for 20d) on extinction after tone conditioning. However, adults had higher levels of contextual fear extinction compared to mid-adolescents while late adolescents performed similar to adults [ 62 ]. Moreover, looking at binge-like exposure in mice (three binges, 3d abstinence), Lacaille et al. [ 65 ] showed comparable impairments in long-term fear memory in adolescents and adults during a passive avoidance task in which one compartment of the testing apparatus was paired with a foot shock once and avoidance of this chamber after a 24 h delay was measured.
In sum, there is limited but fairly consistent evidence for adolescent-specific impairments in hippocampal-linked contextual fear conditioning across two rat studies, while no age differences emerged in context-based fear retention in one study of mice. In contrast, only one of the four studies found evidence of impaired tone fear conditioning in adolescents (that also consumed more alcohol), with most finding no effect of alcohol on tone fear conditioning regardless of age. With only one study examining medial PFC-linked fear extinction, no strong conclusions can be drawn, but initial evidence suggests context-based fear extinction may be diminished in mid-adolescents compared to adults and late adolescents. Research on age-related differences on the effect of alcohol on longer-term fear memory is largely missing.
Spatial learning and memory: The Morris Water Maze (MWM) is commonly used to test spatial learning and memory in rodents. Across trials, time to find the hidden platform in a round swimming pool is used as a measure of spatial learning. Spatial memory can be tested by removing the platform and measuring the time the animal spends in the quadrant where the escape used to be. The sand box maze (SBM) is a similar paradigm in which animals need to locate a buried appetitive reinforcer.
Six rat studies examined spatial learning and memory using these paradigms. Three of the six studies observed age-related differences. Four examined the effects of repeated ethanol challenges 30 minutes prior to MWM training, showing mixed results [ 30 , 66 , 67 , 68 ]. While one found ethanol-induced spatial learning impairments in adolescents only (1.0 and 2.0 g/kg doses) [ 66 ], another found no age-related differences, with both age groups showing impairments after moderate doses (2.5 g/kg) and enhancements in learning after very low doses (0.5 g/kg) [ 67 ]. Sircar and Sircar [ 68 ] also found evidence of ethanol-induced spatial learning and memory impairments in both ages (2.0 g/kg). However, memory impairments recovered after extended abstinence (25d) in adults only. Importantly, MWM findings could be related to thigmotaxis, an anxiety-related tendency to stay close to the walls of the maze. Developmental differences in stress sensitivity may potentially confound ethanol-related age effects in these paradigms. Using the less stress-inducing SBM, adults showed greater impairments in spatial learning compared to adolescents after 1.5 g/kg ethanol doses 30 min prior to training [ 30 ].
Two studies examined the effects of chronic ethanol exposure prior to training with or without acute challenges [ 69 , 70 ]. Matthews et al. [ 70 ] looked at the effect of 20 days binge-like (every other day) pre-exposure and found no effect on spatial learning in either age following an extended abstinence period (i.e., 6–8 weeks). Swartzwelder et al. [ 69 ] examined effects of 5-day ethanol pre-exposure with and without ethanol challenges before MWM training. Ethanol challenges (2.0 g/kg) impaired learning in both age groups regardless of pre-exposure history. Thigmotaxis was also increased in both age groups after acute challenges while pre-exposure increased it in adults only.
In sum, evidence for impaired spatial learning and memory after acute challenges is mixed across six studies. Two studies found support for ethanol having a larger impact in adolescents compared to adults, whereas one study found the opposite and three studies did not observe any differences. Differences in ethanol doses stress responses may partially explain the discrepancies across studies. Importantly, given the sparsity of studies addressing the effects of long-term and voluntary ethanol exposure, no conclusion can be drawn about the impact of age on the relation between chronic alcohol exposure and spatial learning and memory.
Non-spatial learning and memory: Non-spatial learning can also be assessed in the MWM and SBM by marking the target location with a pole and moving it across trials, measuring time and distances traveled to locate the target. By assessing non-spatial learning as well, studies can determine whether learning is more generally impaired by ethanol or whether it is specific to hippocampal-dependent spatial learning processes. A total of six studies assessed facets of non-spatial learning and memory. Two of the six studies observed age-related differences.
In the four studies that examined non-spatial memory using the MWM or SBM in rats, none found an effect of alcohol regardless of dose, duration, or abstinence period in either age group [ 30 , 66 , 67 , 70 ]. Two other studies examined other facets of non-spatial memory in rats [ 65 , 71 ]. Galaj et al. [ 71 ] used an incentive learning paradigm to examine conditioned reward responses and approach behavior towards alcohol after chronic intermittent ethanol (CIE; 4 g/kg; 3d on, 2d off) exposure to mimic binge drinking. To examine reward-related learning and approach behavior, a CS (light) was paired with food pellets and approach behavior to CS only presentation and responses to a lever producing the CS were measured. In both adolescents and adults, the ethanol-exposed rats showed impaired reward-related learning after both short (2d) and extended (21d) abstinence. No effect of alcohol on conditioned approach behavior was observed in either age group during acute (2d) or extended (21d) abstinence. Using a novel object recognition test in mice, Lacaille et al. [ 65 ] assessed non-spatial recognition memory by replacing a familiar object with a novel object in the testing environment. Explorative behavior of the new object was used as an index of recognition. After chronic binge-like exposure (three injections daily at 2 h intervals) and limited abstinence (4d), only adolescents showed reduced object recognition.
Across facets of non-spatial memory, there is little evidence for age-related differences in the effect of chronic alcohol, with four of the six studies finding no age differences. For memory of visually cued target locations in the MWM and SBM paradigms, alcohol does not alter performance in either age. Also, both adolescents and adults appear similarly vulnerable to alcohol-induced impairments in reward-related learning based on the one study. Only in the domain of object memory did any age-related differences emerge, with adolescents and not adults showing reduced novel object recognition after binge-like alcohol exposure in one study. However, more research into object recognition memory and reward-related learning and memory is needed to draw strong conclusions in these domains.
Executive function and higher-order cognition
Executive functions are a domain of cognitive processes underlying higher-order cognitive functions such as goal-directed behavior. Executive functions can include but are not limited to working memory, attentional processes, cognitive flexibility, and impulse control or inhibition [ 72 ]. A core feature of AUD is the transition from goal-directed alcohol use to habitual, uncontrolled alcohol use. Impaired executive functioning, linked to PFC dysfunction [ 73 ], is assumed to be both a risk factor and consequence of chronic alcohol use. A meta-analysis of 62 studies highlighted widespread impairments in executive functioning in individuals with AUD that persisted even after 1-year of abstinence [ 46 ]. Thirteen studies examined facets of executive functioning and higher-order cognition, specifically in the domains of working memory, attentional processes, cognitive flexibility, impulsivity in decision-making, and goal-directed behavior [ 65 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 ].
Working memory: Working memory refers to the limited capacity system for temporarily storing and manipulating information, which is necessary for reasoning and decision-making [ 84 ]. In the Radial Arm Maze test (RAM) [ 85 ], some of the equally spaced arms (typically eight) around a circular platform contain a food reward for animals to find. Spatial working memory is measured by recording the number of revisits to previously visited arms (i.e., working memory error) and first entries into unbaited arms (i.e., reference memory). Alternatively, the hippocampus mediated [ 86 ] spontaneous tendency to alternate arms can be used as a measure of spatial working memory. In this case, revisiting an arm in back-to-back trials in close temporal succession is interpreted as a working memory error. Five studies examined the effects of chronic ethanol exposure on spatial working memory [ 65 , 75 , 79 , 80 , 83 ]. One of the five studies observed age-related differences.
Chronic binge-like alcohol exposure had no effects on spontaneous alterations after prolonged abstinence (2d on, 2d off; 3 weeks abstinence) [ 79 , 80 ] in rats or limited abstinence (three injections daily at 2 h intervals; 24 h abstinence) [ 65 ] in mice, nor on RAM performance in rats (2d on, 2d off) [ 75 , 83 ]. However, acute ethanol challenges (1.5 g/kg) after chronic binge-like exposure (2d on, 2d off) resulted in RAM test impairments in both age groups in rats [ 75 , 83 ], with some evidence for increased working memory errors in adolescents [ 83 ].
In sum, there is little evidence for impairments in working memory function in rats after chronic ethanol exposure, with four of the five studies observing no difference between age groups. While acute intoxication impairs working memory function in both ages, there is evidence from only one study that adolescents may make more working memory errors.
Attentional processes: Attentional processing refers to the selection of information that gains access to working memory [ 87 ]. PPI is a pre-attentional cognitive function which provides an index of sensorimotor gating and measures the ability of a lower intensity sensory stimulus to reduce the magnitude of response to a more intense stimulus presented closely afterward. Reduced sensorimotor gating (reduced PPI) can disrupt information processing and thereby impair cognitive function, while enhanced sensorimotor gating (enhanced PPI) may reflect behavioral inflexibility [ 88 ]. For example, lesions in the medial PFC produce both behavioral inflexibility and enhancements in PPI in rats. Two studies assessed attentional processes by measuring prepulse inhibition (PPI) in rats [ 82 , 89 ]. One study observed age-related differences and one did not.
Slawecki and Ehlers [ 82 ] observed age-related differences in sensorimotor gating following ethanol vapor exposure (2w) and brief abstinence (6d), with adolescents showing enhanced PPI at some decibels reflective of behavioral inflexibility, while adults did not exhibit PPI at any of the intensities tested. Slawecki et al. [ 89 ] did not observe any age-related differences in PPI during the acute phase of ethanol withdrawal (7–10 h abstinence) during a period of chronic ethanol exposure (14d).
In sum, there is limited and mixed evidence from two studies of age-related differences in the pre-attentional process of sensorimotor gating. Only one study found support for adolescent sensitivity to ethanol effects.
Cognitive flexibility: Cognitive flexibility refers to the ability to update information based on environmental factors r changing goals in order to adaptively guide decision-making and is linked to the inability to reduce or abstain from drinking [ 90 ]. Three studies examined facets of cognitive and behavioral flexibility [ 79 , 80 , 81 ]. Two of the three studies observed age-related differences.
In two rat studies, cognitive flexibility was assessed using reversal learning paradigms [ 79 , 80 ]. In the reversal learning paradigm, rats were trained on simple (e.g., visual cue) and more complex discriminations (e.g., visual + scent cue) between rewarded and non-rewarded bowls. After learning the discriminants, the rewards were reversed. Ethanol exposure reduced flexibility in both adolescents and adults for simple discriminations in both studies. Age-related differences emerged for the more complex discriminations in one study, with only adults showing reduced flexibility after prolonged abstinence (21d) following binge-like exposure (5 g/kg, 2d on, 2d off) [ 79 ]. In contrast, both age groups showed reduced flexibility for complex discrimination in the other study after prolonged abstinence (21d) despite adolescents consuming more ethanol orally than adults during the 28 week exposure [ 80 ].
In another study, Labots et al. [ 81 ] used a conditioned suppression of alcohol-seeking task after two months of voluntary ethanol consumption (2 months) in rats to examine flexibility around alcohol-seeking behavior. After stratifying the age groups based on levels of ethanol consumption, medium- and high-consuming, adolescents showed higher levels of conditioned suppression compared to similarly drinking adults, indicating greater behavioral flexibility and control over alcohol-seeking in adolescents after chronic voluntary exposure.
Overall, there is limited evidence for adolescent resilience to the effects of chronic alcohol on cognitive flexibility. Two studies found support for adolescent resilience to ethanol’s effect on behavioral flexibility, whereas another study found no differences between adolescents and adults.
Impulsivity: Impulsivity is a multi-faceted behavioral trait that encompasses impaired response inhibition, preference for an immediate reward over a larger but delayed reward, and premature expression of behaviors which may be maladaptive or in conflict with conscious goals. Impulsivity is a risk-factor for the development of addiction and may also be a consequence of sustained substance use [ 35 ]. Pharmacological evidence points towards overlapping neuronal mechanisms in impulsivity and addictive behavior, particularly within the mesolimbic dopamine system [ 91 ]. Two studies examined impulsive decision-making behavior in rats [ 74 , 78 ]. Both studies observed age-related differences.
One study examined impulsive behavior using a delay-discounting task in which choices are made between immediate small rewards and larger delayed rewards [ 78 ]. Regardless of age, chronic intermittent exposure (2d on, 2d off) had no effect on choice behavior in non-intoxicated rats. Following acute challenges, adolescents but not adults demonstrated a reduced preference for the large reward regardless of ethanol exposure history, reflecting a general adolescent-specific heightened impulsivity during intoxication. Another study examined decision-making under risk conditions using an instrumental training and probability-discounting task [ 74 ]. After prolonged abstinence (20d), rats were trained to press two levers for sucrose rewards and were concurrently trained to choose between two levers with different associated probabilities of reward and reward size, creating a choice between a certain, small reward and an uncertain, large reward (i.e., riskier choice). Ethanol consumption was voluntary and while adolescents initially consumed more ethanol than adults at the beginning of the exposure period, the total amount of consumption was similar by the end of the exposure period. Only adolescents showed increased risky and sub-optimal decision-making compared to age-matched controls, while adults performed similarly to controls.
In sum, both studies found support for ethanol having a larger impact on adolescent compared to adults on impulsive behavior.
Goal-directed behavior: Goal-directed behavior refers to when actions are sensitive to both the outcome value (goal) and contingency between the behavior and the outcome [ 92 ]. Two studies used a sign-tracking and omission contingency learning paradigm to examine goal-directed versus habitual behavior [ 76 , 77 ]. One study observed age-related differences and the other did not. Sign tracking refers to tasks where a cue predicts a reward, but no response is needed for the reward to be delivered. Despite this, after repeated pairings of the cue and reward, animals and humans may respond (e.g., via a lever) when the cue is presented anyway, and even when no reward is known to be available. Sign-directed behavior is considered habitual and has been proposed to underlie the lack of control of alcohol use in addiction [ 93 ]. In humans, sign-tracking behavior is difficult to differentiate from goal-directed behavior based on only the observable behavior, i.e., seeing a cue such as a favorite drink or bar and then having a drink [ 94 ]. In the context of alcohol use, reflexively having a drink when seeing an item that is often associated with the rewarding effects of alcohol (e.g., wine glass, bar, smell of alcohol) despite not consciously desiring the alcohol ‘reward’ is an example of how habitual behavior (possibly driven by sign-tracking) can initiate the behavior as opposed to an intentional goal [ 93 ]. Omission contingency refers to a 2nd phase after sign-tracking when the response is punished and the behavior must be inhibited to avoid punishment. After both forced and voluntary ethanol exposure (6w), no alterations to sign-tracking behavior were observed in adolescent and adult rats [ 76 , 77 ]. One study did observe an age-related difference in omission contingency learning, with adolescents performing better than adults after chronic voluntary ethanol exposure [ 77 ]. This preliminarily suggests that adolescents may be more capable of adapting their behavior to avoid punishment compared to adults after chronic use. However, before behavioral testing began, adolescent rats were abstinent for 17 days, while adults were only abstinence for 10 days which may have influenced the results.
In summary, one study found support for adolescents being less sensitive to ethanol effects on goal-directed behavior compared to adults, whereas one study found no effect of ethanol in either age group.
Across the domains of executive function, there is some evidence that adolescents may be more vulnerable to impairments in certain executive and higher-order cognitive functions following chronic alcohol exposure, with increased risky decision-making after prolonged abstinence [ 74 ], impulsivity during intoxication [ 78 ], and reduced working memory function during intoxication after chronic exposure. In contrast, animals exposed to alcohol during adolescence may better retain cognitive flexibility [ 77 , 79 ] and are better able to regain control over alcohol-seeking in adulthood [ 81 ].
Other behavioral outcomes
Anxiety : AUD is highly comorbid with anxiety disorders [ 95 ], especially in adolescence [ 96 ]. While anxiety is not strictly a cognitive outcome, it is related to altered cognitive functioning [ 97 , 98 ]. Many studies assessing the effects of ethanol on the rodent brain and cognition also include anxiety-related measures. Multiple paradigms have been developed to elicit behaviors thought to reflect anxiety in rodents (e.g., rearing, startle, avoidance, etc.). In the open field test (OFT), anxiety is indexed as the tendency to stay close to perimeter walls as animals have a natural aversion to brightly lit open spaces [ 99 ]. In the elevated plus maze paradigm, rodents are placed at the center of an elevated four-arm maze with two open arms two closed arms [ 100 ]. The open arms elicit unconditioned fear of heights/open spaces and the closed arms elicit the proclivity for enclosed, dark spaces. Anxiety is indexed as entries/duration of time in open vs. closed arms, as well as rearing, freezing, or other postural indices of anxiety. In startle paradigms, the startle response is a defensive mechanism reflecting anxiety which follows a sudden, unpredictable stimulus (e.g., tones, light) [ 101 ]. In light-dark box paradigms, anxiety is elicited using a testing apparatus with a light and dark compartment, relying on the conflict between natural aversions to well-lit spaces and the tendency to explore new areas. Percentage of time spent in the light compartment, latency to return to the dark compartment, movement between compartments (transitions), and rearing-behavior are measured as indices of anxiety [ 102 ]. Anxiety can also be assessed using a social interaction test with an unfamiliar partner, with approach and avoidance behaviors measured to index anxiety [ 103 ]. In the novel object test (NOT) [ 104 ], anxiety is elicited by the introduction of a new object in the rodent’s environment. The amount of contacts and time spent in contact with the object is used as an index of anxiety. Similarly, in the marble-burying test (MBT), novel marbles are placed in an environment and the amount of defensive burying of the objects is used as an index of anxiety [ 105 ].
Eleven studies examined anxiety-like behavior in rodents with mixed results across paradigms [ 70 , 78 , 82 , 83 , 89 , 106 , 107 , 108 , 109 , 110 , 111 ]. Overall, five of the eleven studies observed age-related differences.
Two studies used the OFT, finding no effects of voluntary (2w, 4 h/day access) or forced (12/day vapor) ethanol exposure on anxiety-like behavior in adolescents or adult rats during withdrawal (7–9 h) [ 110 ] or after a brief abstinence period (4 days) [ 107 ]. One study used both the MBT and NOT after voluntary ethanol consumption (2 h/d for 2 weeks; no abstinence) and observed higher anxiety in ethanol-exposed adults and reduced anxiety in ethanol-exposed adolescents compared to controls as indexed by marble burying [ 106 ]. However, no age effects were observed in response to a novel object, with reduced interaction with the novel object in both age groups after chronic exposure.
Four studies used the elevated maze paradigm with mixed results. Only one study observed age-related differences in mice after chronic exposure (8–10w vapor) [ 109 ]. Adolescents showed reduced anxiety compared to adults during the acute withdrawal period, but all mice were kept under chronic social isolation and unpredictable stress conditions, which may have affected the results. Two studies in rats found no effect of intermittent (1 g/kg) or binge-like (5 g/kg) exposure in either age group after short (24 h) [ 70 ] or sustained abstinence (20d) [ 83 ]. A third study observed heightened anxiety in both age groups after intermittent exposure (4 g/kg), with anxiety increasing with prolonged abstinence periods (24 h to 12d) [ 108 ].
Three rat studies used a startle paradigm to assess anxiety. Two observed reduced acoustic startle responses after ethanol exposure (12 h/d vapor) in both age groups during acute withdrawal periods (7–10 h) and following more sustained abstinence (6d) [ 82 , 89 ]. In the other study, light-potentiated startle was also reduced in both ages during days 1–10 of withdrawal after binge-like exposure (2d on, 2d off), but age-related differences emerged when the rats were re-exposed via a 4-day binge (1–4/kg). Then, only adults showed higher levels of light-potentiated startle compared to controls [ 78 ], suggesting that ethanol pre-exposure increases anxiety in adults but not adolescents when re-exposed to ethanol after withdrawal.
Two studies used the light-dark box paradigm with mixed results [ 89 , 111 ]. Only adult rats showed increased mild anxiety-like behaviors during early withdrawal (7–10 h) after chronic vapor exposure 12 h/d) [ 89 ]. In contrast, no age-related differences emerged after voluntary ethanol consumption (18 h/d access; 3d/w for 6 weeks), with male mice showing less anxiety-like behavior in both ages [ 111 ]. In contrast, the one study using the social interaction test observed reduced anxiety in adult mice compared to both adolescents and age-matched controls during early withdrawal (4–6 h) after chronic, unpredictable vapor exposure [ 109 ].
In summary, there is inconsistent evidence for age-related differences in the effect of chronic ethanol exposure on anxiety outcomes in rodents. The substantial differences across studies in how anxiety was elicited and measured make it challenging to draw strong conclusions. In the five studies that found age-related differences, adults tend to show higher levels of anxiety, particularly during early withdrawal; however, the opposite was found in the one study examining anxiety in social interactions. Six studies did not observe any age-related differences. Overall, adolescents may be less sensitive to the anxiety-inducing effects of chronic alcohol exposure.
Social behavior: Two studies were identified that examined the effects of chronic ethanol exposure on social behavior in rats [ 112 , 113 ], with both observing age-related differences. After chronic exposure (1 g/kg, 7d), followed by a brief abstinence period (24–48 h), one study found a decrease in social preference in adolescents only [ 112 ], while the other study found no ethanol-related effects on social behavior (2 g/kg, 10d) [ 113 ]. After acute challenges, age and treatment interactions emerged in both studies, but the directions of the results are inconsistent. In the first study, adolescents showed increased social preference, as indexed by the number of cross-overs between compartments toward and away from a peer, across multiple acute doses (0.5–1.0 g/kg) administered immediately before testing, while adults showed no changes in social preference [ 112 ]. In contrast, Morales et al. [ 113 ] found evidence for age-related temporal differences in social activity after acute challenge, with adults showing decreased social impairment five minutes post injection (1 g/kg) and adolescents (1.25 g/kg) after 25 min compared to age-matched controls.
The findings from these two studies paint a complicated and inconsistent picture of the effects of ethanol on social behavior in adults and adolescents warranting further research. One study found support for a larger effect of chronic ethanol on adolescent social behavior compared to adults, while the other did not observe effects of ethanol in either group. One study found support for a larger effect of chronic plus acute ethanol intoxication on social behavior, with the opposite observed in the other.
Brain outcomes
Neurotransmitter systems.
Glutamate is the brain’s main excitatory neurotransmitter and plays a crucial role in synaptic plasticity (i.e., experience-related strengthening or weakening of synaptic connections). Glutamatergic transmission plays an important role in the formation and maintenance of addictive behaviors and the nucleus accumbens (NAc) is considered an important hub in this, receiving glutamatergic input from cortical-limbic areas and dopaminergic input from the midbrain [ 114 ]. Seven studies investigated glutamate functioning in regions of the brain [ 106 , 107 , 108 , 109 , 115 , 116 , 117 , 118 ]. Four of the seven studies observed age-related differences.
Three studies investigated glutamate-related processes in the NAc [ 106 , 107 , 118 ]. Two weeks of voluntary binge drinking (4-h access, no abstinence) did not affect expression of calcium-dependent kinase II alpha (CaMKIIα) and the AMPA receptor GluA1 subunit in the NAc of mice [ 107 ]. In contrast, Lee et al. [ 106 ] showed that voluntary binge drinking (2-h access, no abstinence) increased mGlu1, mGlu5, and GluN2b expression in the shell of the NAc, as well as PKCε and CAMKII in the core of the NAc in adult mice only. In rats, Pascual et al. [ 118 ] showed reduced NR2B phosphorylation in the NAc of adolescents only after two weeks of chronic intermittent ethanol exposure; an effect that also lasted until 24 h after end of exposure. This indicates that adolescents might be less affected by the effects of ethanol on NAc-related glutamatergic neurotransmission than adults. This may in turn mediate decreased withdrawal symptoms and potentially facilitate increased drinking [ 106 ].
Two studies investigated glutamate-related processes in the (basolateral) amygdala [ 107 , 116 ]. In mice, Agoglia et al. [ 107 ] showed decreased CaMKIIα phosphorylation in adolescents, but increased GluA1 expression in adults after two weeks of voluntary binge drinking (4-h access, no abstinence). Also, drug-induced AMPAR activation resulted in increased binge drinking in adolescents but decreased binge drinking in adults, highlighting the potential importance of glutamatergic signaling in age-related differences in alcohol consumption. However, Falco et al. [ 116 ] reported no difference in NR2A mRNA levels in the basolateral amygdala for either age group after 60-day abstinence.
Alcohol’s effects on frontal cortex functioning is thought to be mediated by alterations in NMDA receptor subunit expression [ 119 , 120 ]. Two studies investigated glutamate-related processes in the frontal cortex of rats [ 115 , 118 ]. Pascual et al. [ 118 ] showed reduced NR2B phosphorylation after two weeks of forced intermittent ethanol exposure in adolescents only. Using a 2-week ethanol vapor paradigm, Pian et al. [ 115 ] found different patterns of NMDAR subunit expression. These patterns were highly dependent on abstinence duration (0 h, 24 h, 2w), however, they only statistically compared results within rather than between age groups. Ethanol exposure was associated with decreased NR1 receptor expression in both age groups, but only the adult group showed a decrease in NR2A and NR2B expression. The NR1 and NR2A expression returned to normal during withdrawal, but in adults NR2B expression increased after two weeks of abstinence.
Conrad and Winder [ 109 ] assessed long-term potentiation (LTP) in the bed nucleus stria terminalis (BNST), a major output pathway of the amygdala towards the hypothalamus and thalamus. Voluntary ethanol exposure resulted in blunted LTP responses in the dorsolateral BNST regardless of age. However, all mice were socially isolated during the experiments to induce anxiety, so it is unclear whether the effects were solely due to ethanol exposure.
Two studies looked at glutamate receptor subunit expression in the hippocampus [ 108 , 115 ]. Pian et al. [ 115 ] observed increased expression of NR1, NR2A, and NR2B in adults after 2 weeks of ethanol exposure. In adolescents, a reduction in NR2A expression was observed. After abstinence, adult levels returned to normal, while in adolescents, decreased NR1 and NR2A expression was seen after 24 h but an increased expression of these subunits was seen after 2 weeks of abstinence. These findings support regional specific effects of age group, with potentially increased sensitivity to the impact of alcohol on glutamatergic mediated hippocampal functioning in adolescents. Unlike expected, van Skike et al. [ 108 ] did not find effects of chronic intermittent ethanol exposure or withdrawal on NMDA receptor subunit expression in the hippocampus and cortex as a whole in adolescent and adult rats. The authors speculate that these null results might be associated with the exposure design (limited exposure and route of administration) and lack of withdrawal periods compared to Pian et al. [ 115 ].
In sum, there is limited and inconsistent evidence for age-related differences in glutamate function across seven studies. The direction of the observed age-related differences varies across regions, with evidence of both increased and decreased sensitivity to ethanol effects in adolescents compared to adults in the four studies that observed age-related differences.
GABA is the brain’s main inhibitory neurotransmitter. GABA A receptors are a primary mediator of alcohol’s pharmacological effects [ 121 ]. A total of four studies looked at GABAergic functioning [ 108 , 116 , 122 , 123 ]. Three of the four studies observed age-related differences.
One study investigated GABA-related processes in the (basolateral) amygdala, showing reduced GABA A α1 and GAD67 (enzyme that converts Glutamate to GABA) mRNA expression in adult rats only, 60 days after 18-days ethanol exposure [ 116 ].
Two studies looked at the rat cortex as a whole [ 108 , 122 ]. Van Skike et al. did not find effects of chronic intermittent ethanol exposure on GABA A receptor expression [ 108 ]. Grobin et al. [ 122 ] showed that, while basal GABA A receptor functioning was not affected by 1 month of chronic intermittent ethanol exposure, GABA A receptors were less sensitive to the neurosteroid THDOC in adolescents. This neuromodulatory effect was not found in adults and did not persist after 33 days of abstinence. However, these results indicate that neurosteroids may play an indirect role in age differences in the GABAA receptor’s response to alcohol.
Two studies focused on the rat hippocampus [ 108 , 124 ]. Fleming et al. [ 124 ] found age-specific effects of chronic intermittent ethanol exposure on hippocampal (dentate gyrus) GABA A receptor functioning. Adolescent rats showed decreased tonic inhibitory current amplitudes after ethanol exposure, which was not the case for young adult and adult rats. Also, only the adolescents showed greater sensitivity to (ex vivo) acute ethanol exposure induced enhanced GABAergic tonic currents. The specificity of these effects to adolescent exposure might indicate adolescent vulnerability to ethanol-induced effects on the hippocampus; however, Van Skike et al. [ 108 ] did not find any effects of chronic intermittent ethanol exposure on GABA A receptor expression in the hippocampus.
In sum, given the limited number of studies and lack of replicated effects, no clear conclusions can be drawn about the role of age on the effects of alcohol on GABAergic neurotransmission. Age-specific effects appear to be regionally distinct. The only available study found support for heightened adult sensitivity to ethanol in the amygdala. In contrast, one study found support for greater adolescent sensitivity in the hippocampus and whole cortex, whereas the other found no age-related differences.
The mesocorticolimbic dopamine system, with dopaminergic neurons in the ventral tegmental area (VTA) projecting to the NAc and prefrontal cortex, plays a key role in AUD, particularly through reward and motivational processes [ 14 ]. Only two studies investigated dopaminergic processes, focusing on the frontal cortex, NAc, and broader striatum [ 118 , 125 ]. Both studies observed age-related differences in certain dopamine outcomes.
Carrara-Nascimento et al. [ 125 ] investigated acute effects of ethanol in adolescent and adult mice 5 days after a 15-day treatment with either ethanol or saline. In the PFC, ethanol pretreated adolescents showed reduced dopamine levels (DA) and related metabolites (DOPAC and HVA) in response to an acute ethanol challenge compared to ethanol pretreated adults and adolescent saline controls. In the NAc, there were no differences between pretreated adolescents and adults, but analyses within each age group revealed that ethanol-pretreatment with an acute challenge decreased DOPAC within the adolescent group. Results from the dorsal striatum also showed no differences between adolescents and adults. However, within the adolescent group, ethanol pre-treatment increased DOPAC and, within the adult group, it increased HVA. Pascual et al. [ 118 ] found similar results looking at the expression of DRD1 and DRD2 dopamine receptors after two weeks of chronic intermittent ethanol exposure in rats. In the NAc and dorsal striatum, DRD2 expression was reduced in adolescent compared to adult exposed rats, while both DRD1 and DRD2 expression were reduced in the frontal cortex.
These results suggest reduced alcohol-induced dopamine reactivity in adolescents in the PFC and NAc based on the two available studies, but more studies are warranted for a more detailed understanding of the relationship between age and dopamine receptor expression following chronic ethanol exposure.
Acetylcholine
Acetylcholine is a known neuromodulator of reward and cognition-related processes [ 126 ]. The composition and expression of nicotinic and muscarinic acetylcholine receptors have been implicated in various alcohol use-related behaviors [ 127 , 128 ]. Only one study investigated cholinergic processes and observed age-related differences. Vetreno et al. [ 129 ] showed global reductions in choline acetyltransferase (ChAT; cholinergic cell marker) expression after adolescent onset, but not adult onset of forced intermittent binge-like exposure (20 days – every other day, 25 days abstinence).
Neuromodulatory processes
Neurodegeneration and neurodevelopment.
Chronic alcohol consumption is thought to lead to brain damage by influencing processes involved in neurodegeneration and neurogenesis. The formation of addictive behaviors is paralleled by the formation of new axons and dendrites, strengthening specific neuronal pathways [ 130 ]. While brain morphology is commonly investigated in humans, it is a proxy of the impact of alcohol on the brain and therefore rarely studied in rodents. Five studies investigated facets of neurodegeneration or development in rodents [ 55 , 65 , 131 , 132 , 133 ]. All five studies observed age-related differences.
Huang et al. [ 131 ] showed reduced cerebral cortex mass in adolescent mice, but shortening of the corpus collosum in adults after 45 days of ethanol injections, suggesting some age-specific regional effects. Using an amino cupric silver staining, significant brain damage was revealed for both adolescent and adult rats after 4 days of binge-like ethanol exposure [ 132 ]. However, adolescents showed more damage in the olfactory-frontal cortex, perirhinal cortex, and piriform cortex.
Looking at hippocampal neurogenesis, ethanol exposure has been shown to initially reduce hippocampal neurogenesis in adult rodents, recovering after 1-month abstinence [ 134 ]. Compared to adults, neurogenesis in the dentate gyrus of the hippocampus was found to be reduced in adolescent exposed mice (Bromodeoxyuridine levels) [ 65 ] and rats (doublecortin levels) [ 133 ]. Lacaille et al. [ 65 ] also measured the expression level of genes involved in oxidative mechanisms after binge-like alcohol exposure. In whole brain samples, they found increased expression of genes involved in brain protection (i.e., gpx3, srxn1) in adults, but increased expression of genes involved in cell death (i.e., casp3) combined with decreased expression of genes involved in brain protection (i.e., gpx7, nudt15) in adolescents. Casp3 protein levels were also higher in the whole brain of adolescent exposed mice [ 65 ] and the adolescent dentate gyrus [ 133 ], suggesting more neurodegeneration and less neurogenesis in adolescents versus adults following ethanol consumption.
Cyclin-dependent kinase 5 (CDK5) is involved in axon, dendrite, and synapse formation and regulation. CDK5 is overexpressed in the prefrontal cortex and the NAc following exposure to substances of abuse including alcohol [ 135 ]. Moreover, CDK5 inhibition has been shown to reduce operant self-administration of alcohol in alcohol-dependent rats [ 136 ]. One study reported higher H4 acetylation of the CDK5 promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal, however, CDK5 mRNA expression was control-like after 2 weeks of abstinence [ 55 ].
In sum, strong conclusions cannot be drawn due to the limited number of studies and lack of replicated effects. However, preliminary evidence points to adolescent vulnerability to damage in the cortex, reduced neurogenesis, and increased neurodegeneration in the hippocampus and the cortex as a whole based on four of the five studies. In contrast, one study found support for adult vulnerability to ethanol’s effects axon, dendrite, and synapse formation and regulation.
Growth factors
Brain-derived neurotrophic factor (BNDF) and nerve growth factor (NGF) are involved in brain homeostasis and neural recovery [ 137 , 138 ]. While ethanol exposure initially increases BDNF and NGF, chronic ethanol exposure seems to reduce BDNF and NGF levels and can thereby result in long-term brain damage and related cognitive problems [ 139 , 140 ]. Four studies investigated growth factor expression in the frontal cortex [ 54 , 55 , 79 , 80 ] and two studies also investigated the hippocampus [ 79 , 80 ]. All four studies of the frontal cortex observed age-related differences. Neither study of the hippocampus observed age-related differences.
In rats, 30 weeks of chronic ethanol exposure reduced prefrontal mBDNF and β-NGF regardless of age, despite adolescents consuming more ethanol [ 80 ]. Moreover, the reduction of mBDNF was correlated with higher blood alcohol levels and was persistent up to 6–8 weeks abstinence. Interestingly, during acute withdrawal (48 h) adolescents but not adults temporarily showed control-like mBDNF levels. This might indicate an attempt to counteract neurodegeneration as a result of ethanol exposure in adolescents. These results were partially replicated using a shorter intermittent exposure paradigm (13 doses, 2 days on/off) [ 79 ]. While intoxication after chronic ethanol exposure reduced prefrontal BDNF, levels recovered after 3-weeks abstinence regardless of age. However, during acute withdrawal (24 h), BDNF was still reduced in early-adolescent onset rats, increased in adult-onset rats, but control-like in mid-adolescent onset-rats, suggesting slower recovery in younger animals. Looking at BDNF gene regulation, a similar study (8 doses, 2 days on/off) reported higher H3 demethylation but lower H4 acetylation of the BDNF promoter in the PFC of adult versus adolescent ethanol-exposed rats during acute withdrawal [ 55 ]. However, prefrontal BDNF mRNA expression returned to control levels after 2 weeks of abstinence. Interestingly, social housing may be protective, as reduced prefrontal BDNF was no longer observed in alcohol-exposed adolescent mice housed in environmentally enriched relative to standard conditions [ 54 ]. Two studies investigated hippocampal BDNF expression but reported no significant interactions between alcohol exposure and age group [ 79 , 80 ].
In sum, the results of the four available studies suggest lower prefrontal BDNF during chronic alcohol use that recovers after abstinence regardless of age. However, the rate of recovery may be influenced by age with slower recovery in adolescents. In the two available studies, no age-related differences were observed in BDNF expression in the hippocampus.
Transcription factors
The transcription factors cFos and FosB are transiently upregulated in response to substance use, and ΔFosB accumulates after chronic exposure, particularly in striatal and other reward-related areas [ 141 ]. Two studies investigated cFos and FosB [ 55 , 142 ] and one study ΔFosB related processes [ 111 ]. All three studies observed age-related differences.
After chronic ethanol exposure (8 doses, 2 days on/off), adolescent compared to adult rats showed increased prefrontal H3 and H4 acetylation of the cFos promotor region and increased H4 acetylation and H3 dimethylation of FosB promotor regions after acute abstinence [ 55 ]. Moreover, mRNA expression of FosB was elevated in adolescents but not adults after 2-weeks abstinence. The upregulating effects of an acute ethanol challenge on prefrontal cFos appears to reduce after chronic pre-treatment to a larger extent in adolescent than adult exposed mice [ 142 ]. This pattern of results was similar in the NAc, but desensitization to ethanol’s acute effects on cFos in the hippocampus was more pronounced in adults. Faria et al. [ 142 ] also looked at Egr-1 (transcription factor, indirect marker of neuronal activity and involved in neuroplasticity), showing a stronger reduction in Egr-1 expression in the PFC, NAc, and hippocampus of adolescent versus adults after repeated ethanol exposure. Regarding ∆FosB, Wille-Bille et al. [ 111 ] found increased ∆FosB in adolescent compared to adult rats in the prelimbic PFC, dorsomedial striatum, NAc core and shell, central amygdala nucleus capsular, and basolateral amygdala after 3 days per week 18 h ethanol exposure sessions for 6 weeks. In sum, the three available studies provide preliminary evidence for increased adolescent vulnerability to ethanol-induced long-term genetic (mRNA expression) and epigenetic (methylation) changes in mesocorticolimbic areas.
Immune factors
Ethanol is known to trigger immune responses in the brain (e.g., increase production of hemokines and cytokines), causing inflammation and oxidative stress [ 143 , 144 , 145 ]. Three studies examined immune factors [ 146 , 147 , 148 ]. Two of the three studies observed age-related differences.
Microglia remove damaged brain tissue and infectious agents and are key to the brain’s immune defense. Only one study investigated microglia levels [ 146 ]. Although direct comparisons between age groups were missing, both adolescent and adult rats showed less microglia in the hippocampus (CA and DG) and peri-entorhinal cortex, and more dysmorphic microglia in the hippocampus after 2 and 4 days of binge-like ethanol exposure [ 146 ]. Notably, age groups were matched on intoxication scores, with adolescents needing more ethanol to reach the same level of intoxication. An in silico transcriptome analysis of brain samples from mice after 4 days of 4 h/day drinking in the dark, suggest overexpression of neuroimmune pathways related to microglia action (toll-like receptor signaling, MAPK signaling, Jak-STAT signaling, T-cell signaling, and chemokine signaling) in adults that was not observed in adolescents, while adolescents consumed more ethanol [ 147 ]. Similarly, ethanol-exposed adult mice showed higher chemokine expression (CCL2/MCP-1) in the hippocampus, cerebral cortex, and cerebellum and higher cytokine expression (IL-6, but not TNF-α) in the cerebellum, while no chemokine or cytokine changes were observed in ethanol exposed adolescent mice [ 148 ]. Both adolescents and adults showed increased astrocyte levels in the hippocampus (CA1) and the cerebellum after ethanol exposure, but changes in astrocyte morphology were only observed in the adult hippocampus.
In sum, two of the studies found support for increased immune responses after ethanol exposure in adults compared to adolescents, whereas the one other study found no difference between the age groups.
HPA-axis functionality
Chronic stress and HPA-axis functionality have been associated with the maintenance of AUD (e.g., reinstatement drug seeking, withdrawal) [ 149 ]. Two studies investigated corticotropin-release factor (CRF) expression in rats [ 116 , 150 ]. One study observed age-related differences and the other did not.
Falco et al. [ 116 ] found decreased CRF mRNA expression in the adult but not adolescent basolateral amygdala 2 months after 18-day restricted ethanol exposure. In contrast, Slawecki et al. did not find any interaction between age and treatment on CRF levels in the amygdala, as well as the frontal lobe, hippocampus, hypothalamus, and caudate 7 weeks after 10-days of ethanol vapor exposure.
No conclusions can be drawn. One study observed found support for reduced effects of ethanol on HPA-axis functionality compared to adults, whereas the other observed no difference between the age groups. Future studies using different (voluntary) exposure paradigms are needed to further investigate the effects of alcohol on HPA activity in relation to age of alcohol exposure.
Neuropeptides
Neuropeptides are a diverse class of proteins that have a modulatory function in many different processes, including but not limited to neurotransmission, stress, immune responses, homeostasis, and pain [ 151 , 152 , 153 ]. Only one study investigated neuropeptides in rats and observed age-related differences [ 150 ].
Slawecki et al. [ 150 ] specifically investigated neuropeptide-Y, substance-P, and interleukine expression in the frontal lobe, hippocampus, hypothalamus, dorsal striatum, and amygdala 7 weeks after 10-days of ethanol vapor exposure in rats [ 150 ]. Interactions between age and treatment were found for the hippocampus and caudate only. Ethanol-induced reductions in hippocampal neuropeptide-Y and increases in caudate neurokinine were more pronounced in adults compared to adolescents suggesting long-lasting effects of ethanol in adults but not adolescents.
Ethanol metabolism
The first metabolite of ethanol is acetaldehyde, which has been theorized to mediate the effects of ethanol on both brain and behavior [ 154 ]. Only one study investigated ethanol metabolism in the brain and did not observe age-related differences [ 155 ].
Rhoads et al. showed that despite the fact that adolescent rats consumed more alcohol brain catalase levels after 3-weeks of ethanol exposure (no abstinence) did not differ between adolescents and adults [ 155 ]. Although the general role of catalase in ethanol metabolism is small, catalase can oxidize ethanol to acetaldehyde in the brain, affecting elimination of ethanol after consumption [ 156 , 157 ]. These findings may therefore imply that ethanol metabolism may not differ between adolescent and adult animals, which should be studied in a more direct manner.
Full proteome analysis
While the previously described studies focused on specific factors involved in neurotransmission, brain health, and plasticity, proteomics allows for the study of the full proteome in a specific region or tissue type. One study investigated the impact of age on ethanol-induced changes in the hippocampal proteome, observing age-related differences [ 158 ]. In this study, rats intermittently and voluntarily consumed beer for 1 month and the hippocampal proteome was analyzed after 2 weeks of abstinence. The results point to the involvement of many of the factors described above and imply age-specific effects of alcohol. Adult beer exposure increased citrate synthase (part of the citric acid, or Krebs, cycle) and fatty acid binding proteins (involved in membrane transport) compared to controls. Adolescent beer exposure increased cytoskeletal protein T-complex protein 1 subunit epsilon (TCP-1), involved in ATP-dependent protein folding, and reduced expression of a variety of other proteins involved in glycolysis, glutamate expression, aldehyde detoxification, protein degradation, and synaptogenesis, as well as neurotransmitter release. These more extensive changes suggest that the adolescent hippocampus might be more vulnerable to the effects of ethanol exposure, but more studies are needed to clarify and replicate these findings and extend the focus to different brain areas.
Neuronal activity and functioning
Ethanol-induced molecular changes may eventually change neuronal activity. Three studies investigated neuronal activity and functioning [ 89 , 159 , 160 ] using electrophysiological methods. All three studies observed age-related differences.
Galaj et al. [ 159 ] assessed firing patterns and the structure of pyramidal neurons in the L2 and L5 layers of the prelimbic cortex of the rat brain using ex vivo electrophysiological recordings and morphological staining. Following chronic intermittent ethanol exposure and brief abstinence (2 days), adolescents, but not adults, showed reduced amplitudes of spontaneous excitatory post-synaptic currents (sEPSCs) in L5 neurons compared to controls, indicating reductions in intrinsic excitability. In line with this, Dil staining showed increased thin spine ratios in the L5 layer in adolescents only. Age differences were more pronounced after prolonged abstinence (21 days), with adolescents showing reduced amplitude and frequency of sEPSCs in L5 neurons while adult’s L5 neurons showed augmented firing patterns (i.e., amplitude and frequency). Furthermore, adolescent rats showed decreased total spine density and non-thin spines, indicating less excitatory postsynaptic receptors in the L5 layer. In contrast, adults showed increases in spine density and non-thin spines.
Li et al. [ 160 ] examined the functioning of CA1 interneurons, which are important for learning and memory processes [ 161 ], in the rat hippocampus using ex vivo whole-cell recordings. After prolonged abstinence (20 days), voltage-gated A-type potassium channel ( I A ) conductance was measured. Differences emerged between age groups (although no statistical interaction effect was directly assessed): EtOH-exposed adolescents and adults both showed lower I A mean peak amplitude compared to the respective control groups. However, adolescents also showed reduced I A density and increased mean decay time, which decreased in adults. Furthermore, only adolescents showed increased depolarization required for activation compared to controls, which can result in higher interneuron firing rates in the CA1 region that could affect learning processes. Additional research is needed to connect these findings to behavioral measures of learning and memory.
Slawecki et al. [ 89 ] was the only study to use in vivo electroencephalogram (EEG) recordings with rats to examine function in the frontal and parietal cortex at different times during a 14-day vapor exposure period. During acute withdrawal (7–10 h abstinence period), following daily exposure no effects emerged in frontal cortical regions throughout the exposure period. In parietal regions, only adolescents showed increased high frequency (16–32 Hz and 32–50 Hz) power on days 8 and 12 compared to controls. Adolescent hyperexcitability during withdrawal may indicate increased arousal in adolescents compared to adults during withdrawal, but more studies linking brain activity to behavioral indices of withdrawal will allow for clearer interpretations.
Overall, strong conclusions cannot be drawn given the disparate paradigms and outcomes utilized. While adolescents and adults appear to differ in the effect of ethanol on neuronal firing, the meaning of these differences is not clear given the lack of connection between these findings and behavioral outcomes.
Human studies
Four studies examined age-related differences of the effect of alcohol on brain or cognition in humans [ 162 , 163 , 164 , 165 ].
Müller-Oehring et al. [ 162 ] examined the moderating role of age on resting state functional connectivity and synchrony in the default mode, central executive, salience, emotion, and reward networks of the brain in a sample of no/low and heavier drinkers aged 12–21 years old. While the study did not compare discrete groups of adolescents and adults, analyses investigating the interaction between continuous age and alcohol exposure history were conducted which provide insight into the effect of alcohol use on functional brain networks from early adolescence to emerging adulthood. Regardless of age, no differences were observed between matched subgroups of no/low drinkers and moderate/heavy drinkers in the default mode, salience, or reward networks. However, in the central executive network, connectivity between the superior frontal gyrus (SFG) and insula increased with age in the no/low drinkers but not in heavier drinkers. Age-related strengthening of this fronto-limbic connection correlated with better performance on a delay discounting task in boys, suggesting that adolescent alcohol use may interfere with typical development of higher-level cognitive functions. In the emotion network, amygdala-medial parietal functional synchrony was reduced in the heavier drinkers compared to the no/low drinkers and exploratory analyses suggested that weaker amygdala-precuneus/posterior cingulate connectivity related to later stages of pubertal development in the no/low drinking group only. Interestingly, in the default mode (posterior cingulate-right hippocampus/amygdala) and emotional networks (amygdala, cerebellum), connectivity in regions that exhibited age-related desynchronization was negatively correlated with episodic memory performance in the heavy drinkers. These results give preliminary evidence that alcohol might have age-dependent effects on resting state connectivity and synchronization in the central executive, emotion, and default mode networks that could potentially interfere with normative maturation of these networks during adolescence.
Three studies examined age effects in alcohol-related implicit cognitions, specifically attentional bias [ 163 , 165 ], alcohol approach bias [ 165 ], and implicit memory associations and explicit outcome expectancies [ 164 ]. Attentional bias refers to the preferential automatic allocation or maintenance of attention to alcohol-related cues compared to neutral cues which is correlated with alcohol use severity and craving [ 166 ]. McAteer et al. [ 163 ] measured attentional bias with eye tracking during presentation of alcohol and neutral stimuli in heavy and light drinkers in early adolescents (12–13 yrs), late adolescents (16–17 yrs), and young adults (18–21 yrs). Regardless of age, heavy drinkers spent longer fixating on alcohol cues compared to light drinkers. Cousijn et al. [ 165 ] measured attentional bias with an Alcohol Stroop task [ 167 ], comparing the speed of naming the print color of alcohol-related and control words. Consistent with the findings of McAteer et al. [ 163 ], adults and adolescents matched on monthly alcohol consumption showed similar levels of alcohol attentional bias. In the same study, Cousijn et al. [ 165 ] did not find any evidence for an approach bias towards alcohol cues in any age group.
Rooke and Hine [ 164 ] found evidence for age-related differences in implicit and explicit alcohol cognitions and their relationship with binge drinking. Using a teen-parent dyad design, adolescents (13–19 yrs) showed stronger memory associations in an associative phrase completion task and more positive explicit alcohol expectancies than adults. Interestingly, both explicit positive alcohol expectancies and implicit memory associations were a stronger predictor of binge drinking in adolescents compared to adults. It is important to note that adolescents also had higher levels of binge drinking than adults in the study.
Cousijn et al. [ 165 ] also investigated impulsivity, drinking motives, risky decision-making, interference control, and working memory. No age differences emerged in the cognitive functioning measures including risky decision-making (Columbia Card Task – “hot” version), interference control (Classical Stroop Task), or working memory (Self-Ordered Pointing Task). However, adolescents were more impulsive (Barrett Impulsiveness Scale) than adults and reported more enhancement motives. Importantly, impulsivity as well as social, coping, and enhancement motives of alcohol use correlated with alcohol use in both ages. However, age only moderated the relationship between social drinking motives and alcohol use-related problems (as measured by the Alcohol Use Disorder Identification Test), with a stronger positive association in adolescents compared to adults. Importantly, the adolescent group had a different pattern of drinking, with less drinking days per month but more drinks per episode than the adult group.
In summary, human evidence is largely missing, with no studies comparing more severe and dependent levels of alcohol use between adolescents and adults. The preliminary evidence is too weak and heterogeneous to draw conclusions, warranting future studies investigating the impact of age.
The current systematic review assessed the evidence for the moderating role of age in the effects of chronic alcohol use on the brain and cognition. The identified 59 rodent studies (Table 1 ) and 4 human studies (Table 2 ) provide initial evidence for the presence of age-related differences. Rodents exposed to ethanol during adolescence show both increased risk and resilience to the effects of ethanol depending on the outcome parameter. However, due to the high variability in the outcomes studied and the limited number of studies per outcome, conclusions should be considered preliminary. Moreover, brain and behavioral outcomes were mostly studied separately, with studies focusing on either brain or behavioral outcomes. The behavioral consequences of changes in certain brain outcomes still need to be investigated. Table 3 provides a comprehensive overview of the strength of the evidence for age-related differences for all outcomes. Below, we will discuss the most consistent patterns of results, make connections between the behavioral and neurobiological findings when possible, highlight strengths and limitations of the evidence base, and identify the most prominent research gaps.
Patterns of results
Age-related differences in learning and memory-related processes appear to be highly domain specific. There is limited but fairly consistent evidence for adolescent-specific impairments in contextual fear conditioning, which could be related to hippocampal dysfunction. Results for other hippocampus-related memory processes such as spatial memory are mixed and largely based on forced exposure with acute challenge studies rather than voluntary long-term exposure to alcohol. The evidence base is currently insufficient to draw conclusions about the role of age in alcohol’s effects on non-spatial types of learning and memory. Alcohol generally did not impact performance in the non-spatial variants of the MWM and SBM paradigms or in reward-learning, but the results of the limited studies in the object-learning domain highlight potential impairments and the importance of age therein. For example, adolescents but not adults demonstrated impaired object memory in the only study using the novel object recognition task [ 65 ]. Acute challenges after chronic pre-exposure to alcohol also appear to impair performance in the working memory domain, with one study suggesting heightened adolescent sensitivity to working memory impairment [ 83 ]. Thus, although the domain-specific evidence is limited by the relative lack of research, overall patterns suggest that learning and memory functions that are primarily hippocampus-dependent may be differentially affected by adolescent compared to adult alcohol use. Studies focusing on neural hippocampal processes corroborate these findings, reporting more extensive changes in protein expression [ 158 ], less desensitization of cFos upregulation [ 142 ], larger changes in GABAa receptor subunit expression [ 124 ], longer lasting changes in NMDA receptor expression [ 115 ], and larger reductions in neurogenesis [ 65 , 133 ] in the hippocampus of adolescent compared to adult ethanol-exposed rodents. On the other hand, ethanol-induced changes in the hippocampus recovered more quickly in younger animals after abstinence [ 150 ] and adolescent mice showed less signs of ethanol-induced neuroinflammation compared to adults [ 148 ].
Higher rates of adolescent alcohol use, especially binge drinking, may be facilitated by a heightened sensitivity to the rewarding properties of alcohol in combination with a reduced sensitivity to the negative effects of high doses [ 47 ]. In line with this, there is limited but consistent evidence that adolescents show less CTA in response to chronic ethanol and consequently voluntarily consume more ethanol [ 50 ]. Importantly, distinct vulnerability periods within adolescence for altered CTA may exist [ 168 , 169 ], with early adolescents potentially being least sensitive to aversive effects. Future studies using chronic exposure paradigms comparing different stages of adolescence to adults are needed. In contrast to CTA, there is insufficient evidence of age-related differences in the motivational value of alcohol based on CPP paradigms, with only one of five studies reporting stronger CPP in adolescents than adults [ 52 ]. Adolescents may be more sensitive to the effects of environmental factors on the motivational value of alcohol than adults, as adolescents housed in enriched environments acquired CPP while those in standard housing did not, an effect that was not found in adults [ 54 ]. Evidence for environmentally enriched housing being protective against these changes in adolescents provides an important indication that environmental factors matter and are important factors to consider in future research on the motivational value of ethanol on both the behavioral and neural level. Complementary studies on the functioning of brain regions within the mesolimbic dopamine pathway and PFC, which play an important role in motivated behavior, indicate limited but consistent evidence for age-related differences. Adolescents showed less dopamine reactivity in the PFC and NAc compared to adults after chronic ethanol exposure. Furthermore, there is limited but consistent evidence that adolescents are more vulnerable to epigenetic changes in the frontal cortex and reward-related areas after chronic ethanol exposure. For instance, adolescents may be more sensitive to histone acetylation of transcription factors in motivational circuits underlying the rewarding effects of alcohol [ 55 ], which may contribute to addictive behaviors [ 170 , 171 ]. Chronic alcohol use is also associated with lower BDNF levels in the PFC and subsequent increases in alcohol consumption, implicating BDNF as an important regulator of alcohol intake [ 172 ]. While evidence is limited, chronic alcohol use consistently reduced prefrontal BDNF in both age groups. However, the rate of recovery of BDNF levels after abstinence appears to be slower in adolescents.
Regarding executive functioning, there is limited but fairly consistent evidence from animal studies that adolescents are more vulnerable to long-term effects of chronic exposure on decision-making and are more impulsive than adults during acute intoxication and after prolonged abstinence following chronic exposure. Impulsivity is associated with functional alterations of the limbic cortico-striatal systems [ 91 ], with involvement of both the dopaminergic and serotonergic neurotransmitter systems [ 173 ]. While no studies investigating serotonergic activity were identified, the consistent reduction in dopamine reactivity observed in the PFC and NAc in adolescents compared to adults parallel the behavioral findings. There is also limited but fairly consistent evidence that adolescents are more resilient to impairments in cognitive flexibility than adults following chronic exposure to alcohol, and that adolescents may more easily regain control over their alcohol-seeking behavior than adults. These behavioral findings provide preliminary support for the paradox of adolescent risk and resilience in which adolescents are at once more at risk to develop harmful patterns of drinking, but are also more resilient in that they may be more equipped to flexibly change behavior and with time regain control over alcohol consumption. However, studies assessing processes that might be related to brain recovery provide little conclusive evidence for potential underlying mechanisms of these behavioral findings. While adolescents appear more vulnerable to ethanol-induced brain damage [ 131 , 132 ], show reduced neurogenesis [ 65 , 133 ], and show less changes in gene expression associated with brain recovery [ 65 , 133 ], adults show relatively higher immune responses after repeated ethanol exposure [ 147 , 148 ]. The limited evidence for adolescent resilience to alcohol’s effects on cognitive flexibility diverge from the conclusions of recent reviews that focused mostly on adolescent-specific research. Spear et al. [ 18 ] concluded that adolescents are more sensitive to impairments in cognitive flexibility; however, this was based on adolescent-only animal studies. Similarly, the systematic review of Carbia et al. [ 19 ] on the neuropsychological effects of binge drinking in adolescents and young adults also revealed impairments in executive functions, particularly inhibitory control. However, as pointed out by the authors, the lack of consideration of confounding variables (e.g., other drug use, psychiatric comorbidities, etc.) in the individual studies and the lack of prospective longitudinal studies limit our ability to causally interpret these results. This further highlights the difficulty of conducting human studies which elucidate causal associations of the effects of alcohol, and the need for animal research that directly compares adolescents to adults to bolster interpretation of findings from human research.
Only a few studies have investigated age-related differences in cognitive functioning in humans. These studies focused on mostly non-dependent users and studied different outcomes, including cognitive biases and implicit and explicit alcohol-related cognitions. Overall, there was limited but consistent evidence that age does not affect alcohol attentional or approach biases, with heavy drinkers in both age groups allocating more attention to alcohol cues compared to controls [ 163 , 165 ]. In contrast, in line with a recent meta-analysis of the neurocognitive profile of binge-drinkers aged 10–24 [ 23 ], there is limited evidence that age affects alcohol associations. One study found age effects on implicit (memory associations) and explicit (expectancies) cognition in relation to alcohol use. Adolescents showed stronger memory associations and more positive expectancies than adults [ 164 ]. These expectancies were also predictive of higher binge drinking in adolescents but not adults, highlighting the importance of future research into age differences in alcohol-related cognitions and their consequences on alcohol consumption. However, the quality of the evidence was rated as weak based on the methodological design of the included studies.
Regarding anxiety-related outcomes, results are inconsistent across studies and paradigms. When age-differences are observed, adolescents often show reduced anxiety compared to adults during both acute withdrawal and sustained abstinence following chronic ethanol exposure. However, the direction of age-related effects of alcohol may also be anxiety-domain specific. In social settings, adults show reduced anxiety compared to adolescents. Research on the neurocircuitry of anxiety processes implicates the extended amygdala, especially the BNST, in anxiety behaviors with an emphasis on the role of GABAergic projections to the limbic, hindbrain, and cortical structures in rodents [ 174 ]. Despite adolescents showing less non-social anxiety than adults after ethanol exposure, no age-differences were observed for LTP in the BNST [ 109 ]. Also, GABA receptor expression in the hippocampus and whole cortex was not altered by ethanol exposure in either age group [ 108 ]. However, the anxiolytic effects of NMDA antagonists [ 175 ] also highlight the importance of glutamatergic activity in anxiety processes [ 176 ]. In line with behavioral findings, adolescents were less sensitive to changes in glutamate expression: adults showed heightened expression in the NAc, which has been suggested to underlie the higher levels of anxiety observed in adults compared to adolescents [ 106 ]. Importantly, across the various studies, different paradigms were used to assess anxiety, potentially contributing to the inconsistent results. Furthermore, most of the identified studies used a forced ethanol exposure paradigm. As alcohol-induced anxiety is likely also dependent on individual trait anxiety, voluntary consumption studies in high and low trait anxiety animals are important to further our understanding of the interaction between alcohol use and anxiety. Of note, the observed pattern suggestive of reduced anxiety in adolescents compared to adults diverges from conclusions of previous reviews such as Spear et al. [ 18 ] which concluded that adolescents are more likely to show augmented anxiety after alcohol exposure based on animal studies with adolescent animals only. Importantly, anxiety was included as a secondary outcome in this review because of the high comorbidity between anxiety disorders and alcohol addiction, warranting the inclusion of age-related differences in the relation between alcohol and anxiety. However, the search strategy was not specifically tailored to capturing all studies assessing age-related differences in the effect of alcohol on anxiety.
Translational considerations, limitations, and future directions
The reviewed studies revealed a high degree of variability in study designs and outcomes, hindering integration and evaluation of research findings. We were unable to differentiate our conclusions based on drinking patterns (i.e., comparing binge drinking, heavy prolonged use, AUD). The prevalence of binge-drinking in adolescence is very high and is associated with neurocognitive alterations [ 177 ]. Studies investigating the potential differential impact of binge-drinking compared to non-binge-like heavy alcohol use in adolescence and adulthood are critical for understanding the risks of chronic binge-like exposure in adolescence, even if it does not progress to AUD.
It is also important to acknowledge the limitations of the choice of adolescent and adult age ranges in our inclusion criteria. Rodent studies had to include an adolescent group exposed to alcohol between the ages of PND 25–42 and an adult group exposed after age PND 65. Ontogenetic changes may still be occurring between PND 42–55, and this period may more closely correspond to late adolescence and emerging adulthood in humans (e.g., 18–25 years). Studies that compared animals in this post-pubertal but pre-adulthood age range were not reviewed. Studies investigating age-related differences in the effects of ethanol on brain and cognitive outcomes in emerging adulthood are also translationally valuable given the high rates and risky patterns of drinking observed during this developmental period [ 178 ]. Indeed, an important future direction is to examine whether there are distinct vulnerability periods within adolescence itself for the effects of ethanol on brain and cognitive outcomes. Given that emerging adulthood is a period of continued neurocognitive maturation and heightened neural plasticity, studies comparing this age range to older adults (e.g., over 30) are also necessary for a more thorough understanding of periods of risk and resilience to the effects of alcohol.
Furthermore, we did not conduct a risk of bias assessment to examine the methodological quality of the animal studies. The applicability and validity of the risk of bias tools for general animal intervention studies, such as the SYRCLE risk of bias tool [ 179 ], remain in question at the moment. The lack of standardized reporting in the literature for many of the criteria (e.g., process of randomizing animals into intervention groups) would lead to many studies being labeled with an ‘unclear risk of bias’. Furthermore, there is still a lack of empirical evidence regarding the impact of the criteria in these tools on bias [ 179 , 180 ]. This is a significant limitation in evaluating the strength of the evidence for age-related differences based on the animal studies, which highlights the importance of more rigorous reporting standards in animal studies.
Moreover, most work is done in male rodents and is based on forced ethanol exposure regimes. In a recent opinion article, Field and Kersbergen [ 181 ] question the usefulness of these types of animal models to further our understanding of human substance use disorders (SUD). They argue that animal research has failed to deliver effective SUD treatment and that social, cultural, and other environmental factors crucial to human SUD are difficult, if not impossible, to model in animals. While it is clear that more sophisticated multi-symptom models incorporating social factors are needed to further our understanding of SUD and AUD specifically, a translational approach is still crucial in the context of investigating the more fundamental impact of alcohol use on brain and cognition. In humans, comparing the impact of alcohol use on brain and cognition between adolescents and adults is complicated by associations between age and cumulative exposure to alcohol; i.e., the older the individual, the longer and higher the overall exposure to alcohol. Although animal models may be limited in their ability to model every symptom of AUD, they can still provide critical insights into causal mechanisms underlying AUD by allowing direct control over alcohol exposure and in-depth investigation of brain mechanisms.
The intermittent voluntary access protocol resembles the patterns of alcohol use observed in humans, and also result in physiologically relevant levels of alcohol intake [ 182 , 183 , 184 ]. Only a minority of the studies included in this review employed a voluntary access protocol, with one study using beer instead of ethanol in water [ 158 ], which better accounts for the involvement of additional factors (e.g., sugar, taste) in the appeal of human alcohol consumption. Voluntary access protocols can also model behavioral aspects of addictive behavior such as loss of control over substance use and relapse [ 185 , 186 , 187 ], an important area in which little is known about the role of age. Ideally, one would also investigate choices between ethanol and alternative reinforcers, such as food or social interaction, that better mimic human decision-making processes [ 188 ]. However, studies on the effects of ethanol on social behavior are limited and show inconsistent results and studies assessing reward processes often lack a social reward component as an alternative reinforcer.
On a practical level, rodents mature quickly and choice-based exposure paradigms are more complex and time-consuming than most forced exposure paradigms. Consequently, by the time final behavioral measurements are recorded, both the adolescent and adult exposure groups have reached adulthood. To combat this, many of the included studies use forced ethanol exposure, such as ethanol vapor, to quickly expose rodents to very high doses of ethanol. Although the means and degrees of alcohol exposure may not directly translate to human patterns of alcohol use, such studies do allow for the assessment of the impact of high cumulative doses of ethanol within a relatively short period of time which allows for more time in the developmental window to test age-related differences in the outcomes. When considering the translational value of a study, it is therefore important to evaluate studies based on the goal, while not ignoring the practical constraints.
While human research is challenging due to the lack of experimental control and the inherent confounds in observational studies between age and alcohol exposure history, large-scale prospective longitudinal studies offer a gateway towards a better understanding. Comparisons of different trajectories of drinking from adolescence to adulthood (i.e., heavy drinking to light drinking, light drinking to heavy drinking, continuously heavy drinking, and continuously light drinking) could offer insight into the associated effects on cognitive and brain-related outcomes. Of course, different drinking trajectories are likely confounded with potentially relevant covariates which limits causal inference. Direct comparisons of low and heavy adolescent and adult drinkers, supported by a parallel animal model can help to bolster the causality of observed age-related differences in human studies. In addition, changes in legislation around the minimum age for alcohol consumption in some countries provide a unique opportunity to investigate how delaying alcohol use to later in adolescence or even young adulthood impacts cognitive functioning over time. Importantly, future studies investigating the moderating role of age in humans should carefully consider the impact of psychiatric comorbidities. While adolescence into young adulthood is the period in which mental health issues often emerge [ 189 , 190 ], there is some evidence that the prevalence of comorbidities is higher in adults with AUD [ 95 ]. This is an important to control for when considering age-related differences on cognition and the brain given the evidence of altered cognitive functioning in other common mental illnesses [ 191 , 192 ].
Concluding remarks
The aim of this systematic review was to extend our understanding of adolescent risk and resilience to the effects of alcohol on brain and cognitive outcomes compared to adults. In comparison to recent existing reviews on the impact of alcohol on the adolescent brain and cognition [ 17 , 18 , 19 , 22 , 23 ], a strength of the current review is the direct comparison of the effects of chronic alcohol exposure during adolescence versus adulthood. This approach allows us to uncover both similarities and differences in the processes underlying alcohol use and dependence between adolescents and adults. However, due to the large degree of heterogeneity in the studies included in sample, designs, and outcomes, we were unable to perform meta-analytic synthesis techniques.
In conclusion, while the identified studies used varying paradigms and outcomes, key patterns of results emerged indicating a complex role of age, with evidence pointing towards both adolescent vulnerability and resilience. The evidence suggests adolescents may be more vulnerable than adults in domains that may promote heavy and binge drinking, including reduced sensitivity to aversive effects of high alcohol dosages, reduced dopaminergic neurotransmission in the NAc and PFC, greater neurodegeneration and impaired neurogenesis, and other neuromodulatory processes. At the same time, adolescents may be more resilient than adults to alcohol-induced impairments in domains which may promote recovery from heavy drinking, such as cognitive flexibility. However, in most domains, the evidence was too limited or inconsistent to draw clear conclusions. Importantly, human studies directly comparing adolescents and adults are largely missing. Recent reviews of longitudinal human research in adolescents, however, revealed consistent evidence of alterations to gray matter, and to a lesser extent white matter, structure in drinkers [ 17 , 18 ], but also highlight the limited evidence available in the domains of neural and cognitive functioning in humans [ 17 ]. Future results from ongoing large-scale longitudinal neuroimaging studies like the ABCD study [ 193 ] will likely shed valuable light on the impact of alcohol use on the adolescent brain. However, our results also stress the need for direct comparisons with adult populations. Moreover, while the lack of experimental control and methodological constraints limit interpretations and causal attributions in human research, translational work aimed at connecting findings from animal models to humans is necessary to build upon the current knowledge base. Furthermore, the use of voluntary self-administration paradigms and incorporation of individual differences and environmental contexts are important steps forward in improving the validity of animal models of alcohol use and related problems. A more informed understanding of the effects of alcohol on adolescents compared to adults can further prevention efforts and better inform policy efforts aimed at minimizing harm during a crucial period for both social and cognitive development.
Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012.
Article Google Scholar
Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. World Health Organization; 2004. https://doi.org/10.1590/S0042-96862004001100011 .
Fleury MJ, Djouini A, Huỳnh C, Tremblay J, Ferland F, Ménard JM, et al. Remission from substance use disorders: a systematic review and meta-analysis. Drug Alcohol Depend. 2016;168:293–306.
Article PubMed Google Scholar
Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2006;118:e755–e763.
Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–46.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edn. Arlington, VA: American Psychiatric Association; 2013. https://doi.org/10.1176/appi.books.9780890425596.dsm04 .
Conrod P, Nikolaou K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry Allied Discip. 2016;57:371–94.
Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–30.
Article CAS PubMed PubMed Central Google Scholar
Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci. 2013;127:23–32.
Article PubMed PubMed Central Google Scholar
Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004(Suppl. 43):84–105.
Mastwal S, Ye Y, Ren M, Jimenez DV, Martinowich K, Gerfen CR, et al. Phasic dopamine neuron activity elicits unique mesofrontal plasticity in adolescence. J Neurosci. 2014;34:9484–96.
Article PubMed PubMed Central CAS Google Scholar
Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–50.
Article CAS PubMed Google Scholar
Vergés A, Haeny AM, Jackson KM, Bucholz KK, Grant JD, Trull TJ, et al. Refining the notion of maturing out: results from the national epidemiologic survey on alcohol and related conditions. Am J Public Health. 2013;103:e67–e73.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35:217–38.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9.
de Goede J, van der Mark-Reeuwijk KG, Braun KP, le Cessie S, Durston S, Engels RCME, et al. Alcohol and brain development in adolescents and young adults: a systematic review of the literature and advisory report of the health council of the Netherlands. Adv Nutr. 2021. https://doi.org/10.1093/advances/nmaa170 .
Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci. 2018;19:197–214.
Carbia C, López-Caneda E, Corral M, Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci Biobehav Rev. 2018;90:332–49.
Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage Clin. 2014;5:420–37.
Article PubMed Central Google Scholar
Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res. 2014;38:2509–16.
Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharm Biochem Behav. 2020;192:172906.
Article CAS Google Scholar
Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: a systematic review and meta-analysis. Neuropsychol Rev. 2019;29:357–85.
Cservenka A, Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front Psychol. 2017;8:1111.
Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol Alcohol. 2013;48:433–44.
Maeda K-I, Satoshi O, Hiroko T. Physiology of reproduction. Academic Press; 2000.
Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63.
Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–80.
Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;70:121–34.
Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. In: Annals of the New York Academy of Sciences. New York Academy of Sciences; 2004. p. 441–4.
Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–7.
Dumontheil I. Adolescent brain development. Curr Opin Behav Sci. 2016;10:39–44.
Shillington AM, Woodruff SI, Clapp JD, Reed MB, Lemus H. Self-reported age of onset and telescoping for cigarettes, alcohol, and marijuana: across eight years of the national longitudinal survey of youth. J Child Adolesc Subst Abus. 2012;21:333–48.
Livingston MD, Xu X, Komro KA. Predictors of recall error in self-report of age at alcohol use onset. J Stud Alcohol Drugs. 2016;77:811–8.
De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
Rodriguiz RM, Wetsel WC. Assessments of cognitive deficits in mutant mice. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; 2006. p. 223–82.
Leung RK, Toumbourou JW, Hemphill SA. The effect of peer influence and selection processes on adolescent alcohol use: a systematic review of longitudinal studies. Health Psychol Rev. 2014;8:426–57.
Cousijn J, Luijten M, Feldstein Ewing SW. Adolescent resilience to addiction: a social plasticity hypothesis. Lancet Child Adolesc Heal. 2018;2:69–78.
Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–71.
Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46.
Vanderschuren LJMJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–95.
Gorey C, Kuhns L, Smaragdi E, Kroon E, Cousijn J. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37–58.
Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–9.
Ambrose ML, Bowden SC, Whelan G. Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol Clin Exp Res. 2001;25:185–91.
Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–13.
Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: setting the stage for alcohol use disorders? Child Dev Perspect. 2011;5:231–8.
Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–51.
Moore EM, Forrest RD, Boehm SL. Genotype modulates age-related alterations in sensitivity to the aversive effects of ethanol: an eight inbred strain analysis of conditioned taste aversion. Genes, Brain Behav. 2013;12:70–77.
Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9.
Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol. 2011;45:45–55.
Carrara-Nascimento PF, Olive MF, Camarini R. Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev Psychobiol. 2014;56:36–48.
Leichtweis KS, Carvalho M, Morais-Silva G, Marin MT, Amaral VCS. Short and prolonged maternal separation impacts on ethanol-related behaviors in rats: sex and age differences. Stress. 2020;23:162–73.
Pautassi RM, Suárez AB, Hoffmann LB, Rueda AV, Rae M, Marianno P, et al. Effects of environmental enrichment upon ethanol-induced conditioned place preference and pre-frontal BDNF levels in adolescent and adult mice. Sci Rep. 2017;7:1–12.
Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–19.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning to context. Behav Brain Res. 2000;108:1–19.
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6.
Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–802.
Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43.
Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiol Behav. 2006;88:466–72.
Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19.
Broadwater M, Spear LP. Consequences of adolescent or adult ethanol exposure on tone and context fear retention: effects of an acute ethanol challenge during conditioning. Alcohol Clin Exp Res. 2014;38:1454–60.
Broadwater M, Spear LP. Tone conditioning potentiates rather than overshadows context fear in adult animals following adolescent ethanol exposure. Dev Psychobiol. 2014;56:1150–5.
Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M, Vaudry D. Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. J Neurochem. 2015;132:629–41.
Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. In: Chapple L, editors. Alcoholism: clinical and Experimental Research. Blackwell Publishing Ltd; 1998. p. 416–21.
Acheson SK, Ross EL, Swartzwelder HS. Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol. 2001;23:167–75.
Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–10.
Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48:353–60.
Matthews DB, Watson MR, James K, Kastner A, Schneider A, Mittleman G. The impact of low to moderate chronic intermittent ethanol exposure on behavioral endpoints in aged, adult, and adolescent rats. Alcohol. 2019;78:33–42.
Galaj E, Barrera E, Morris D, Ma YY, Ranaldi R. Aberrations in incentive learning and responding to heroin in male rats after adolescent or adult chronic binge-like alcohol exposure. Alcohol Clin Exp Res. 2020;44:1214–23.
Diamond A. Executive functions. 2013;64:135–68. https://doi.org/10.1146/annurev-psych-113011-143750 .
Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471–82.
Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res. 2014;38:1622–9.
Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS ONE. 2013;8:e62940.
Pickens CL, Cook A, Gaeddert B. Dose-dependent effects of alcohol injections on omission-contingency learning have an inverted-U pattern. Behav Brain Res. 2020;392:112736.
Pickens CL, Kallenberger P, Pajser A, Fisher H. Voluntary alcohol access during adolescence/early adulthood, but not during adulthood, causes faster omission contingency learning. Behav Brain Res. 2019;370:111918.
Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res. 2014;266:19–28.
Fernandez GM, Lew BJ, Vedder LC, Savage LM. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience. 2017;348:324–34.
Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149987 .
Labots M, Cousijn J, Jolink LA, Leon Kenemans J, Vanderschuren LJMJ, Lesscher HMB. Age-related differences in alcohol intake and control over alcohol seeking in rats. Front Psychiatry. 2018;9:419.
Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–36.
White AM, Ghia AJ, Levin ED, Scott Swartzwelder H. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–6.
Baddeley A. Working memory. Science. 1992;255:556–9.
Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol Anim Behav Process. 1976;2:97–116.
Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12.
Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78.
Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49.
Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51.
Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35:875–81.
Jupp B, Dalley JW. Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. Br J Pharm. 2014;171:4729–66.
Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18.
Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Curr Drug Abus Rev. 2013;6:201–19.
Tomie A, Jeffers P, Zito B. Sign-tracking model of the addiction blind spot. In: Tomie JMA, editors. Sign tracking and drug addiction. Maize Books; 2018. p. 8–34.
Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry. 2019;6:1068–80.
Wolitzky-Taylor K, Bobova L, Zinbarg RE, Mineka S, Craske MG. Longitudinal investigation of the impact of anxiety and mood disorders in adolescence on subsequent substance use disorder onset and vice versa. Addict Behav. 2012;37:982–5.
Park J, Moghaddam B. Impact of anxiety on prefrontal cortex encoding of cognitive flexibility. Neuroscience 2017;345:193–202.
Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. The complex interaction between anxiety and cognition: Insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93.
Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33.
Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67.
Elsey JWB, Kindt M. Startle reflex. In: Zeigler-Hill V, Shackelford TK, editors. Encyclopedia of personality and individual differences. Springer International Publishing; 2018. p. 1–5.
Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharm Biochem Behav. 1980;13:167–70.
File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharm. 2003;463:35–53.
Misslin R, Ropartz P. Responses in mice to a novel object author. Behavior. 1981;78:169–77.
Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharm Biochem Behav. 1991;38:63–67.
Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK. Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci. 2016;10:265.
Agoglia AE, Holstein SE, Reid G, Hodge CW. CaMKIIα-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcohol Clin Exp Res. 2015;39:1680–90.
Van Skike CE, Diaz-Granados JL, Matthews DB. Chronic intermittent ethanol exposure produces persistent anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2015;39:262–71.
Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–93.
Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607.
Wille-Bille A, de Olmos S, Marengo L, Chiner F, Pautassi RM. Long-term ethanol self-administration induces ΔFosB in male and female adolescent, but not in adult, Wistar rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;74:15–30.
Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30.
Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–24.
PubMed PubMed Central Google Scholar
Neuhofer D, Kalivas P. Metaplasticity at the addicted tetrapartite synapse: a common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol Learn Mem. 2018;154:97–111.
Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–54.
Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–73.
Chin VS, Van Skike CE, Berry RB, Kirk RE, Diaz-Granados J, Matthews DB. Effect of acute ethanol and acute allopregnanolone on spatial memory in adolescent and adult rats. Alcohol. 2011;45:473–83.
Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31.
Akkus F, Mihov Y, Treyer V, Ametamey SM, Johayem A, Senn S, et al. Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Transl Psychiatry 2018;8. https://doi.org/10.1038/s41398-017-0066-6 .
Leurquin-Sterk G, Ceccarini J, Crunelle CL, De Laat B, Verbeek J, Deman S, et al. Lower limbic metabotropic glutamate receptor 5 availability in alcohol dependence. J Nucl Med. 2018;59:682–90.
Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–74.
Grobin AC, Matthews DB, Montoya D, Wilson WA, Morrow AL, Swartzwelder HS. Age-related differences in neurosteroid potentiation of muscimol-stimulated 36Cl- flux following chronic ethanol treatment. Neuroscience. 2001;105:547–52.
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol. 2011;45:577–83.
Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37:1154–60.
Carrara-Nascimento PF, Hoffmann LB, Flório JC, Planeta CS, Camarini R. Effects of ethanol exposure during adolescence or adulthood on locomotor sensitization and dopamine levels in the reward system. Front Behav Neurosci. 2020;14:31.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012;76:116–29.
Wu J, Gao M, Taylor DH. Neuronal nicotinic acetylcholine receptors are important targets for alcohol reward and dependence. Acta Pharmacol Sin. 2014;35:311–5.
Walker LC, Berizzi AE, Chen NA, Rueda P, Perreau VM, Huckstep K, et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol Psychiatry. 2020;88:898–909.
Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS ONE. 2014;9:113421.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Huang C, Titus JA, Bell RL, Kapros T, Chen J, Huang R. A mouse model for adolescent alcohol abuse: stunted growth and effects in brain. Alcohol Clin Exp Res. 2012;36:1728–37.
Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23.
Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305.
Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–29.
Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–35.
Goulding SP, de Guglielmo G, Carrette LLG, George O, Contet C. Systemic administration of the cyclin-dependent kinase inhibitor (S)-CR8 selectively reduces escalated ethanol intake in dependent rats. Alcohol Clin Exp Res. 2019;43:2079–89.
Joe KH, Kim YK, Kim TS, Roh SW, Choi SW, Kim YB, et al. Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1833–8.
Huang MC, Chen CH, Chen CH, Liu SC, Ho CJ, Shen WW, et al. Alterations of serum brain-derived neurotrophic factor levels in early alcohol withdrawal. Alcohol Alcohol. 2008;43:241–5.
Miller R, King MA, Heaton MB, Walker DW. The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Res. 2002;950:137–47.
Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35.
Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–37.
Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, et al. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–40.
Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. In: Alcoholism: clinical and experimental research. John Wiley & Sons, Ltd; 2006. p. 720–30.
Davis RL, Syapin PJ. Chronic ethanol inhibits CXC chemokine ligand 10 production in human A172 astroglia and astroglial-mediated leukocyte chemotaxis. Neurosci Lett. 2004;362:220–5.
Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–43.
Marshall SA, McClain JA, Wooden JI, Nixon K. Microglia dystrophy following binge-like alcohol exposure in adolescent and adult male rats. Front Neuroanat. 2020;14:52.
Agrawal RG, Owen JA, Levin PS, Hewetson A, Berman AE, Franklin SR, et al. Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcohol Clin Exp Res. 2014;38:428–37.
Kane CJM, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res. 2014;38:384–91.
Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 2017;122:136–47.
Slawecki CJ, Jiménez-Vasquez P, Mathé AA, Ehlers CL. Effect of ethanol on brain neuropeptides in adolescent and adult rats. J Stud Alcohol. 2005;66:46–52.
van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115.
Souza-Moreira L, Campos-Salinas J, Caro M, Gonzalez-Rey E. Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology. 2011;94:89–100.
Carniglia L, Ramírez D, Durand D, Saba J, Turati J, Caruso C, et al. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm. 2017;2017. https://doi.org/10.1155/2017/5048616 .
Hipolito L, Sanchez M, Polache A, Granero L. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–27.
Rhoads DE, Contreras C, Fathalla S. Brain levels of catalase remain constant through strain, developmental, and chronic alcohol challenges. Enzyme Res. 2012;2012. https://doi.org/10.1155/2012/572939 .
Vasiliou V, Ziegler TL, Bludeau P, Petersen DR, Gonzalez FJ, Deitrich RA. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics. 2006;16:51–58.
Zimatkin SM, Buben AI. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–32.
Hargreaves GA, Quinn H, Kashem MA, Matsumoto I, McGregor IS. Proteomic analysis demonstrates adolescent vulnerability to lasting hippocampal changes following chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33:86–94.
Galaj E, Guo C, Huang D, Ranaldi R, Ma YY. Contrasting effects of adolescent and early-adult ethanol exposure on prelimbic cortical pyramidal neurons. Drug Alcohol Depend. 2020;216:108309.
Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher ML, et al. Long-term modulation of A-type K+ conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcohol Clin Exp Res. 2013;37:2074–85.
Artinian J, Lacaille JC. Disinhibition in learning and memory circuits: new vistas for somatostatin interneurons and long-term synaptic plasticity. Brain Res Bull. 2018;141:20–26.
Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, et al. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb Cortex. 2018;28:1049–63.
McAteer AM, Hanna D, Curran D. Age-related differences in alcohol attention bias: a cross-sectional study. Psychopharmacology. 2018;235:2387–93.
Rooke SE, Hine DW. A dual process account of adolescent and adult binge drinking. Addict Behav. 2011;36:341–6.
Cousijn J, Green KH, Labots M, Vanderschuren LJMJ, Kenemans JL, Lesscher HMB. Motivational and control mechanisms underlying adolescent versus adult alcohol use. NeuroSci. 2020;1:44–58.
Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20.
Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: a prospective study. Dev Cogn Neurosci. 2015;16:36–45.
Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci. 2015;16:174–82.
Saalfield J, Spear L. The ontogeny of ethanol aversion. Physiol Behav. 2016;156:164–70.
McQuown SC, Wood MA. Epigenetic regulation in substance use disorders. Curr Psychiatry Rep. 2010;12:145–53.
Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–50.
Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628:60–67.
Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience 2012;215:42–58.
Robinson OJ, Pike AC, Cornwell B, Grillon C. The translational neural circuitry of anxiety. J Neurol Neurosurg Psychiatry. 2019;90:1353–60.
PubMed Google Scholar
Martínez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, et al. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol Behav. 2002;76:219–24.
Bergink V, Van Megen HJGM, Westenberg HGM. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–83.
Scott AJ, Jordan M, Lueras BJN. Effects of binge drinking on the developing brain. Alcohol Res. 2018;39:87–96.
Google Scholar
Krieger H, Young CM, Anthenien AM, Neighbors C. The epidemiology of binge drinking among college-age individuals in the United States. Alcohol Res. 2018;39:23–30.
Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9.
Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environ Health Perspect. 2013;121:985–92.
Field M, Kersbergen I. Are animal models of addiction useful? Addiction. 2020;115:6–12.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23.
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63.
Spoelder M, Hesseling P, Baars AM, Lozeman-van’t Klooster JG, Rotte MD, Vanderschuren LJMJ, et al. Individual variation in alcohol intake predicts reinforcement, motivation, and compulsive alcohol use in rats. Alcohol Clin Exp Res. 2015;39:2427–37.
Fredriksson I, Venniro M, Reiner DJ, Chow JJ, Bossert JM, Shaham Y. Animal models of drug relapse and craving after voluntary abstinence: a review. Pharm Rev. 2021;73:1050–83.
Vanderschuren LJMJ, Ahmed SH. Animal models of the behavioral symptoms of substance use disorders. Cold Spring Harb Perspect Med. 2021;11:a040287.
Kuhn BN, Kalivas PW, Bobadilla AC. Understanding addiction using animal models. Front Behav Neurosci. 2019;13. https://doi.org/10.3389/fnbeh.2019.00262 .
Venniro M, Shaham Y. An operant social self-administration and choice model in rats. Nat Protoc. 2020;15:1542–59.
Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet 2012;379:1056–67.
Costello EJ, Egger H, Angold A. 10-Year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry. 2005;44:972–86.
Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, Munakata Y. Opposite effects of anxiety and depressive symptoms on executive function: the case of selecting among competing options. 2014;28:893–902. https://doi.org/10.1080/026999312013859568 .
McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8.
Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7.
Download references
Acknowledgements
This work was supported by grant 1RO1 DA042490-01A1 awarded to Janna Cousijn and Francesca Filbey from the National Institute on Drug Abuse/National Institutes of Health. The grant supported the salaries of authors Lauren Kuhns, Emese Kroon, and Janna Cousijn. Thank you to Claire Gorey (CG) for running the initial search and aiding in the screening process.
Author information
Authors and affiliations.
Neuroscience of Addiction (NofA) Lab, Department of Psychology, University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon, Gabry Mies & Janna Cousijn
The Amsterdam Brain and Cognition Center (ABC), University of Amsterdam, Amsterdam, The Netherlands
Lauren Kuhns, Emese Kroon & Janna Cousijn
Department of Animals in Science and Society, Division of Behavioural Neuroscience, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands
Heidi Lesscher
Department of Psychology, Education & Child Studies, Erasmus University Rotterdam, Rotterdam, The Netherlands
Janna Cousijn
You can also search for this author in PubMed Google Scholar
Contributions
LK conducted the systematic searches; LK, EK, GM, and JC screened the citations for exclusion and inclusion; LK, EK, HL, and JC wrote the review; LK, EK, HL, GM, and JC revised the manuscript.
Corresponding author
Correspondence to Lauren Kuhns .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kuhns, L., Kroon, E., Lesscher, H. et al. Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review. Transl Psychiatry 12 , 345 (2022). https://doi.org/10.1038/s41398-022-02100-y
Download citation
Received : 26 October 2021
Revised : 21 June 2022
Accepted : 28 July 2022
Published : 25 August 2022
DOI : https://doi.org/10.1038/s41398-022-02100-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Emotional and cognitive influences on alcohol consumption in middle-aged and elderly tanzanians: a population-based study.
- Patrick Kazonda
- Till Bärnighausen
Scientific Reports (2024)
Ethanol’s impact on the brain: a neurobiological perspective on the mechanisms of memory impairment
- Mahdiyeh Hedayati-Moghadam
- Fateme Razazpour
- Yousef Baghcheghi
Molecular Biology Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Volume 42 Issue 1 October 27, 2022
Alcohol’s Negative Emotional Side: The Role of Stress Neurobiology in Alcohol Use Disorder
Part of the Topic Series: NIAAA 50th Anniversary Festschrift
Rajita Sinha
Yale University School of Medicine, New Haven, Connecticut
This article is part of a Festschrift commemorating the 50th anniversary of the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Established in 1970, first as part of the National Institute of Mental Health and later as an independent institute of the National Institutes of Health, NIAAA today is the world’s largest funding agency for alcohol research. In addition to its own intramural research program, NIAAA supports the entire spectrum of innovative basic, translational, and clinical research to advance the diagnosis, prevention, and treatment of alcohol use disorder and alcohol-related problems. To celebrate the anniversary, NIAAA hosted a 2-day symposium, “Alcohol Across the Lifespan: 50 Years of Evidence-Based Diagnosis, Prevention, and Treatment Research,” devoted to key topics within the field of alcohol research. This article is based on Dr. Sinha’s presentation at the event. NIAAA Director George F. Koob, Ph.D., serves as editor of the Festschrift.
Introduction
The word “alcohol” often conjures up positive feelings and associations with fun, socializing, relaxing, and partying. Yet there is another side to drinking alcohol, especially with risky, hazardous levels of consumption. This side is associated with distress and may include anxiety, loneliness, pain, and depressive symptoms. 1 This has been labeled the “dark side,” or “negative emotional, stress side,” of alcohol intake. 2 These two paradoxical, dialectically opposing alcohol experiences map onto the biphasic drug effects of alcohol, with alcohol being both a stimulant and a depressant drug. They also represent a shift from positive to negative situations that may drive alcohol intake, especially as alcohol intake increases from low or moderate “social” levels of drinking to binge, heavy, and chronic consumption. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines drinking in moderation as an intake of two drinks or less per day for men and one drink or less per day for women. Binge drinking is generally defined as five or more drinks per occasion for men and four or more drinks per occasion for women. Heavy drinking is generally defined as more than four drinks per day or more than 14 drinks per week for men and as more than three drinks per day or more than seven drinks per week for women. 3
One aspect of the research the author has conducted with the support of NIAAA, and which is the topic of this article, has focused on identifying the physiological and neural effects, as well as the subjective and cognitive effects, of binge and chronic alcohol use. This research also has explored the factors that influence these effects and investigated whether these effects can be reversed or normalized to allow for recovery from any of the long-term changes that occur with binge and chronic alcohol misuse.
The worldwide coronavirus (COVID-19) pandemic is a chronic, ongoing stressor. Research has shown that alcohol consumption has increased significantly during this period, especially among individuals who regularly binge drink or drink heavily. 4,5 While onsite alcohol sales were down as businesses closed, e-commerce profits increased more than 30% during the COVID-19 pandemic. 4,5 Who is most susceptible to increased drinking episodes during COVID-19–related stress? This question highlights the need to understand the well-known bidirectional relationship between stress or trauma and alcohol intake, and why those with binge and chronic alcohol use are most vulnerable to increased alcohol use under high levels of stress and with traumatic exposure.
This article reviews human research investigating neurobiological and psychological changes related to alcohol misuse that are associated with greater distress and stress-related alcohol craving and their role in predicting risk of binge drinking, relapse, and impact on treatment outcomes. The author presents the effects of stress and trauma on brain stress responses and their associations with resilient coping and describes the impact of binge and chronic alcohol use on brain and peripheral stress responses and their role in promoting alcohol craving and relapse risk. Specific clinical and biobehavioral markers of both risk of developing alcohol use disorder (AUD) and relapse are also reviewed. Finally, the article discusses recent findings on treatments that focus on reversing stress and craving disruptions related to chronic alcohol misuse to improve treatment outcomes.
Alcohol and Stress—Shift From Positive to Negative Effects
It is well known that one or two standard alcoholic drinks have a stimulating and physiologically arousing effect; for example, heart rate increases acutely, and blood pressure changes have been documented. These responses are part of the autonomic nervous system readouts that occur with alcohol intake, but also are observed in challenging situations such as when faced with acute stressful life events. 6,7 The arousing response to alcohol is associated with a sense of feeling energized and stimulated as well as increases in sociability. 6 With increasing levels of alcohol intake in one sitting, however, alcohol also stimulates the hypothalamic-pituitary-adrenal (HPA) axis, and increases in cortisol are observed. 8,9 Alcohol also activates brain emotion and stress pathways, including the amygdala, under emotional arousing and stressful states. 10,11 In addition, acute alcohol use stimulates the brain cortico-striatal pathways involved in reward, motivation, and goal-directed behaviors. These include the ventral and dorsal striatum, the orbitofrontal cortex (OFC), and the ventromedial prefrontal cortex (VmPFC). 10-13 The emotion/stress pathway and the reward/motivation pathways closely interact, and such interactions are involved in emotional cue-related drinking motivation. 11,12
Binge and hazardous alcohol drinking patterns are associated with well-documented changes both in the brain stress and emotion regions, such as the amygdala, 8,12 and in associated brain networks, including the ventral and dorsal striatum as well as the OFC, VmPFC, and dorsolateral prefrontal cortex. 9,12,14,15 These brain changes are associated with blunted autonomic and cortisol responses to stress and to acute alcohol intake, 6,8 as well as with increases in negative emotional and stress responses and greater alcohol craving. 6,9,14-17 Together, these changes are part of the psychobiological adaptations in humans that occur with increasing patterns of binge and hazardous alcohol intake.
Stress, Alcohol Craving, and Binge Alcohol Intake
Acute stress exposure stimulates the autonomic, endocrine, and brain emotion and motivation regions that process and regulate negative emotion and distress responses, and it also activates stress coping. 6,12,18 Additionally, acute stress exposure increases physiological arousal, including cortisol responses, and activates brain stress pathways involved in emotional arousal, emotional learning, and memory. This activation occurs via circuits involving the hypothalamus, amygdala, hippocampus, insula, and prefrontal regions, including the OFC, VmPFC, and inferior frontal cortices. Also activated is the premotor supplementary motor area, which is involved in behavioral intent, response selection, and action. 6,18,19 Previous studies reported that there are dynamic time-dependent changes in the cortico-striatal regions involving the ventral and dorsal striatum and the VmPFC during stress versus non–stress conditions; these changes were associated with active, goal-directed stress coping. 18 Additionally, greater dynamic responses in these brain stress-reward pathways were associated with lower daily numbers of alcoholic drinks consumed, lower reports of emotional conflicts, and lower emotional eating, whereas blunted ventral striatum and VmPFC responses during stress were associated with greater reports of binge drinking, emotion dysregulation, and emotional eating. 18 Based on these findings, the dynamic neural responses in the striatum and VmPFC are thought to document neurophysiological flexibility during stress, and their associations with behavioral coping suggest that this circuit is part of the resilient stress-coping pathway involved in behavioral control and self-regulation of stress, emotions, and reward impulses. 6,18
These adaptations to alcohol also vary by sex, as fundamental differences between men and women exist in brain organization, structure, and functional networks 20 as well as in the responses of brain stress, emotion, and reward regions 21 and in patients with cocaine use disorder. 22 Moreover, sex differences in the responses to stress and to alcohol-related stimuli have been documented in people who drink moderately. Unlike in animal studies, males in human studies show greater adrenocorticotropic hormone (ACTH) and cortisol responses to stress, 23 whereas females show higher autonomic physiologic arousal to stress; a greater response to stress cues in the amygdala, insula, OFC, and VmPFC; and greater VmPFC response to alcohol cues. 24-28 This suggests that the psychological and biological responses to alcohol and to stress vary by sex and that although men and women report similar levels of alcohol motivation when matched for recent drinking history, the psychological and neurobiological pathways that facilitate alcohol use are different for men and women who drink moderately.
Regardless of sex, repeated escalated alcohol use induces changes in both peripheral and brain stress systems. 2,12,16 Higher binge levels of alcohol use increase basal cortisol levels and blunt the peripheral stress responses; these changes also predict greater craving and behavioral motivation for alcohol use in people who binge drink or drink heavily (see Figure 1). 8,9 Additionally, changes in the amygdala responses to emotional cues and ventral striatal responses to alcohol have been reported with higher binge levels of alcohol use. 14,29 Along with these neural changes, increased salience of alcohol and greater alcohol craving levels have been observed in response to stress as well as in response to alcohol and to alcohol cues, which then promote increased alcohol intake and escalation to risky drinking. 8,15,17 These brain stress system, physiologic, and behavioral effects of binge drinking history need to be further examined by sex to better understand the recent data on greater escalation of binge drinking in women compared to men. 30
Figure 1. Baseline cortisol levels and responses to stress differ between moderate drinkers and binge/heavy drinkers. (A) Fasting morning plasma levels of cortisol (μg/dL) were higher in binge/heavy drinkers (orange bars) compared to moderate drinkers (blue bars) (***p < .001). (B) Cortisol responses to stress and alcohol cues, but not to neutral cues, were blunted in binge/heavy drinkers compared with moderate drinkers (**p < .01). (C) In binge/heavy drinkers, the behavioral motivation for alcohol use as reflected in the amount of alcohol consumed post stress in an ad lib drinking task was greater in individuals with a more blunted cortisol response to stress (r 2 = .11, p = .0022). Source: Adapted with permission from Blaine et al. (2019). 8
Effects of Stress and Trauma on Brain Pathways and AUD Risk
Stress and trauma are associated with greater levels of risky alcohol intake as well as greater severity of AUD. 19 Numerous different types of traumatic stress and life events as well some temperament and individual-level variables relate to risk of binge drinking and developing AUD (see Table 1). Exposure to repeated stress and trauma also contributes to changes in the brain and body’s responses to stress and emotions as well as to changes in alcohol motivation and adaptive coping responses.
| Adverse Life Events | Childhood and Life Trauma | Chronic Stressors | Stressful Internal States |
|---|---|---|---|
Source: Included with permission from Milivojevic & Sinha (2018). 37
Greater levels of cumulative adversity, stressful life events, and trauma are associated with lower brain volume and greater negative emotion and subjective stress responses. They also are associated with dysregulated neural and peripheral physiological responses to stress and to alcohol cues in the brain regions involved in stress, emotion, reward regulation, and self-control, including the OFC, VmPFC, supplementary motor area, amygdala, insula, and striatum. 31-33 Furthermore, altered or blunted ACTH and cortisol and autonomic responses to stress and to alcohol and drug cues are observed with greater trauma or stress. 19,33 These stress- and trauma-related brain and peripheral alterations co-occur alongside emotional and behavioral dysregulation and higher alcohol motivation. As a result, people with more risky drinking exposed to stress or trauma are at greater risk of emotion dysregulation as evidenced by more arguments, fights, emotional eating, and higher maximum drinks consumed per occasion (see Figure 2). 18,34
Figure 2. Associations between brain stress responses and resilient coping. (A) Dynamic activation in the ventromedial prefrontal cortex (VmPFC) during stress challenge (represented by red and yellow) was a sign of resilient coping, whereas a lack of dynamic changes in the VmPFC during stress, suggesting inability to mobilize during stress, was a sign of risky coping. (B) Greater dynamic activation of the VmPFC was associated with greater self-reported active coping. (C) Lack of dynamic activation of the VmPFC was more pronounced in binge drinkers. (D) Greater emotional dysregulation (measured by greater frequency of arguments or fights) also was associated with less dynamic activation of the VmPFC. Source: Adapted with permission from Sinha et al. (2016). 18
Several interacting brain networks are activated during stress, including those involved in emotion experiences (e.g., amygdala, insula), emotional memory (e.g., amygdala, hippocampus), reward and motivation regions (e.g., ventral and dorsal striatum), and goal-directed behavior (e.g., OFC, VmPFC). 13,18,19,21,29 These regions form networks and patterns of activation that enable emotional and motivational coping, and both stress and alcohol directly act on these networks to influence active coping, motivation, and flexible control of behavior, such as exercising self-control with drinking. The accumulating evidence shows that stress and trauma exposure alter these emotional and motivational responses involved in adaptive stress coping, such that people become more vulnerable to craving and consuming higher levels of alcohol, which increases risk of hazardous and risky drinking.
The research described above resulted in the development of a model explaining the role of glucocorticoids in drinking behavior on the basis of changes in peripheral cortisol levels and responses across the full spectrum of alcohol consumption levels. 8 At baseline, people who binge drink or drink heavily have higher cortisol levels than those who drink moderately (see Figure 1A), indicating a shift in HPA axis functioning. This also suggests possible changes in brain glucocorticoid pathways in humans that may increase risk of hazardous drinking. As stated earlier, alcohol consumption stimulates cortisol release; however, in response to either stress or alcohol exposure, the increase in cortisol is lower in people who binge drink or drink heavily than in those who drink moderately. Thus, when given one standard alcoholic drink, those drinking at binge levels do not feel its effects as robustly as do people who drink moderately. 8,9 As cortisol is critical for survival, humans have well-preserved neurobehavioral signals with the brain stress system pathways 12 that seek to enhance cortisol release in response to stress. In people with blunted cortisol responses due to heavy drinking, this mechanism may signal greater motivation for alcohol to increase alcohol-related cortisol responses. 9 Thus, there is a neurophysiologic drive to enhance wanting alcohol in order to increase cortisol and HPA axis functioning in people who drink heavily. This disruption in alcohol-related cortisol signaling and the need to drive the homeostatic HPA axis rhythm back to functional levels may be one component of the enhanced motivation for alcohol in those who drink alcohol at binge and heavy levels. This conceptual model suggests that normalizing the brain and body’s stress and motivational coping responses may reduce risk of hazardous drinking. Researchers are seeking to develop and evaluate novel strategies to achieve this normalization and to reduce the risk of heavy drinking.
Effects of Stress and Alcohol Cues in AUD
Researchers also have investigated the role of stress biology and stress responses in people with AUD. Chronic heavy drinking or binge drinking increases the risk of disrupted alcohol-related autonomic and HPA axis responses as described in previous sections. These disruptions contribute to clinical symptoms associated with the negative emotional side of AUD, 15 such as increased levels of anxiety, negative mood, sleep difficulties, emotional reactivity, and impulsivity, along with high levels of craving for alcohol. 1,35 Furthermore, these disruptions increase the risk of relapse and heavy drinking during treatment and posttreatment, thereby jeopardizing long-term recovery. 6,36,37 Alcohol relapse refers to return to heavy drinking (at binge levels) after any period of abstinence, whereas treatment failure refers to maintaining or returning to binge and hazardous drinking levels during or after treatment. 3 These observations have led researchers to investigate which factors contribute to early risk of dropout and recovery failure during treatment.
A series of studies assessed brain and body responses as well as cognitive, emotional, and motivational responses to both stress and alcohol cues in a laboratory study of human participants with AUD who were entering treatment and control participants without AUD. The analyses also included structural and functional magnetic resonance imaging as well as real-world daily assessment of stress and motivational responses using smartphones. These analyses using multiple approaches across different samples of individuals with AUD found that stress exposure increased alcohol craving. This response was accompanied by higher emotional, mood, and anxiety symptoms and lower ability to regulate emotions and control alcohol cravings. 36,37 Furthermore, the biological stress response was significantly disrupted during the early recovery period. Thus, individuals in early recovery exhibited a higher basal heart rate and higher free cortisol levels, but lower levels of endogenous bound cortisol. Additionally, these individuals did not show a significant normal response to stress or alcohol challenge. 6,37 Thus, the biological responses that support emotion and mood regulation are disrupted during this early recovery phase, and the greater these levels of dysfunction, the higher the risk of relapse or heavy drinking. Notably, sex differences in these biological responses have been reported, where women with AUD showed a more blunted ACTH and cortisol level than men with AUD; however, women had much higher basal norepinephrine levels, which in turn affected their response to stress and to alcohol cues. 26,38
Another series of experiments examined brain correlates of later alcohol relapse and treatment failure. These analyses found that the volume of gray matter cells in the medial prefrontal brain regions—which are involved in regulating emotions, reward, and actions—was lower among individuals entering treatment compared with healthy control participants. 39 Also, individuals with the lowest gray matter volume in the medial prefrontal brain region tended to be most likely to relapse and not do well in treatment. 39 Analyses assessing the function of these brain regions during experimental exposure to stress and to alcohol cues (compared to neutral cues) detected disrupted, hyperactive VmPFC responses to neutral relaxing cues, but blunted, hypoactive VmPFC responses to stress and cue exposure. These observations suggest that the brain pathways that help regulate emotions and desires showed dysfunction and that the greater the VmPFC disruption, the higher the risk of alcohol relapse and heavy drinking. 40,41
The studies described above have led to the characterization of a risk profile to identify individuals who are most vulnerable for alcohol relapse and heavy drinking during treatment. Thus, risk was determined by specific clinical measures—such as alcohol craving and withdrawal, 42,43 mood, anxiety, and sleep difficulties—and biological markers 37 as well as by additional moderating factors, including childhood maltreatment (see Table 2). 44 Furthermore, this research supported the conceptualization that the effects of binge drinking and chronic alcohol use on stress biology occur along a continuum, with higher levels of alcohol intake associated with more significant chronic stress pathophysiology, which in turn contributes to greater risk of alcohol relapse and treatment failure. 35
| Clinical and Biological Markers | Moderating Factors |
|---|---|
AUD Treatments Targeting Stress, Craving, and Loss of Control of Alcohol Intake
Critical basic science and translational work by Koob and colleagues 45 had focused on stress pathophysiology to develop novel therapeutics for AUD. Similarly, the findings described above motivated additional research to evaluate whether reversal of the chronic alcohol-related disruptions in stress psychobiology that are associated with increased alcohol craving and relapse risk could improve treatment and treatment outcomes for individuals most vulnerable to alcohol-related stress pathophysiology. Previous research by Arnsten had shown that noradrenergic agents such as guanfacine and prazosin could rescue the prefrontal cortex from the toxic effects of high uncontrollable stress. 46 Because the effects of chronic alcohol exposure are similar to those of high chronic stress, it seemed plausible that pharmacologic targets that reduce prefrontal norepinephrine and the toxic effects of stress-related damage also could be of benefit in improving the stress and craving-related pathology associated with AUD. Studies to test these hypotheses have shown positive results. Guanfacine, an alpha-2 adrenergic agonist that reduces brain norepinephrine in the prefrontal cortex, improved prefrontal functioning and reduced alcohol and drug craving. 47,48 Furthermore, guanfacine had some sex-specific effects, with greater benefits in women than in men. 49,50
Similarly, prazosin—an alpha-1-adrenergic antagonist that had been shown to improve working memory and prefrontal functioning during stress 46 as well as withdrawal-related drinking in laboratory animals 51 —reduced stress-related craving and stress dysfunction in AUD. 52,53 Based on these findings, an NIAAA-supported, 12-week proof-of-concept, double-blind, placebo-controlled, randomized trial of prazosin versus placebo (16 mg/day, three times a day dosing, titrated over 2 weeks) was conducted with 100 individuals with AUD. The study found that alcohol withdrawal symptoms were a moderating factor impacting prazosin efficacy in improving drinking outcomes over 12 weeks; that is, prazosin treatment benefit was determined by the presence of alcohol withdrawal symptoms at treatment entry. Thus, individuals with more severe alcohol withdrawal symptoms at treatment initiation experienced greater reductions in heavy drinking days and drinks per occasion during the 12-week treatment period. 54 In addition, prazosin reduced alcohol craving, anxiety, and negative mood compared with placebo in participants with high alcohol withdrawal symptoms, but had no impact in those with no or low levels of alcohol withdrawal symptoms. Finally, prazosin appeared to reverse VmPFC and dorsal striatal dysfunction, improving medial prefrontal response to stress and reducing dorsal striatal response to alcohol cues in participants treated with prazosin compared with those receiving placebo. 55 These findings support further development of prazosin in the treatment of severe AUD. However, they also underscore the need to pursue further research to identify behavioral and pharmacologic strategies to prevent and treat chronic alcohol effects on stress pathophysiology in AUD.
Conclusions
This article summarizes research by the author’s group demonstrating that binge, heavy, and chronic drinking leads to adaptations in brain, biological, and psychological stress responses. These adaptations are associated with alcohol’s negative emotional aspects, as evidenced by greater alcohol craving, higher alcohol withdrawal, greater negative mood and anxiety symptoms, as well as sleep difficulties that are commonly reported by individuals with AUD entering treatment. These changes occur in brain stress, reward, and motivation pathways that represent the stress pathophysiology of AUD. This stress pathophysiology directly targets brain circuits that underlie people’s ability to cope with stress and day-to-day challenges and are involved in jeopardizing recovery from AUD.
This research also has identified various clinical and biobehavioral markers that are associated with relapse and treatment failure and has allowed for identification of individuals who may be at greatest risk of treatment failure. Additionally, identification of these markers has led to research seeking to develop new strategies to target and reverse the stress pathophysiology of AUD to optimize interventions for AUD. Current and future work is focused on developing and testing specific treatments that can target this particular stress pathophysiology and help individuals who are most vulnerable to jeopardizing their recovery in the early phase of AUD treatment.
Acknowledgments
This article is a summary of the presentation delivered at the NIAAA 50th Anniversary Science Symposium on December 1, 2020. It serves as a tribute to NIAAA in commemoration of their persistent commitment to developing the science of alcohol effects and associated harm, and to developing novel cutting-edge strategies in support of prevention and treatment of, and recovery from, alcohol use disorder. I was honored to present at this symposium that captured some of the innovative research supported by NIAAA over the years. It is especially personally meaningful as the discoveries presented here would not have been possible without the financial and intellectual support provided by NIAAA and its dedicated staff to my work and lab over the past 25 years. It has been a real privilege to receive this support from NIAAA to conduct this work and to have this opportunity to share the research findings at this important symposium.
Correspondence
Address correspondence concerning this article to Rajita Sinha, Yale University School of Medicine, New Haven, CT 06519. Email: [email protected]
Disclosures
The author declares no competing financial or nonfinancial interests.
Publisher's note
This article was based on a presentation at the NIAAA 50th Anniversary Science Symposium, “Alcohol Across the Lifespan: 50 Years of Evidence-Based Diagnosis, Prevention, and Treatment Research,” held on November 30–December 1, 2020. Links to the videocast are available on the NIAAA 50th Anniversary Science Symposium agenda webpage.
Opinions expressed in contributed articles do not necessarily reflect the views of NIAAA, National Institutes of Health. The U.S. government does not endorse or favor any specific commercial product or commodity. Any trade or proprietary names appearing in Alcohol Research: Current Reviews are used only because they are considered essential in the context of the studies reported herein.
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl). 2001;158(4):343-359. https://doi.org/10.1007/s002130100917 .
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology . 2001;24(2):97-129. https://doi.org/10.1016/S0893-133X(00)00195-0 .
- National Institute on Alcohol Abuse and Alcoholism. Alcohol and Your Health: Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking .
- Grossman ER, Benjamin-Neelon SE, Sonnenschein S. Alcohol consumption and alcohol home delivery laws during the COVID-19 pandemic. Subst Abus . 2022;43(1):1139-1144. https://doi.org/10.1080/08897077.2022.2060432 .
- Sohi I, Chrystoja BR, Rehm J, et al. Changes in alcohol use during the COVID-19 pandemic and previous pandemics: A systematic review. Alcohol Clin Exp Res. 2022;46(4):498-513. https://doi.org/10.1111/acer.14792 .
- Wemm SE, Sinha R. Drug-induced stress responses and addiction risk and relapse. Neurobiol Stress . 2019;10:100148. https://doi.org/10.1016/j.ynstr.2019.100148 .
- Tasnim S, Tang C, Musini VM, Wright JM. Effect of alcohol on blood pressure. Cochrane Database Syst Rev . 2020;7(7):CD012787. https://doi.org/10.1002/14651858.CD012787.pub2 .
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha, R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol. 2019;24(5):1096-1108. https://doi.org/10.1111/adb.12665 .
- Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology . 2017;122:136-147. https://doi.org/10.1016/j.neuropharm.2017.01.037 .
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage . 2011;55(1):371-380. https://doi.org/10.1016/j.neuroimage.2010.11.062 .
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci . 2008;28(18):4583-4591. https://doi.org/10.1523/JNEUROSCI.0086-08.2008 .
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology . 2010;35(1):217-238. Erratum in: 2010;35(4):1051. https://doi.org/10.1038/npp.2009.110 .
- Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7-21. https://doi.org/10.31887/DCNS.2016.18.1/shaber .
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology . 2012;37(2):467-477. https://doi.org/10.1038/npp.2011.206 .
- Goldfarb EV, Scheinost D, Fogelman N, Seo D, Sinha R. High-risk drinkers engage distinct stress-predictive brain networks. Biol Psychiatry Cogn Neurosci Neuroimaging . 2022;S2451-9022(22)00049-0. https://doi.org/10.1016/j.bpsc.2022.02.010 .
- Kwako LE, Koob GF. Neuroclinical framework for the role of stress in addiction. Chronic Stress (Thousand Oaks) . 2017;1:2470547017698140. https://doi.org/10.1038/npp.2011.206 .
- Wemm SE, Tennen H, Sinha R, Seo D. Daily stress predicts later drinking initiation via craving in heavier social drinkers: A prospective in-field daily diary study. J Psychopathol Clin Sci . 2022; advance online publication. https://doi.org/10.1037/abn0000771 .
- Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A . 2016;113(31):8837-8842. https://doi.org/10.1073/pnas.1600965113 .
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci . 2008;1141:105-130. https://doi.org/10.1196/annals.1441.030 .
- Cahill L. Equal ≠ the same: Sex differences in the human brain. Cerebrum. 2014;2014:5.
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci . 2010;30(2):431-438. https://doi.org/10.1523/JNEUROSCI.3021-09.2010 .
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: A voxel-based morphometric study. Addict Biol. 2013;18(1):147-160. https://doi.org/10.1111/adb.12008 .
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biol Psychol . 2005;69(1):113-132. https://doi.org/10.1016/j.biopsycho.2004.11.009 .
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2011;32(11):1998-2013. https://doi.org/10.1002/hbm.21165 .
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: An assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res . 2008;32(7):1242-1250. https://doi.org/10.1111/j.1530-0277.2008.00679.x .
- Guinle MIB, Sinha R. The role of stress, trauma, and negative affect in alcohol misuse and alcohol use disorder in women. Alcohol Res . 2020;40(2):05. https://doi.org/10.35946/arcr.v40.2.05 .
- Holsen LM, Lancaster K, Klibanski A, et al. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience . 2013;250:733-742. https://doi.org/10.1016/j.neuroscience.2013.07.042 .
- Goldfarb E, Seo D, Sinha R. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress. 2019;11:100177. https://doi.org/10.1016/j.ynstr.2019.100177 .
- Lannoy S, Duka T, Carbia C, et al. Emotional processes in binge drinking: A systematic review and perspective. Clin Psychol Rev . 2021;84:101971. https://doi.org/10.1016/j.cpr.2021.101971 .
- Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry . 2017;74(9):911-923. https://doi.org/10.1001/jamapsychiatry.2017.2161 .
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate and insula regions. Biol Psychiatry . 2012;72(1):57-64. https://doi.org/10.1016/j.biopsych.2011.11.022 .
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: Association with health symptoms. Neuropsychopharmacology . 2014;39(3):670-680. https://doi.org/10.1038/npp.2013.250 .
- Seo D, Rabinowitz A, Douglas R, Sinha R. Limbic response to stress linking life trauma and hypothalamus-pituitary-adrenal axis function. Psychoneuroendocrinology . 2019;99:38-46. https://doi.org/ 10.1016/j.psyneuen.2018.08.023 .
- Hermes G, Fogelman N, Seo D, Sinha R. Differential effects of recent versus past traumas on mood, social support, binge drinking, emotional eating and BMI, and on neural responses to acute stress. Stress . 2021;24(6):686-695. https://doi.org/10.1080/10253890.2021.1877271 .
- Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol . 2013;23(4):649-654. https://doi.org/10.1016/j.conb.2013.05.001 .
- Sinha, R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep . 2011;13(5):398-405. https://doi.org/10.1007/s11920-011-0224-0 .
- Milivojevic V, Sinha R. Central and peripheral biomarkers of stress response for addiction risk and relapse vulnerability. Trends Mol Med . 2018;24(2):173-186. https://doi.org/10.1016/j.molmed.2017.12.010 .
- Fox HC, Hong KA, Siedlarz KM, et al. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol . 2009;44(6):575-585. https://doi.org/10.1093/alcalc/agp060 .
- Rando K, Hong KA, Bhagwagar Z, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: A prospective study. Am J Psychiatry . 2011;168(2):183-192. https://doi.org/10.1176/appi.ajp.2010.10020233 .
- Seo D, Lacadie CM, Tuit K, Hong K, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry . 2013;70(7):727-739. https://doi.org/10.1001/jamapsychiatry.2013.762 .
- Blaine SK, Wemm S, Fogelman N, et al. Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am J Psychiatry . 2020;177(11):1048-1059. https://doi.org/10.1176/appi.ajp.2020.19070703 .
- Martins JS, Fogelman N, Wemm S, Hwang S, Sinha R. Alcohol craving and withdrawal at treatment entry prospectively predict alcohol use outcomes during outpatient treatment. Drug Alcohol Depend. 2022;231:109253. https://doi.org/10.1016/j.drugalcdep.2021.109253 .
- Wemm SE, Larkin C, Hermes G, Tennen H, Sinha R. A day-by-day prospective analysis of stress, craving and risk of next day alcohol intake during alcohol use disorder treatment. Drug Alcohol Depend . 2019;204:107569. https://doi.org/10.1016/j.drugalcdep.2019.107569 .
- Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry , 2014;71(8):917-925. https://doi.org/10.1001/jamapsychiatry.2014.680 .
- Koob GF. Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev . 2021;73(1):163-201. https://doi.org/10.1124/pharmrev.120.000083 .
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci . 2009;10(6):410-422. https://doi.org/10.1038/nrn2648 .
- Fox HC, Seo D, Tuit K, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: Preliminary findings. J Psychopharmacol . 2012;26(7):958-972. https://doi.org/10.1177/0269881111430746 .
- McKee SA, Potenza MN, Kober H, et al. A translational investigation targeting stress reactivity and prefrontal cognitive control for smoking cessation. J Psychopharmacol . 2015;29(3):300-311. https://doi.org/10.1177/0269881114562091 .
- Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39(6):1527-1537. https://doi.org/10.1038/npp.2014.1 .
- Milivojevic V, Fox HC, Jayaram-Lindstrom N, Hermes G, Sinha R. Sex differences in guanfacine effects on stress-induced Stroop performance in cocaine dependence . Drug Alcohol Depend. 2017;179:275-279. https://doi.org/10.1016/j.drugalcdep.2017.07.017 .
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. α 1 -noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol . 2008;42(2):91-97. https://doi.org/10.1016/j.alcohol.2007.12.002 .
- Fox, HC, Anderson GM, Tuit K, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcohol Clin Exp Res . 2012;36(2):351-360. https://doi.org/10.1111/j.1530-0277.2011.01628.x .
- Milivojevic V, Angarita GA, Hermes G, Sinha R, Fox HC. Effects of prazosin on provoked alcohol craving and autonomic and neuroendocrine response to stress in alcohol use disorder. Alcohol Clin Exp Res. 2020;44(7):1488-1496. https://doi.org/10.1111/acer.14378 .
- Sinha R, Wemm S, Fogelman N, et al. Moderation of prazosin’s efficacy by alcohol withdrawal symptoms. Am J Psychiatry . 2021;178(5):447-458. https://doi.org/10.1176/appi.ajp.2020.20050609 .
- Sinha R, Fogelman N, Wemm S, Angarita G, Seo D, Hermes G. Alcohol withdrawal symptoms predict corticostriatal dysfunction that is reversed by prazosin treatment in alcohol use disorder. Addict Biol. 2022;27(2):e13116. https://doi.org/10.1111/adb.13116 .
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Trusted Health Information from the National Institutes of Health
Why alcohol-use research is more important than ever
Nih's george koob talks about how addiction changes the brain and the rise in alcohol-related deaths.

Alcohol use disorder is a common but serious condition that affects how the brain functions.

George Koob, Ph.D.
Alcohol use disorder (AUD) affects roughly 15 million people in the U.S. People with the condition may drink in ways that are compulsive and uncontrollable, leading to serious health issues.
"It's the addiction that everyone knows about, but no one wants to talk about," says George Koob, Ph.D., the director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
As NIAAA celebrates an important milestone this year—its 50th anniversary—the institute's research is more important than ever. Like NIAAA reported earlier this year, alcohol-related health complications and deaths as a result of short-term and long-term alcohol misuse are rising in the U.S.
"Alcohol-related harms are increasing at multiple levels—from emergency department visits and hospitalizations to deaths," Dr. Koob says. He spoke about NIAAA efforts that are working to address this and how people can get help.
What has your own research focused on?
I started my career researching the science of emotion: how the brain processes things like reward and stress. Later, I translated this to alcohol and drug addiction and investigating why some people go from use to misuse to addiction, while others do not.
What are some major breakthroughs NIAAA has made in this area?
We now understand how alcohol affects the brain and why it causes symptoms of AUD . This has far-reaching implications for everything from prevention to treatment. We also understand today that AUD physically changes the brain. This has been critical in treating it as a mental disorder, like you would treat major depressive disorder.
Other breakthroughs have been made in screening and intervention, and in the medications available for treatment. All of this has led to a better understanding of how the body changes when one misuses alcohol and the proactive actions we can take to prevent alcohol misuse.
What is a misconception that people have about AUD?
Many people don't realize how common AUD is. There are seven times more people affected by AUD than opioid use disorder, for example. It doesn't discriminate against who it affects. People also don't realize that AUD is a brain disorder that actually changes how the brain functions. Severe AUD is associated with widespread injury to the brain, though some of the effects might be partially reversible.
What's next for NIAAA?
For five decades, the institute has studied how alcohol affects our health, bringing greater awareness to alcohol-related health issues and providing better options for diagnosis and treatment. Recent research has focused on areas such as the genetics of addiction, links between excessive alcohol use and mental health and other disorders, harm to long-term brain health that can be caused by adolescent alcohol use, and the effects of prenatal alcohol exposure, among others.
"We want everyone from pharmacists and nurses to addiction medicine specialists to know more about alcohol and addiction." - George Koob, Ph.D.
Currently, we are working on a number of initiatives. One is education. We want everyone from pharmacists and nurses to addiction medicine specialists to know more about alcohol and addiction. We're also working on prevention resources for middle school-aged adolescents. Other goals include understanding recovery and what treatments work best for people and why. We're also learning more about alcohol's effects on sleep and pain, and we have ongoing efforts in medication development.
Finally, we're learning more about the impact of alcohol on women and older adults. Women have begun to catch up to men in alcohol consumption and alcohol-related harms. Women are more susceptible to some of the negative effects that alcohol has on the body, from liver disease to certain cancers. Further, more older adults are binge drinking and this places them at greater risk of alcohol-medication interactions, falls, and health problems related to alcohol misuse.
How can someone get help?
If alcohol is negatively affecting you or someone you know, seek help from someone you respect. For example, a primary care doctor or clergy member. There are a number of online resources from NIAAA, like the NIAAA Alcohol Treatment Navigator® , an online resource to help people understand AUD treatment options and search for professionally led, evidence-based alcohol treatment nearby. There's also Rethinking Drinking SM , an interactive website to help individuals assess and change their drinking habits. Also, know that there is hope. Many people recover from AUD and lead vibrant lives.
July 16, 2020
You May Also Like

4 discoveries beyond the brain
Neurodegenerative diseases—such as Alzheimer’s disease, Parkinson’s disease (PD), Lewy body dementia (LBD), and amyotrophic lateral sclerosis (also known as ALS...

Meet Walter J. Koroshetz, M.D., Director of the National Institute of Neurological Disorders and Stroke
Dr. Walter J. Koroshetz has been fascinated by the brain and how it works from a young age. This curiosity,...

Living with Tourette syndrome is nothing to be embarrassed about
Kelsey Christensen went from a shy child who avoided the spotlight to a successful television journalist. Residents of the Twin...
Trusted health information delivered to your inbox
Enter your email below

Drinking alcohol before conceiving a child could accelerate their aging – new research in mice
Professor of Physiology, Texas A&M University
Disclosure statement
Michael Golding receives funding through a Medical Research Grant from the W. M. Keck Foundation and a research grant from the NIH through the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Texas A&M University provides funding as a founding partner of The Conversation US.
View all partners
The conditions within a person’s home, family and community affect their ability to stay healthy. Scientists studying these social determinants of health are trying to understand whether nature or nurture has a stronger effect on a person’s ability to fight disease.
I am a developmental physiologist studying the ways that drinking affects fetal development and lifelong health. Although researchers have long recognized that a father’s alcohol abuse negatively affects his children’s mental health and social development , it hasn’t been clear if paternal drinking has any lasting biological effects on his offspring’s physical health.
My lab’s recently published research shows that chronic alcohol use from both parents has an enduring effect on the next generation by causing their offspring to age faster and become more susceptible to disease.
Fetal alcohol spectrum disorders
According to the National Institutes of Health, nearly 11% of adults in the U.S. have an alcohol use disorder. Heavy drinking causes multiple health issues , including liver disease, heart problems, declining cognitive function and accelerated aging .
Parents may pass these health problems on to their children. Fetal alcohol spectrum disorders refer to a wide range of alcohol-related physical, developmental and behavioral deficits that affect as many as 1 in 20 U.S. schoolchildren .

Children with fetal alcohol spectrum disorders experience an early onset of adult diseases , including type 2 diabetes and heart disease. Cardiovascular disease first appears during adolescence for people with these disorders, while the rest of the population is affected typically in their 40s and 50s. Children with fetal alcohol spectrum disorders are also more likely to be hospitalized and have lifespans that are 40% shorter than children without these conditions.
However, it has been unclear whether these health problems are because of life circumstances – people with fetal alcohol spectrum disorders have high rates of psychiatric disorders , which cause stress that makes them more susceptible to aging and disease – or if their parents’ substance use directly causes lasting negative effects to their health. In other words, can a parent’s alcohol abuse before conception directly influence their offspring’s physical health and lifespan?
Mom and dad drinking
In our study, my colleagues and I used a mouse model to measure the effects that alcohol use by mom, dad or both parents around the time of conception have on their offspring aging and chronic disease. The mice chose when and how much alcohol to drink.
We found that paternal and maternal drinking both cause harmful changes to their offspring’s mitochondria . Mitochondria – often called the battery of the cell – control many aspects of aging and health . Like a cellphone battery, mitochondria deteriorate over time and cause cells to lose their ability to repair damage and control metabolism.
Our experiments in mice show that dad’s drinking causes a defect in mitochondrial function that first emerges during fetal development and persists into adult life , causing the offspring to age faster. For example, paternal alcohol exposure caused a twofold increase in age-related liver disease, suggesting that parental alcohol use – particularly by the father – could have significant implications on aging and age-related diseases.
Importantly, we found that when both parents drank, the effects on their offspring were worse than when only one parent consumed alcohol. For example, we observed a threefold increase in age-related liver scarring when both parents consumed alcohol.
Treating fetal alcohol syndrome
People with fetal alcohol syndrome face lifelong challenges , including problems with hand-eye coordination and difficulties with memory and attention.
Early educational interventions for children with fetal alcohol spectrum disorders, like using visual and auditory materials instead of print, can provide additional structure to help facilitate learning.
Although my team and I examined chronic alcohol exposure, we do not know if moderate alcohol use also causes mitochondrial problems. We also don’t know if these same effects emerge in people who haven’t been diagnosed with fetal alcohol spectrum disorders but whose parents drank heavily. Whether paternal drinking influences human embryonic development is still unclear, although emerging studies are beginning to suggest it does.
The next step is to explore if interventions that focus on mitochondrial health, such as exercise and specific diets , can improve health outcomes for people with fetal alcohol spectrum disorders.
- Fetal development
- Alcohol abuse
- Fetal Alcohol Spectrum Disorder
- Fetal Alcohol Syndrome
- New research
- STEEHM new research

Director of STEM

Community member - Training Delivery and Development Committee (Volunteer part-time)

Chief Executive Officer

Finance Business Partner

Head of Evidence to Action
- Search Menu
- Sign in through your institution
- Author Guidelines
- Submission Site
- Open Access
- About Alcohol and Alcoholism
- About the Medical Council on Alcohol
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the MCA
- Journals on Oxford Academic
- Books on Oxford Academic
Advance articles
Email alerts.
- Recommend to your Library
Affiliations
- Online ISSN 1464-3502
- Copyright © 2024 Medical Council on Alcohol and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Alcohol Res Health
- v.33(4); 2011

Advances in Alcoholism Treatment
Researchers are working on numerous and varied approaches to improving the accessibility, quality, effectiveness, and cost-effectiveness of treatment for alcohol use disorders (AUDs). This overview article summarizes the approaches reviewed in this issue, including potential future developments for alcoholism treatment, such as medications development, behavioral therapy, advances in technology that are being used to improve treatment, integrated care of patients with AUDs and co-occurring disorders, the role of 12-step programs in the broader realm of treatment, treating patients with recurring and chronic alcohol dependence, strategies to close the gap between treatment need and treatment utilization, and how changes in the health care system may affect the delivery of treatment. This research will not only reveal new medications and behavioral therapies but also will contribute to new ways of approaching current treatment problems.
Alcoholism treatment, as it exists today, rests on decades of research exploring the most effective ways to help people reduce their alcohol use or to stop drinking. That research has paved the way for the development and application of new methods and therapies and will continue to influence treatment practice in the future.
This article reviews the origins of alcoholism treatment and major studies of behavioral therapies and medications for treating alcohol dependence. It then provides a preview of the topics covered in this issue, including the potential future developments for alcoholism treatment such as medications development, behavioral therapy, advances in technology that are being used to improve treatment, integrated care of patients with alcohol use disorders (AUDs) and co-occurring disorders, the role of 12-step programs in the broader realm of treatment, treating patients with recurring and chronic alcohol dependence, strategies to close the gap between treatment need and treatment utilization, and how changes in the health care system may affect the delivery of treatment.
Origins of Alcoholism Treatment
Alcoholics Anonymous (AA) was founded by Bill Wilson and Bob Smith in Akron, Ohio, in 1935. AA’s program of spiritual and character development, the 12 Steps, is based on the premise that turning one’s life and will over to a personally meaningful “higher power,” is the key to recovery. Another essential idea is that sobriety or recovery depends on the admission of powerlessness with respect to alcohol or other substances of abuse.
The Minnesota Model of addiction treatment was created in a State mental hospital in the 1950s. It was first practiced in a small nonprofit organization called the Hazelden Foundation In this approach, professional and trained nonprofessional (recovering) staff cooperated in applying the principles of AA. The model called for an individualized treatment plan with active family involvement in a 28-day inpatient setting and participation in AA both during and after treatment. Throughout the 1950s, Hazelden took the stance that (1) alcoholism is a disease and not a symptom of an underlying disorder and that it should be treated as a primary condition and (2) alcoholism affects people physically, mentally, and spiritually and that treatment for alcoholism should take all three aspects into account.
Around the same time that AA and Hazelden treatment methods were being refined and popularized, the study of alcohol abuse and alcoholism was expanding. Alcohol research, including the study of alcoholism treatment, found a home at the National Institutes of Health in 1970, when the National Institute on Alcohol Abuse and Alcoholism (NIAAA) was founded.

Scope of the Problem
AUDs are prevalent in the United States and often go untreated. NIAAA’s National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a large general-population survey conducted in 2001–2002, estimated the prevalence of alcohol abuse and dependence at 4.65 percent and 3.81 percent, respectively ( Grant et al. 2004 ).
Using NESARC results, Cohen and colleagues (2007) reported that only 14.6 percent of those with a lifetime history of alcohol abuse or dependence have received treatment. In another study that used NESARC results, Dawson and colleagues (2005) reported on people who experienced the onset of alcohol dependence at some point before the year prior to the survey. In this group, 25 percent still were alcohol dependent, 27.3 percent were in partial remission, 11.8 percent were in full remission but drinking at levels or patterns that put them at high risk for relapse, 17.7 percent were low-risk drinkers, and 18.2 percent were abstainers during the year prior to the survey.
Only 25.5 percent of these respondents reported ever receiving treatment. Among them, 3.1 percent participated in 12-step programs, 5.4 percent received formal treatment only, and the remaining 17 percent participated in both 12-step and formal treatment programs ( Dawson et al. 2006 ).
Findings from this survey show that there is a wide range of recovery from alcohol dependence in the general population, from partial remission to full abstinence. The track of this disease is not clear cut—some people appear to recover from alcoholism without formal treatment. Others may cycle into and out of dependence throughout their lifetime despite repeated attempts to achieve sobriety ( NIAAA 2006 ).
Comparing Treatment Options: Project MATCH and the COMBINE Study
Because no single treatment approach is effective for everyone with alcohol dependence, clinicians and researchers proposed that assigning patients to treatment based on specific needs and characteristics would improve outcomes. NIAAA initiated Project MATCH in 1989 to test this theory. Patients—who were characterized according to factors such as severity of alcohol involvement, cognitive impairment, psychiatric severity, gender, motivational readiness to change, and social support for drinking versus abstinence—were randomly assigned to 12-step facilitation, cognitive–behavioral therapy, or motivational enhancement therapy. Patients were followed at 3-month intervals for 1 year after completion of the 12-week treatment period and were evaluated for changes in drinking patterns, functional status/quality of life, and treatment services utilization. The study found that patients with low psychiatric severity were best suited to 12-step facilitation therapy. These patients had more abstinent days than those treated with cognitive–behavioral therapy. Overall, Project MATCH participants showed significant improvement in percentage of abstinent days and decreased number of drinks per drinking days, with few significant outcome differences among the three treatment groups ( Project MATCH Research Group 1997 ).
Following Project MATCH, the next step for evaluating treatment options was a large-scale study of medications for alcohol dependence. Combining Medications and Behavioral Interventions for Alcoholism, or the COMBINE Study, evaluated the efficacy of naltrexone and acamprosate, both alone and in combination, with medical management (i.e., patients had brief sessions with a health care professional) with and without behavioral therapy. The behavioral treatment integrated aspects of cognitive–behavioral therapy, motivational interviewing, and 12-step facilitation. Patients who received naltrexone, behavioral therapy, or both demonstrated the best drinking outcomes after 16 weeks of treatment. Acamprosate showed no evidence of efficacy, with or without behavioral therapy ( Anton et al. 2006 ).
In addition to naltrexone (and an injectable, long-acting form of naltrexone) and acamprosate, disulfiram (Antabuse ® ) also is approved to treat alcohol dependence. Naltrexone helps to reduce the craving for alcohol after someone has stopped drinking. Acamprosate is thought to work by reducing symptoms that follow lengthy abstinence, such as anxiety and insomnia. Disulfiram discourages drinking by making the patient feel sick after drinking alcohol. Other types of drugs are available to help manage symptoms of withdrawal.
As shown in COMBINE, no single medication or treatment strategy is effective in every case or in every person. As research exploring the neuroscience of alcoholism continues to pave the way for new medications, studies also have sought to better understand why some behavioral interventions are more effective than others. The articles to follow in this special issue examine a broad range of topics relevant to developing and applying new treatment tools and methods.
Medications Management
With the high prevalence of AUDs in the United States and low rates of treatment seeking, the value of identifying and treating alcohol problems in primary care settings is well known. As Stephanie O’Malley, Ph.D., and Patrick G. O’Connor, M.D., M.P.H., report in their article to follow (pp. 300–312 ), it is important to have effective approaches available to assist patients identified in primary care. Multiple studies have supported the efficacy of brief-intervention counseling in primary care settings. Research also supports the use of medications in primary care and suggests that, with counseling, this approach to treating alcohol problems is cost-effective and facilitates patients receiving continuing care. In addition to the four medications currently approved for treating alcohol dependence, efforts are underway to identify new medications that may be more effective. Other medications with some clinical evidence of efficacy include topiramate (an antiseizure medication); selective serotonin reuptake inhibitors (approved for depression); ondansetron (a serotonin receptor antagonist approved for nausea); baclofen (a γ-aminobutyric acid-b receptor agonist used for muscle spasticity), and atypical neuroleptics such as aripiprozole and quetiapine. Nicotinic compounds, including agonists, partial agonists, and antagonists, currently are under investigation. In addition, researchers are evaluating the therapeutic potential of corticotrophin-releasing factor antagonists and neurokinin 1 antagonists, which may address the relationship between stress and alcohol consumption.
Behavioral Therapy
All behavioral approaches to the treatment of AUDs combine general behavioral principles (e.g., reinforcement and punishment) with therapeutic techniques designed to facilitate healthy behavior change. Coping skills training, cognitive–behavioral treatment, brief interventions, and relapse prevention also introduce concepts from cognitive therapy and social-learning theory. For example, the cognitive concept of self-efficacy, or belief in one’s ability to abstain from alcohol, plays a prominent role in both cognitive–behavioral treatments and relapse prevention. Likewise, a person’s expectations regarding the effects of alcohol (i.e., expectancies) often are identified and challenged during the course of cognitive–behavioral interventions. Coping skills training and relapse prevention primarily focus on identifying high-risk situations for drinking and then building a repertoire of coping skills to help patients approach risky situations without using alcohol. Brief interventions also utilize many cognitive– behavioral tools; however, in these cases, treatment occurs over a short period of time (often an hour or less).
In their article on behavioral therapy, Katie Witkiewitz, Ph.D., and G. Alan Marlatt, Ph.D. (pp. 313–319 ) describe and report on the efficacy of interventions including contingency management; couples, marital, and family therapy; facilitated self-change; and brief intervention. All of these treatments can be delivered in individual sessions or group formats, and many of them have been adapted to be delivered in a variety of treatment settings, including residential, outpatient, computerized, medical, and workplace settings. New methods of delivery and successful adjuncts to existing behavioral treatments also have been developed, including computerized cognitive–behavioral treatments, Web-based, guided, self-change, and mindfulness-based approaches. Choosing the most appropriate treatment for a given patient remains a challenge. Although research in this area has previously focused on comparing the effectiveness of different therapies, it is important for future research to also consider how people change as well as the mechanisms of change at work during the course of behavioral treatments.
The Use of Emerging Technologies in Alcohol Treatment
The delivery of alcohol treatment, whether that treatment is medication, behavioral therapy, or a combination of both, can be facilitated by the use of communication tools such as the telephone, e-mail, and the Internet. These tools also can be used to identify people with alcohol problems. In their article, John A. Cunningham, Ph.D., Kypros Kypri, Ph.D., and Jim McCambridge, Ph.D. (pp. 320–326 ), describe the growing use of emerging technologies or electronic tools used to provide services to help problem drinkers. Among the applications being used are Internet- and computer program–based screening instruments (e.g., www.AlcoholScreening.org ), online social support groups, Internet-based interventions, telephone contact, e-mail, and text messaging.
Emerging technologies can be used in primary care, the emergency department, prenatal care settings, college settings, and traditional addiction-treatment settings. Research is needed to demonstrate efficacy, to explore how to use these tools most effectively, and how to integrate them into traditional treatment modalities.
In a sidebar to the topic of emerging technologies, David Gustafson, Ph.D., and colleagues (pp. 327–337 ) describes a cell phone–based support system to be given to patients as they leave residential treatment. The technology, called A-CHESS (Addiction Comprehensive Health Enhancement Support System), is designed to provide coping competence, social support, and autonomous motivation. A-CHESS contains a proactive computer-based relapse prevention system, data transfer from A-CHESS to a care manager’s computer, vehicles for the patient to maintain contact with his/her care manager, audio/visual delivery of content to provide access to those with reading difficulties, and anywhere/anytime access through a smartphone. The researchers hypothesize that A-CHESS will reduce days of risky drinking by reducing negative affect, which will be mediated by social support, autonomous motivation, and improved coping strategies.
Integrating Care for People With Co-Occurring Alcohol and Other Drug, Medical, and Mental Health Conditions
Treatment support and management is especially important for people with AUDs and co-occurring disorders (CODs) such as mental health and medical problems. Stacy Sterling, M.P.H., M.S.W.; Felicia Chi, M.P.H.; and Agatha Hinman (pp. 338–349 ), state in their article that care for patients’ AUDs, mental health, and medical problems primarily is provided in separate treatment systems and integrated care addressing all of a patient’s CODs in a coordinated fashion is the exception in most settings. A variety of barriers impede further integration of care for patients with CODs. These include differences in education and training of providers in the different fields, organizational factors, existing financing mechanisms, and the stigma still often associated with AUDs and CODs. Many programs are recognizing the disadvantages of separate treatment systems and are attempting to increase integrated approaches. Although few studies have been done in this field, findings suggest that patients receiving integrated treatment may have improved outcomes.
The Role of Mutual-Help Groups in Extending the Framework of Treatment
Despite the advances in pharmacological and behavioral treatment reported throughout this issue, peer-run mutual-help groups (MHGs) such as AA continue to play an important role in helping millions of Americans achieve recovery. Indeed, MHGs are the most commonly sought source of help for alcohol and drug use problems in the United States. In their article, John F. Kelly, Ph.D., and Julie D. Yeterian (pp. 350– 355 ) describe the nature and prevalence of MHGs, particularly AA, and review evidence for their effectiveness, cost-effectiveness, and for the mechanisms through which they may exert their effects. The article also provides details about how health care professionals, including primary care providers, can facilitate their alcohol-dependent patients’ participation in such groups and reviews the evidence for the benefits of doing so. In contrast to professional treatments, people typically have access to MHGs at times when they are at higher risk of relapse, such as evenings and weekends, and many MHGs encourage members to contact each other by phone between meetings whenever help is needed. Consequently, these organizations provide an adaptive community-based system that is highly responsive to changes in relapse risk.
Treating Alcoholism As a Chronic Disease: Approaches to Long-Term Continuing Care
MHGs can be especially valuable for patients with chronic, recurring AUDs involving multiple cycles of treatment, abstinence, and relapse. James R. McKay, Ph.D., and Susanne Hiller-Sturmhöfel, Ph.D. (pp. 356–370 ), describe in their article how efforts to disrupt this cycle can include strategies to reduce the risk of relapse, including initial intensive inpatient or outpatient care based on 12-step principles, followed by continuing care involving MHGs, 12-step group counseling, or individual therapy. Although these programs can be effective, many patients drop out of initial treatment or do not complete continuing care. Thus, researchers and clinicians have begun to develop alternative approaches to enhance treatment retention in both initial and continuing care. One focus of these efforts has been the design of extended treatment models. These approaches increasingly blur the distinction between initial and continuing care and aim to prolong treatment participation by providing a continuum of care. Other researchers have focused on developing alternative treatment strategies (e.g., telephone-based interventions) that go beyond traditional settings.
The Recovery Spectrum: From Self-Change to Seeking Treatment
As reported above, a large percentage of people with AUDs go untreated in the United States. Jalie A. Tucker, Ph.D., M.P.H., and Cathy A. Simpson, Ph.D. (pp. 371–379 ) explain in their article that most people with alcohol problems recognize their situation long before they seek treatment, implying that interventions could be provided earlier. Closing the gap between treatment need and service utilization therefore is a public health priority that depends on understanding relationships between help-seeking and recovery patterns and processes at both the population and individual levels. The authors suggest that a spectrum of services—including screening and brief intervention, guided self-change programs, and e-health options—is needed to match the needs and preferences of the under-treated population.
In a sidebar to this article, Robert J. Meyers, Ph.D., Hendrik G. Roozen, Ph.D., and Jane Ellen Smith, Ph.D. (pp. 380–388 ) describe the community reinforcement approach (CRA), which helps people rearrange their lifestyles so that healthy, drug-free living becomes rewarding and thereby competes with substance use. This approach also encourages people to become progressively involved in alternative non–substance-related social activities and to focus on the enjoyment of work and family activities. A variation of CRA, the community reinforcement and family-training approach, works through friends and family members promoting treatment entry for treatment-resistant individuals.
Health Services and Financing of Treatment
Recognizing the need for treatment and finding an appropriate treatment setting and provider are important steps in the recovery process. Another important factor influencing treatment access is cost and how services are financed and paid for. Since the 1960s, changes in these factors have driven changes in the delivery of treatment. Recent developments, including the passage of Federal parity legislation and health care reform, as well as increasing use of performance contracting, promise to bring additional changes. In their article, Maureen T. Stewart, Ph.D., and Constance M. Horgan, Sc.D. (pp. 389– 394 ) outline the current state of the substance abuse treatment system and highlight implications of these impending changes for access to and quality of treatment services. With the rise of managed care, private insurance coverage has been declining as a share of total treatment expenditures since 1986. People without insurance coverage or with limited insurance coverage for substance abuse treatment can pay out of pocket for services or through publicly funded addiction treatment programs. Performance-based contracts have been implemented to try to improve program accountability and provide incentives for high-quality care by tracking activities that are thought to facilitate positive patient outcomes.
The Federal parity law was designed to remove barriers to utilization, remove financial burdens on patients, and reduce stigma around addictive and mental disorders by requiring group health plans that offer mental health/addiction services to cover these services in a comparable manner to medical/surgical services. This is likely to result in changes to the management of treatment services under private and public insurance, as insurers will have to apply similar processes to medical and behavioral health care. National health care reform will increase insurance access by expanding Medicaid eligibility and mandating individual insurance coverage.
Treatment for AUDs has made significant advances in the last 20 years. Researchers are working on numerous and novel approaches to improving the effectiveness, accessibility, quality, and cost-effectiveness of treatment. Practitioners now have at their disposal a full menu of evidence-based options to treat AUDs. In addition, recent work on the organization and delivery of alcohol services will play an increasingly important role as health care reform unfolds. This domain of alcohol research will not only reveal new medications and behavioral therapies, but will also lay the foundation for the development of exciting and potentially radical new approaches to a longstanding public health problem.
F inancial D isclosure
The authors declare that they have no competing financial interests.
- Anton RF, O’Malley SS, Ciraulo DA, et al. Effect of combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2006; 295 (17):2003–2017. [ PubMed ] [ Google Scholar ]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2007; 86 (2–3):214–221. [ PubMed ] [ Google Scholar ]
- Dawson DA, Grant BF, Stinson FS, et al. Recovery from DSM–IV alcohol dependence: United States, 2001–2002. Addiction. 2005; 100 (3):281–292. [ PubMed ] [ Google Scholar ]
- Dawson DA, Grant BF, Stinson FS, et al. Estimating the effect of help-seeking on achieving recovery from alcohol dependence. Addiction. 2006a; 101 (6):824–834. [ PubMed ] [ Google Scholar ]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Alcohol Alert 70: National Epidemiologic Survey on Alcohol and Related Conditions. Bethesda, MD: 2006. [ Google Scholar ]
- Grant BF, Dawson DA, Stinson FS, et al. The 12-month prevalence and trends in DSM–IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004; 74 (3):223–234. [ PubMed ] [ Google Scholar ]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997; 58 (1):7–29. [ PubMed ] [ Google Scholar ]
Exploring what works, what doesn’t, and why.

As drinking habits shift, global alcohol industry fights to stay ahead
- Share on LinkedIn
- Share on Twitter
- Share on Facebook
- Share on WhatsApp
- Share through Email
This story was reported by The Examination , a nonprofit newsroom that investigates global health threats. Sign up to get The Examination ’s newsletter in your inbox.
For a growing number of American and European youth, drinking simply isn’t as cool as it once was.
Young people—increasingly aware of the myriad physical and mental health issues linked to alcohol—are shunning drinking in favor of healthier lifestyle choices. The rise of mocktails , nonalcoholic beer, and legal weed offer plenty of alternatives.
That’s encouraging for public health experts, since alcohol is one of the world’s most significant preventable causes of death: Excess drinking kills 2.6 million people each year, according to a status report the World Health Organization published in June , accounting for one in every 21 deaths worldwide.
But the alcohol industry is doing everything it can to stay relevant. In pursuit of new profit centers, booze, beer, and wine makers are marketing to demographics they’ve historically underserved, including women, and looking to new markets in the Global South, where burgeoning economies with young populations offer growth opportunities. Conveniently, many of these countries also place few or no limitations on how alcohol is sold and marketed.
Sign up for Harvard Public Health
Delivered to your inbox weekly.
- Email address By clicking “Subscribe,” you agree to receive email communications from Harvard Public Health.
- Name This field is for validation purposes and should be left unchanged.
During an investor meeting last year, a representative from Heineken, the Dutch brewer, said the company’s fastest-growing markets were in Asia Pacific and Africa. A slide deck titled “Africa drives global beer growth” showed the volume of Heineken sales on the continent had quadrupled in the last 20 years.
“Africa is the next frontier of growth,” Roland Pirmez, president of Heineken’s Africa, Middle East, and Eastern European region, said in a slide deck from the presentation, citing the continent’s growing population, rapid urbanization, and increasing prosperity.
Jürgen Rehm, a professor at the University of Toronto and senior scientist at the Centre for Addiction and Mental Health in Canada, said that industry marketing had led people in Asia and Africa to begin drinking at younger ages than prior generations who had grown up with different norms and less discretionary income.
“The overall concern is that alcohol consumption is normalized globally and that a majority of the world’s adults may be drinkers 10 years from now,” Rehm wrote in an email. This result will have “serious consequences” for alcohol-related disease and economic prosperity, he said.
Heineken did not respond to questions from The Examination about these concerns.
Changing demographics
The harms of excessive drinking have been well known for centuries, but science measuring the risks is becoming more precise. In the last decade, numerous studies have suggested that even “moderate” levels of consumption—as little as two drinks per day for men and one for women—increase the risk of heart disease and cancer. That knowledge has begun to sink in. In the West, drinking is no longer an unquestioned rite of passage for young people: European and American adolescents are drinking less compared with a generation ago. A study of drinking habits in 28 European countries found that weekly use among adolescents peaked in 2007 and has since declined.
Boys, in particular, who historically drank more than girls, are cutting back. A recent WHO survey from Europe, Central Asia, and Canada found that girls, for the first time, were more likely than boys to have tried alcohol by age 15.
Adults are also picking up the slack. In the U.S., millennials are consuming more alcohol than previous generations in their 30s and 40s. The uptick has been particularly pronounced among women, especially those with a college education .
Katherine Keyes, a professor of epidemiology at Columbia University, published a review of several studies and found binge drinking and alcohol-related hospitalizations have been rising fastest among U.S. women 26 to 35 and 45 to 64.
“What we’ve seen is this shift where drinking declines during adolescence but then the acceleration of drinking during the transition to adulthood is getting faster,” Keyes said.
The COVID-19 pandemic exacerbated dangerous drinking habits. Between 2020 and 2021, women aged 40 to 64 experienced significant increases in urgent complications from alcoholic liver disease, according to an analysis of insurance claims data published in the Journal of the American Medical Association .
From a man’s drink to “mommy juice”
This shift in drinking behavior didn’t occur by chance. Alcohol companies that once alienated women with ads suffused with sexist imagery are now marketing directly and more effectively to women, according to a 2020 report by investment research and brokerage firm Bernstein Research.
Diageo, one of the world’s largest spirit conglomerates, regularly participates in the United Nation’s annual International Women’s Day, and has wrapped itself in its messaging . Recent advertising campaigns by 20 alcohol makers frame drinking as post-feminist —adopting language like “girl power” and “strong women” while also reinforcing gender norms by dousing their campaigns in pink—and as a way for mothers to destress and briefly escape the burdens of parenting. In one social media campaign by Bailey’s Nigeria, the company celebrated Mother’s Day by asking drinkers to comment on “why your mum deserves a Baileys Treat from us.”
Companies are also pivoting to products that appeal to women. Beer makers, which historically struggled to attract female consumers, have eagerly invested in ready-to-drink beverages like hard seltzers—a product category that appeals equally to both genders . Soda companies also have leaped into the fray , producing alcoholic offerings of their own.
Lawmakers attempting to rein in excess drinking are up against a tsunami of spending. Alcohol makers spend billions to market their products. AB InBev—which produces Budweiser and Corona, among hundreds of other labels—spent $6.8 billion on advertising in 2022, according to Marketing Week .
Unlike tobacco companies, which since the 1998 Tobacco Master Settlement Agreement have been prohibited from exempting the money they spend on advertising from their U.S. taxes, the alcohol industry is under no such restrictions. That meant the 10 largest alcohol producers could write off taxes on $1.5 billion in advertising expenses in 2017, according to the public health advocacy organization Vital Strategies . Whereas the majority of developed countries restrict beer advertising on television and radio, most of the countries with no advertising restrictions are in Africa and Central and South America, according to the WHO. A third of countries have a total or partial ban on beer company sponsorships at sporting events, but in many developing economies there are no restrictions.
Raising taxes
Economists and scientists say one of the most effective ways to curb excess drinking is to raise taxes on alcohol. Taxes are “best buy” interventions for reducing noncommunicable diseases , according to the WHO, as they are highly cost-effective and relatively easy to implement.
Taxes have been crucial to curbing drinking rates in Eastern Europe—a region once home to some of the world’s heaviest drinkers. Since 2002, former Soviet states Estonia, Latvia, and Lithuania have raised taxes on alcohol and seen alcohol-attributable deaths fall . Lithuania later implemented additional excise tax increases and saw an extra three percent decline in mortality by any cause.
Countries have experimented with other pricing policies, too. In Russia, after the government set a minimum price per unit for spirits, alcohol consumption per capita fell by 26 percent from 2010 to 2016. A similar policy In Scotland reduced alcohol sales by three percent and cut hospital admissions and deaths from chronic diseases, according to an independent review.
Although global alcohol companies like Diageo preach “the importance of moderation,” this shifts the responsibility for excess alcohol use onto the drinker. Meanwhile, companies fiercely oppose measures shown to reduce excess drinking, such as raising alcohol taxes. Diageo has lobbied to cut taxes on its products in Mexico; Anheuser-Busch and other alcohol businesses have largely funded efforts to kill alcohol tax bills in New Mexico and Oregon .
Recent advertising campaigns by 20 alcohol makers frame drinking as post-feminist—adopting language like “girl power” and “strong women.”
Politicians have listened. In the U.S., state alcohol taxes have fallen more than 30 percent in real terms since 1990 . In 2020, a bipartisan group of federal lawmakers awarded permanent tax cuts to many alcohol sellers. As of August 1, the alcohol industry had 280 registered lobbyists in Washington, D.C.
Asked for comment, a Diageo spokesperson responded, “We are committed to changing the way people drink for the better,” citing the company’s educational website DrinkIQ and its growing portfolio of nonalcoholic beverages. “The right information empowers consumers to make the right choices.”
Global health policymakers have not made combatting excessive drinking a priority. Governments and philanthropic organizations spend $4.17 on alcohol policy for each death due to excessive drinking, compared with $11,000 for each death caused by HIV/AIDS, according to a recent analysis .
The WHO has set an ambitious goal to cut drinking by 20 percent by 2030, but member states have done little to further it . Its recent status report found that nearly half of reporting countries have no alcohol policies in place.
Warning labels add cancer to the list of risks
Where governments have hesitated to limit alcohol sales practices by other means, health advocates have turned to a new weapon: information. A growing number of countries are requiring labels warning drinkers about alcohol’s potential health impacts, which a recent systematic review in The Lancet shows have the potential to raise awareness and reshape behaviors. And alcohol makers are struggling to argue why consumers shouldn’t be fully informed about the products they are buying.
The efforts are a patchwork so far but could be gaining steam. Last fall, Australia and New Zealand began enforcing a requirement for stronger pregnancy warning labels on alcoholic beverages . Ireland went a step further, mandating that in 2026, beverages carry labels informing drinkers that alcohol causes cancer . And this spring in Alaska, lawmakers passed a new requirement that bars post signs warning patrons about the link between alcohol and cancer. The fight can still be an uphill battle. In 2015, after Thailand proposed adding stronger warning labels to alcohol products, other countries with large alcohol export industries disputed the measure in the World Trade Organization, and Thailand ultimately withdrew it. (The government recently rebooted the effort but is again facing fierce opposition from businesses .)
Setting global standards to rein in alcohol makers
Some have called for a global treaty to protect people from alcohol-related harms, modeled after the WHO’s Framework Convention on Tobacco Control . The 20-year-old treaty signed by countries with 90 percent of the world’s population has helped curb smoking rates in many places. It includes many of the same strategies public health experts recommend for regulating alcohol, such as raising taxes and regulating marketing.
One of the most striking parts of the FCTC is that it explicitly excludes tobacco companies from policy negotiations, said Ben Hawkins, a researcher at the University of Cambridge. In contrast, the alcohol industry often has a seat at the table during policy negotiations.
Maristela Monteiro, a retired senior adviser on alcohol at the Pan American Health Organization, said alcohol companies are in dire need of regulation.
“They’re stronger than the tobacco corporations,” she said. “They learned from the tobacco case, and they know that they need to be ahead of the game.”
Image: bodnarphoto / Adobe Stock
More in Policy & Practice

SNAP helps Americans eat. But can it help them eat better?

As food stamps turn 60, four reasons to celebrate
Is alcohol good or bad for you? Yes.
Loading metrics
Open Access
Peer-reviewed
Research Article
Commercial determinants of mental ill health: An umbrella review
Roles Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom
Roles Formal analysis, Methodology, Supervision, Writing – review & editing
Affiliation Department of Public Health, Environments and Society, London School of Hygiene and Tropical Medicine, London, United Kingdom
Roles Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing
Affiliation School of Social and Political Science, University of Edinburgh, Edinburgh, United Kingdom
Roles Methodology, Writing – review & editing
Roles Conceptualization, Funding acquisition, Methodology, Writing – review & editing
- Kate Dun-Campbell,
- Greg Hartwell,
- Nason Maani,
- Alice Tompson,
- May CI van Schalkwyk,
- Mark Petticrew

- Published: August 28, 2024
- https://doi.org/10.1371/journal.pgph.0003605
- Reader Comments
Mental ill health has complex and interrelated underlying causes, with wider determinants of health often overlooked as risk factors. The ‘commercial determinants of health’ are gradually receiving more attention and recognition but there is a relative lack of awareness of the commercial determinants of mental health. This aim of this umbrella review was to synthesise systematic review level evidence for the association between commercial determinants and mental health outcomes. This umbrella review included evidence from high, middle, and low-income countries. We included terms related to broader commercial activities and terms focused on six key unhealthy commodities (tobacco, alcohol, ultra-processed foods, gambling, social media, fossil fuels) and the impacts of fossil fuel consumption (climate change, air pollution, wider pollution). We included 65 reviews and found evidence from high quality reviews for associations between alcohol, tobacco, gambling, social media, ultra-processed foods and air pollution and depression; alcohol, tobacco, gambling, social media, climate change and air pollution with suicide; climate change and air pollution with anxiety; and social media with self-harm. There was a lack of evidence examining wider practices of commercial industries. Our umbrella review demonstrates that by broadening the focus on commercial determinants, the influence of commercial products and activities on mental ill health can be better understood. The lack of research examining broader commercial practices on mental ill health is an area that should be addressed. Our review highlights the existing base of high-quality evidence for many of these unhealthy commodities’ impacts on mental ill health and indicates that commercial determinants is a valuable framework for understanding the drivers of mental ill health.
Citation: Dun-Campbell K, Hartwell G, Maani N, Tompson A, van Schalkwyk MC, Petticrew M (2024) Commercial determinants of mental ill health: An umbrella review. PLOS Glob Public Health 4(8): e0003605. https://doi.org/10.1371/journal.pgph.0003605
Editor: Godfred Boateng, York University, CANADA
Received: January 31, 2024; Accepted: July 24, 2024; Published: August 28, 2024
Copyright: © 2024 Dun-Campbell et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data extracted from reviews (as in summary table) but no new data generated.
Funding: This work was supported by the Three NIHR Research Schools Mental Health Programme (award number: MH004) The grant was received by GH, NM, and MP. . MVS is funded by a National Institute for Health Research Doctoral Fellowship (Ref NIHR300156). MP is a co-investigator in the Spectrum consortium, which is funded by the UK Prevention Research Partnership (UKPRP), a consortium of UK funders (UK Research and Innovation research councils: Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, and Natural Environment Research Council; charities: British Heart Foundation, Cancer Research UK, Welcome, and The Health Foundation; Government: Scottish Government Chief Scientist Office, Health and Care Research Wales, National Institute of Health Research and Public Health Agency. AT is also supported by the Spectrum consortium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Factors that determine mental health are both complex and interrelated [ 1 ]. Globally, around 1 in 8 people are living with a diagnosed mental health disorder [ 1 ] - although this is likely an underestimate of the true proportion of people living with mental ill health. Existing research shows a focus on individual susceptibility and life experiences such as childhood trauma. This can overlook the context in which these experiences occur including the ways in which wider social, political, economic and commercial forces shape mental health and inequalities [ 2 , 3 ]. Recent research, reflecting a growing interest in the social determinants of health (SDOH), has focused on these wider factors – such as household income, employment and housing [ 4 , 5 ]. The commercial determinants of mental health (CDMH) in particular, have not yet been afforded a similar level of attention [ 6 ].
The commercial determinants of health (CDOH) can be thought of as “the systems, practices, and pathways through which commercial actors drive health and equity” [ 7 ]. This includes both the direct and indirect effects of the consumption of produced commodities - such as tobacco, alcohol, fossil fuels and unhealthy foods - and the drivers of consumption such as marketing and advertising [ 8 ]. In addition to analysis of specific unhealthy commodities, CDOH research also includes analysis of the role of commercial actors in shaping the political, structural, and cultural environments which affect health [ 9 ].
These commercial influences can impact not just physical but also mental health, since unhealthy commodity products directly affect and/or harm mental health [ 10 – 12 ]. There is already an evidence base, for instance, for the impact of alcohol consumption on depression and suicide [ 13 ] and smoking on depression and anxiety [ 14 ]. Yet the effects are also indirect; for example, the producers of harmful commodities frequently adopt framings of individual responsibility to place the blame for product harms on individuals themselves. This is often done though ‘responsibility’ campaigns and slogans such as “Gamble/Drink Responsibly” [ 15 ].
Despite the evidence for impacts of unhealthy commodity consumption on health outcomes, existing frameworks for the social determinants of health generally do not consider commercial determinants; nor do they typically include mental health [ 16 ]. There is a strong case for drawing together the existing evidence on mental ill health and commercial determinants. This is of value both for informing the further development of existing SDOH frameworks and identifying points at which to intervene on the CDMH.
This umbrella review therefore aimed to synthesise systematic review evidence on the effects of commercial determinants on mental ill health outcomes to map and identify gaps in the existing evidence base.
The review was developed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines and the protocol registered on PROSPERO 2022 CRD42022320288 [ 17 ].
Eligibility criteria
Inclusion criteria were:
- Population: High, middle, and low-income countries.
- Intervention/exposure: Commercial determinants of mental health, including market strategies and non-market strategies, across six key unhealthy commodities (tobacco, alcohol, ultra-processed foods, gambling, social media, fossil fuels), and the results of fossil fuel consumption (climate change, air pollution, wider pollution).
- Outcome: Mental health outcomes, anxiety, depression, self-harm, and suicide. Any type of measure was included (e.g., self-reported or assessed by a clinician).
- Study design: Full-text articles, in the English language, between 2012-2023 (limited to the past 10 years to ensure a manageable number of results; and in the case of social media to ensure there were sufficient studies), systematic review, meta-analysis, narrative review, scoping review.
Exclusion criteria
Studies that examined mental well-being, or severe mental illness, including bipolar disorder, schizophrenia, and other psychotic disorders, and eating disorders were excluded. As noted- for reasons of feasibility- the review focused on six industries with major relevance to health: tobacco, alcohol, social media, ultra-processed foods, gambling, and fossil fuel products. We also chose to only include adverse impacts from large-multinational manufacturers [ 18 ]. Although the private sector often undertakes important social functions aligned with health benefits, these positive health impacts are already incentivised through the commercial incentives of profit-seeking, just as negative health impacts are. As Maani et al. have therefore argued [ 15 ], focusing research on areas where profit and health are misaligned is likely to contribute to greater short-term net health benefits. Similarly, the most significant scientific insights can be expected by focusing on the largest commercial entities rather than the manifold small and medium organisations than constitute a far smaller fraction of overall commercial impacts on health.
Search strategy
The literature search was developed in Medline and adapted for use in other databases (see S1 – S3 Figs for full search strategy). Search terms relating to the influence of commercial actors were developed based on Lee et al.’s (2022) ‘Conceptual Framework for the Study of the Commercial Determinants of Health’ – including market and non-market strategies [ 8 ]. KDC ran pilot searches to develop additional terms, then conducted searches of Medline, PubMed, PsychInfo, Scopus and the Cochrane database on 28 th March 2022 (Repeat searches were run on 7 th August 2023). See Fig 1 for PRISMA.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
PRISMA outlining the number of reviews found for each database searched, the number excluded, and the final number included.
https://doi.org/10.1371/journal.pgph.0003605.g001
Study selection
One reviewer (KDC) conducted initial screening by title. Two reviewers (KDC and GH) screened reviews in Covidence [ 19 ] by title and abstract; each screened 50% of the reviews with a sample of 100 screened by both to check agreement. This sample had high inter-rater reliability with Cohen’s Kappa of 0.8 (strong agreement). The full-text screening was undertaken by two independent reviewers (KDC and GH) using Covidence. We discussed disagreements between the reviewers, with high reliability (Cohen’s Kappa = 0.71, indicating substantial agreement). At this stage of the search, we had an unmanageable volume of reviews so decided to limit the included reviews to those that focused specifically on our exposures and outcomes of interest (as opposed to prevalence studies examining a very wide range of exposures or outcomes, of which only one or a small part would be relevant).
Data collection process and data items
We extracted information on the name of the review, authors, date, number of studies included, type of studies included (e.g., cohort, cross-sectional), measure of exposure, measure of outcome, pooled effect estimate (if available), summary of results, proposed mechanism for effecting mental health and funding. One reviewer (KDC) extracted the data, and results were grouped for each industry and sent to a second reviewer (GH) for review.
Quality assessment
We used the Scottish Intercollegiate Guidelines Network (SIGN) checklist for systematic reviews and meta-analyses [ 20 ] to assess each study’s quality.
Quality was rated as ‘low’, ‘acceptable’ and ‘high’ using the criteria set out in the guidance notes - “High quality (++): Majority of criteria met. Little or no risk of bias. Acceptable (+): Most criteria met. Some flaws in the study with an associated risk of bias. Low quality (-): Either most criteria not met, or significant flaws relating to key aspects of study design. Reject (0): Poor quality study with significant flaws. Wrong study type. Not relevant to guideline” [ 20 ]. For example, a review was unable to achieve a ‘high’ rating if the review did not assess the quality of its included studies. We included all reviews deemed to be of “high” and “acceptable” quality. Low-quality reviews were only included if they contained an exposure or outcome which was not well represented in the sample.
Synthesis of results
We used narrative synthesis to combine the findings across the included reviews. It was decided during our study design that meta-analysis was not appropriate due to heterogeneity in the measurement of exposures and outcomes across included studies – this is outlined in our protocol on PROSPERO 2022 CRD42022320288 [ 17 ].
Our search returned 11,666 reviews across the five databases. Once duplicates were removed, 9,663 records were screened by title, of which 2,366 were uploaded to Covidence [ 19 ] for abstract screening. We then assessed 318 full texts for eligibility. This left a total of 220 reviews, of which 158 were excluded for being non-specific (as per ‘study selection’ above).
We included 65 reviews in the final synthesis, see Fig 2 for summary of reviews and Fig 3 for review characteristics and main findings. Fourteen reviews examined the impact of smoking on mental health outcomes. Eight were rated as high and six as acceptable quality. We included eleven reviews of alcohol consumption and mental health outcomes. We rated four as high, four as acceptable, and three as low-quality. Five reviews examined ultra-processed food as an exposure; we assessed three as high and two as acceptable quality. Three reviews examined the impact of gambling on mental health outcomes, we assessed one as high and two as acceptable quality. We identified 11 reviews that included social media as an exposure. Of these, we rated five as high, five as acceptable, and one as low-quality. We identified 21 reviews related to fossil fuel products and their impacts. 10 focused on air pollution, eight on climate ‘change’, and four on pesticides. We rated 11 reviews as high, nine as acceptable and one as low-quality.
A summary table of the number of reviews included by each exposure and outcome and the number of reviews of each quality. Note numbers may not total the number of reviews included as some reviews reported multiple outcomes.
https://doi.org/10.1371/journal.pgph.0003605.g002
Outlining the characteristics and main findings of each of the reviews included in our umbrella review. * As rated by SIGN criteria ** Includes suicidal ideation, planning, attempt and completed suicide *** Rounded to 2 decimal places by KDC **** Note this is the pooled effect using the fixed effects model as reported by the authors - given the high heterogeneity, it could be more appropriate to use the random effects model CI: Confidence interval; OR: Odds Ratio; PM: Particulate Matter; RR: Risk Ratio; HR: Hazard Ratio.
https://doi.org/10.1371/journal.pgph.0003605.g003
Smoking and mental ill health
We included fourteen reviews examining the impact of smoking on mental health outcomes [ 22 – 35 ].
There was evidence from high quality reviews for associations between second-hand smoke with depression in children and adolescents, second-hand smoke exposure in pregnancy and smoking in pregnancy with postnatal depression, smoking in pregnancy with suicidal ideation, and smoking and suicide. There was evidence from high quality reviews that smoking cessation improved symptoms of depression, anxiety and mixed anxiety and depressive disorder.
Of the included studies examining anxiety, there was evidence from an acceptable quality review that daily smokers had 5 times the odds of panic disorder at age 24 than non-daily smokers (Odds Ratio (OR) 5.1; 95% Confidence Interval (CI) 2.4-10.5) [ 31 ]. The same review reported 15 times the odds for smokers smoking more than 1 pack per day, than under 1 pack, for generalised anxiety disorder (OR 15.58; 95%CI 2.31-105.14). Another acceptable quality review reported two out of four included prospective observational studies found smoking status was predictive of anxiety [ 28 ].
One high [ 23 ] and three acceptable quality [ 24 , 25 , 30 ] reviews examined the association between smoking and depression. In addition, one review of prospective studies found a statistically significant 62% higher odds of depression at follow up among smokers vs never smokers. (OR 1.62 95%CI 1.10-2.40). A smaller effect size was found(32%), when only prospective studies with both baseline and follow up data were included (OR 1.32, 95%CI 1.02-1.71) [ 30 ].
Exposure to secondhand smoke
Three reviews examined second-hand smoke (SHS) [ 23 – 25 ]. One focused on children and adolescents specifically [ 23 ]; in this review there was no pooled effect size but six of the included eight studies found a positive association and a dose-response relationship between SHS exposure and depressive symptoms in children and adolescents (SHS exposure in the home and in public places were included as measures). Two included acceptable quality reviews found statistically significant associations between exposure to SHS and depression - one found 32% higher odds of depressive symptoms in those exposed to SHS (OR 1.32, 95%CI 1.25–1.39) and evidence of a dose-response effect [ 24 ], the other 60% higher odds of depressive symptoms with exposure to SHS (OR 1.60, 95%CI 1.35–1.90) [ 25 ].
Smoking in pregnancy
Two high-quality reviews examined smoking in pregnancy and reported statistically significant results. One found that engaging in prenatal smoking was associated with more than twice the odds of postpartum depression [ 22 ] and the second found that women exposed to second-hand smoke during pregnancy had 77% higher odds of postpartum depression and 75% higher odds of antenatal suicidal ideation [ 26 ]. In addition, one review [ 28 ] found 37 of the 51 studies they included (73%) reported that smoking increased the risk of subsequent depression. Among included prospective observational studies two out of four found smoking status was predictive of anxiety.
Three high-quality systematic reviews reported an association between smoking and suicide outcomes (including suicidal ideation, planning, attempt, and death by suicide) [ 27 , 29 , 32 ]. One review of prospective cohort studies found an 81% higher risk for completed suicide in those who were current smokers at the time of death vs non-smokers (Relative risk (RR) 1.81; 95%CI 1.50-2.19), with the risk of suicide increasing by 24% for each additional ten cigarettes smoked daily. Both these findings were statistically significant [ 29 ]. A second high-quality review, also including only prospective cohort studies, found that, compared with never-smokers, current smokers experienced 2.4 times the risk of death by suicide (RR 2.41; 95%CI 2.08-2.80) and nearly twice the risk of suicidal ideation (RR 1.84; 95%CI 1.21-2.78), with both findings statistically significant [ 27 ]. The third review [ 32 ] replicated these findings for suicidal ideation and death by suicide, with statistically significantly higher odds for current vs non-smokers - current smokers experienced more than twice the odds of suicidal ideation (OR 2.05 95%CI 1.53-2.58), planning (OR 2.36, 95%CI 1.69-30.02) and attempt (OR 2.84; 95%CI 1.49-4.19) and nearly twice the odds of death from suicide (RR 1.83; 95% CI 1.64-2.02).
Three reviews examined the effect of smoking cessation on mental health outcomes [ 33 – 35 ]. Two high-quality reviews [ 34 , 35 ] reported statistically significant reductions in risk of anxiety, mixed anxiety and depression, and depression from baseline smoking to follow-up after cessation. For example, Taylor et al. (2021) [ 35 ] found that the strength of the evidence was greatest for a reduction in risk of in mixed anxiety and depression. One acceptable quality review found lower odds of depression amongst those who had stopped smoking vs current smokers (OR 0.63; 95%CI 0.54-0.75) [ 33 ].
Alcohol and mental ill health
We included eleven reviews examining the impact of alcohol on mental health outcomes [ 36 – 46 ]. There was evidence from high quality reviews for associations between alcohol consumption with depression and suicide.
Amongst reviews examining depression, a high-quality systematic review and meta-analysis including only prospective cohort studies [ 46 ] found a statistically significant 57% higher risk of subsequent symptoms of depression in people with alcohol use disorder (RR 1.57; 95%CI 1.41-1.76). Examining dose effects, compared with non-heavy drinkers, heavy drinkers had a 13% higher risk of developing later depressive symptoms (RR 1.13; 95% CI 1.05-1.22). One acceptable quality review [ 39 ] found that prenatal alcohol exposure (via maternal drinking) was associated with increased depression and anxiety in children aged three years and over in 69% (9/14) of the included studies. One low-quality review examining older adults (≥50 years) [ 41 ] found statistically significant increased hazard ratios for depressive symptoms amongst long-term abstainers (Hazard ratio (HR) 1.14; 95%CI 1.08-1.21) and occasional (HR 1.16; 95%CI 1.10-1.21) and heavy alcohol drinkers (HR 1.22; 95%CI 1.13-1.30) when compared with moderate drinkers.
Two high-quality reviews [ 40 , 45 ] reported statistically significant associations between alcohol consumption and completed suicide. In one review that only included cohort studies, alcohol use was associated with a statistically significantly 74% increased odds (OR 1.74; 95%CI 1.31-2.31) of completed suicide [ 40 ]. A second review including cohort, case-control and cross-sectional studies found higher odds of suicidal ideation (OR 1.86; 95%CI 1.38-2.35), three times higher odds of suicide attempt (OR 3.13; 95% CI 2.45-3.81) and higher odds of completed suicide (OR 2.59; 95% CI 1.95-3.23) for people with alcohol use disorder, all statistically significant [ 45 ]. Amongst the acceptable quality reviews [ 36 , 37 ] examining suicide as an outcome, both found statistically significant associations between acute alcohol ingestion and risk of suicide attempt [ 36 ], as well as between any alcohol use and suicidal behaviours (ideation, attempt and completed suicide) [ 37 ].
Two included reviews looked at population-level impacts on suicidal outcomes. One high quality [ 42 ] and one acceptable quality [ 44 ] review found that alcohol policies restricting access to alcohol were associated with lower rates of suicide at the population level. The high-quality review [ 42 ] included “enforcing minimum legal drinking age (MLDA), dram shop laws, restrictions on hours of trading, privatization, outlets, and complete alcohol bans”. It did not include a pooled effect size but reported on studies that showed decreases of 3 suicides per 100,000 and 55.5 per 100,000, and a RR of 0.91 (95%CI 0.76-1.08) [ 42 ]. The acceptable quality review [ 44 ] included analyses of alcohol price and taxation, minimum legal drinking age laws, outlet density, ‘other alcohol policies’ and evaluations of changes in alcohol policy mix in countries other than the US; again it did not have a pooled effect size but reported lower suicide rates following these policies.
Of the two low-quality reviews with suicide as the outcome, one [ 43 ] estimated a greater suicide risk amongst ‘alcohol abusers’ vs the general population. The other [ 38 ] found that in 27% of suicide post-mortem samples, the blood alcohol level was above zero.
We did not identify any reviews reporting alcohol’s impact on anxiety in adults.
Ultra-processed foods and mental ill health
We included five reviews examining the impact of ultra-processed foods (UPFs) on mental health outcomes. There was evidence from high quality reviews for associations between UPF consumption and depression [ 47 – 51 ].
One high quality review [ 47 ] conducted a meta-analysis of prospective studies and found a 22% higher risk of subsequent depression associated with ultra-processed food consumption (HR 1.22; 95% CI 1.16-1.28). When including all studies, there was a higher odds of depression and anxiety together (OR 1.53; 95% CI 1.43-1.63) and separately (depression OR 1.44; 95% CI 1.14-1.82, anxiety OR 1.48; 95% CI 1.37-1.59).
One high-quality review [ 51 ] found a statistically significant 31% higher risk of depression amongst high consumers of sugar-sweetened beverages when compared with low consumers and with non-consumers (RR 1.31; 95%CI 1.24-1.39). This review also found a dose-response relationship, with an increased risk of 5% for 2 cups per day and 25% for 3 cans per day compared with non-drinkers of sugar sweetened beverages. The second high-quality review [ 49 ] found a statistically significant 8% higher risk of depression amongst people who ate red and processed meats vs those who did not (OR 1.08; 95% CI 1.04-1.12).
An acceptable quality review [ 48 ] analysed several different diets that posed a potential risk for depressive symptoms. The authors referred to these as “pro-inflammatory” diets and they included “sweets; refined flour; high-fat products; red and processed meat” [ 48 ]. The second acceptable quality review [ 50 ] examined dietary sugars but did not exclusively focus on sugar-sweetened beverages. These reviews reported no pooled effect sizes and found mixed results, though several included prospective cohort studies reported positive associations between added dietary sugars and subsequent risk of depression.
No identified reviews in this group examined self-harm or suicide as outcomes or specifically focused on anxiety. Several different mechanisms for these associations were proposed by the included reviews including systemic inflammation [ 48 , 50 ], disruption of the gut microbiota, disrupted dopamine function, insulin resistance, oxidative stress or generation of toxic advanced glycation end-products [ 51 ].
Gambling and mental ill health
We included three reviews examining the impact of gambling on mental health outcomes [ 52 – 54 ]. There was evidence from high quality reviews for associations between gambling with depression, and general mental health outcomes.
One high-quality review [ 52 ], which did not calculate pooled effect sizes, reported on a study that found onset of ‘problem gambling’ was significantly associated with nearly double the odds of incident major depressive disorder (Adjusted odds ratio (AOR) 1.98; 95%CI 1.14-3.44), and almost 4 times the odds of any mental disorder (AOR 3.84; 95%CI 1.89-7.79) at 3.5 years of follow-up [ 52 ]. This review also included several studies which found no association between gambling and later depression or anxiety. The review reported a 15 times higher standardised mortality ratio (SMR) for death by suicide in 20–74-year-olds who had a gambling disorder, compared with the public. For people aged 20-49, the SMR was even higher at 19.3 [ 52 ].
Amongst the acceptable quality reviews, one was not exclusively focused on gambling and mental health and included gaming and conduct problems (which are defined as aggressive or antisocial behaviour that impacts on functioning) in its analysis [ 53 ]. Focusing on gambling and depression, this review did not report any pooled effects, but 10 out of the 12 included cross-sectional studies found statistically significant positive associations between problem gambling and depressive symptoms [ 53 ]. The second acceptable quality review [ 54 ] examined online gambling, including only cross-sectional studies, and found several studies reporting a positive association between online gambling and depressive symptoms [ 54 ].
Social media and mental ill health
We included eleven reviews examining the impact of social media on mental health outcomes [ 55 – 65 ]. There was evidence from high quality reviews for associations between social media with depression, suicidal ideation, and self-harm.
There was mixed evidence for an association between social media use and depression. Of the four high-quality reviews [ 59 – 63 ], one [ 59 ] which focused on adolescents (11-18 years) and included only cross-sectional studies found a weak positive correlation ( r = 0.11 p<0.01). Another high-quality review [ 60 ], including mostly cross-sectional studies, reported mixed results - with a general association between social media and depression, but no pooled effect size - and noted potential confounders and methodological issues within the included studies [ 60 ]. One high-quality review [ 61 ] focusing on young people (10-24 years) examined effect sizes for different measures of online media use (including social media) – with greater effect sizes seen when only including studies that used a measure of ‘addiction’ rather than just time spent. A sub analysis based on media type found the effect size of social media to be significantly smaller than internet use.
Another high-quality review focused on adolescents [ 61 ] found a positive association between time spent on social media and depression symptoms – OR 1.60 (95%CI 1.45-1.75) with a stronger association for girls (OR 1.72; 95% CI 1.41-2.09) than boys (OR 1.20; 95% CI 1.05-1.37).
The finding that time spent on social media had a small significant positive association with depressive symptoms was replicated across four reviews – two high and two acceptable quality, with three reporting the same effect size [ 56 , 59 , 65 ]; r = 0.11 p<0.01, with time spent on social media accounting for around 11% of the depressive symptoms. Among the acceptable-quality reviews, two found statistically significant positive correlations between social media use and depression ( r = 0.11, 95%CI 0.086 – 0.13, and r = 0.11, 95% CI 0.08 - 0.14, p< 0.001) [ 56 , 65 ].
The remainder of the acceptable quality reviews including depression found a mix of positive and negative associations between social media use and depression [ 55 , 57 , 58 ] but for most of the reviews, these associations were positive.
There was evidence of an association between social media use and anxiety. An acceptable quality review [ 58 ] of studies conducted in China reported a significant positive correlation between the two across four included studies, with bivariate correlations ranging between 0.19-0.56.
A high-quality review [ 62 ] found nearly 3 times the odds of suicidal ideation amongst adolescents with “problematic” social media use (a definition was not given for this term), which was statistically significant (OR 2.81; 95%CI 1.72- 4.59) [ 55 ]. In addition, non-significant associations were found between high frequency of social media use and suicidal ideation (OR 1.45; 95%CI 0.95-2.23), suicidal plans (OR 1.47; 95%CI 0.33-6.43) and self-harm (OR 2.03; 95% CI 0.79-5.21).
A range of mechanisms for the associations between social media use and mental ill health were described in these studies, with many mentioning the mediating impacts of insomnia and other sleep disorders [ 60 , 62 ], cyber bullying [ 62 , 64 ] and sexting [ 57 , 62 ].
Fossil fuel products, impact of their use and mental ill health
We included 21 reviews examining the impact of air pollution, ambient temperature increases, and pesticides on mental health outcomes [ 66 – 85 ]. There was evidence from high quality reviews for associations between air pollution with depression, anxiety and suicide; ambient temperature increases with risk of suicide; and pesticides with depression and suicide.
Air pollution and mental ill health.
We found six high quality reviews examining air pollution and depression [ 67 , 70 , 72 – 74 , 81 ]. The reviews found statistically significant but small associations between short term exposure to particulate matter (PM)10 [ 65 , 72 ], PM2.5 [ 67 ], NO2 [ 67 , 70 ] SO2 [ 67 ], or CO [ 67 ] and depression. Two reviews also found associations between long-term exposure to PM2.5 or NO2 and depression [ 67 , 72 ]. One of the reviews estimated a 10% increased risk in depression per 10μg/m3 increase in long term PM2.5 exposure [ 73 ]. While the effect sizes were small, the authors highlighted that the population level exposure contributes to a large burden of mental ill health.
One review focused specifically on the impact of air pollution on perinatal mental health and found an association between PM2.5 and NO2 on postnatal depression [ 74 ].
Two acceptable quality reviews [ 68 , 82 ] and one low-quality one [ 71 ] supported these findings. There was a strong association between air pollution and hospital admissions for depression in an acceptable quality review (no effect size calculated) [ 66 ].
There was also a general association between air pollution and anxiety in these reviews [ 73 , 76 ]. A statistically significant positive association was found between long-term air pollution and anxiety in two studies included within one high-quality review (the review did not perform meta-analyses due to the low number of studies) [ 73 ].
Three high-quality reviews found positive associations between air pollution and suicide. One of these found small positive associations per increased Inter Quartile Range (IQR) in PM2.5, PM10 and NO2 [ 75 ]. The second high quality review found statistically significant pooled effect sizes at days 0-2 per 10μg/m3 increase in PM10 [ 73 ]. The third found small positive associations between suicide and each 10μg/m3 increase in mean NO2 at a lag of 1-3 days in mean SO2 at 1-4 days and mean PM2.5 at 1 day [ 69 ]. Several mechanisms for this were proposed including reduced respiratory function leading to oxidative stress and hypoxia, the latter of which can in turn lead to depleted levels of serotonin [ 69 ]. Other suggested mechanisms were via reduced neurophysiological function, stress response pathways, neuroinflammation, decreased cerebral blood flow, cerebral oedema, and swollen nerve cells [ 75 ].
Temperature increases and mental ill health.
Evidence from high, acceptable, and low-quality reviews found ambient temperature increases to be associated with poor mental health outcomes, including risk of suicide. One high quality review found a 9% increased risk of suicide per increase of 7.1C in temperature [ 75 ]. Reviews also showed ambient temperature increases to be associated with mental ill health in adults. One high quality review found that each 1C increase in temperature led to an increased risk of mental health related mortality and morbidity [ 86 ].
Pesticides and mental ill health.
One high quality review looked at the impacts of pesticide exposure on anxiety, depression, and suicide in farmers [ 86 ] and found an association between exposure to pesticides and depression and suicide. Two acceptable-quality reviews examined pesticide exposure finding evidence of a positive association between pesticide exposure and depression and suicide [ 84 , 85 ]. Both reviews noted inconsistencies in the methodological approaches of included studies and mixed findings.
Wider practices/commercial actions and mental health
We identified two reviews examining impacts of introducing policies focused on reducing consumption in any of these six CDoH areas; these both looked at alcohol policy with suicide as the outcome, as discussed in the alcohol results section. Included policies were changes to alcohol pricing, changes to alcohol availability, changes to drink driving countermeasures, increased taxation, regulation of advertising and anti-alcohol advertising [ 42 , 43 ]. No other identified reviews examined the wider impacts of commercial practices and actions on mental health.
Fig 4 provides an overview of the highest quality of evidence (as rated by our reviewers) for positive relationships between respective exposures and outcomes. In summary, there is evidence from high quality reviews linking: tobacco, social media, UPF, pesticides, climate change and air pollution with anxiety; alcohol, tobacco, gambling, social media, ultra-processed foods, and air-pollution with depression; alcohol, tobacco, gambling, social media, climate change and air pollution with suicide; and social media with self-harm.
This figure outlines the highest quality of review evidence found for each exposure and outcome (as assessed by the review authors). Green reflects high quality, amber acceptable quality and no colour reflects no studies found. *From individual studies (no pooled effect sizes) ** Outcome was offspring anxiety and depression following maternal prenatal exposure.
https://doi.org/10.1371/journal.pgph.0003605.g004
Funding of reviews
Information was also collected on funding of reviews. Funding was not declared in 12 of the included reviews, 15 reported that they had received no funding, 4 that there were no competing financial interests (without outlining funders), and 2 that they had received funding from private foundations. None of the reviews declared any funding from industry but authors in 2 of the reviews reported they had previously received payment from pharmaceutical companies. The remaining 32 received funding from universities, governmental bodies, research organisations (e.g., Medical Research Council, NIHR) or international bodies (WHO and EU). None of the included reviews considered the funding of their included studies or stratified their results depending on industry funding.
To our knowledge, this is the first umbrella review examining the impact of commercial determinants on mental ill health. We found evidence from high quality reviews for associations between alcohol, tobacco, gambling, social media, ultra-processed foods, and air pollution with depression; alcohol, tobacco, gambling, social media, climate change and air pollution with suicide; climate change and air pollution with anxiety; and social media with self-harm. There was a lack of evidence examining wider practices of commercial industries and their impact on mental health.
We found evidence from high quality reviews for associations between five out of the six commodities examined and suicide [ 27 , 40 , 42 , 45 , 52 , 62 , 70 , 76 , 86 ] – the two highest quality reviews with alcohol as an exposure both found significant associations with completed suicide, for instance, while smoking reviews demonstrated a dose-response relationship with this outcome. One review [ 27 ] called for smoking to be included in risk assessments for suicide.
While suicide is clearly an extremely important outcome, it is important also to note the evidence base for other less serious but far more common mental health outcomes, such as anxiety and depression.
Overall, for exposures, there were fewer reviews identified for gambling and ultra-processed foods, with high heterogeneity of study design and definition of exposures. The studies included in the social media reviews were meanwhile mostly cross-sectional in design, with many using self-reported measures of both social media exposure and mental health outcomes, but there was high quality evidence for associations between social media use and depression, as well as suicide. The temporality of some of these relationships were, however, less clear than for other exposures (e.g., tobacco).
These identified gaps in the literature may, in part, reflect the well documented influence of industry on research, including research agendas [ 87 , 88 ]. For example, the limited amount of review-level evidence on gambling and mental health, particularly of high-quality, is remarkable given the nature of these products and their impacts. In this respect, it is noteworthy that the gambling industry has been the main funder of such research in many countries for approximately 40 years [ 89 , 90 ]. It was also striking that only one of the CDoH industries (alcohol) had been the focus of any research analysing relevant policies to tackle its impacts. It is also important to consider the wider mental health burden incurred by those close to someone affected by mental ill-health (for instance, the devastating impact of suicides on family and friend networks), a literature not included in this review. The mental health impacts on affected others should effectively be understood in the same way as other secondary exposures. Further consideration is similarly needed of the mental ill-health associated with the physical health impacts from these commercial determinants (e.g., lung cancer, liver cirrhosis, and violence). Finally, there are likely to also be mental ill-health burdens associated with wider harms such as the impact on communities from clustering of alcohol or gambling venues or the noise created by fossil-fuel based transport systems. Such considerations could inform future systems approaches to research on this topic and more comprehensive mappings of the commercial determinants of health.
Suggested pathways
Several similarities were identified in the potential pathways by which consuming these products may impact on health. For example, for tobacco products, alcohol, and ultra-processed foods - most studies highlighted the role of inflammation. For products that are not directly consumed into the body, e.g., social media or gambling, the pathways for mental health outcomes may be via wider impacts such as relational or financial factors.
Yet this review also identified key gaps in the evidence base in relation to pathways around wider commercial impacts on mental health. Such an agenda will also require a shift in how harm - and pathways or chains of harm - are conceptualised, as well as greater appreciation of the differences between how health and disease impact on individuals versus populations. Further research should focus on wider systems, practices and pathways through which commercial actors influence physical and mental health.
Methodological challenges
This review identified many methodological challenges in measuring commercial determinants of health. Even when considering relatively easier exposures, such as smoking or alcohol, there are substantial differences in approaches to measurement. It becomes more challenging still when considering more difficult exposures, such as air pollution and ultra-processed food consumption; when consumption is part of our everyday experience, it can be difficult to recall or measure accurately. For example, variable measurements were used for alcohol, including both self-reported unit intake and blood alcohol levels, while some studies used “ever use” of alcohol as an exposure. This latter measure obscures important insights given the substantial proportion of the population that has consumed alcohol at any point in their lives. Indeed, substantial methodological challenges are involved in measuring alcohol consumption, with intake often underreported and misremembered - alcohol consumed in the home, for instance, is unlikely to be served in recommended or standard measures. Social media was another exposure with specific methodological challenges. There are two components to social media that can be difficult to disentangle: (1) the platforms on which users can engage and the ways these platforms influence people’s use of social media and its roles in their lives, and (2) the content created by the users themselves to which others are then exposed.
Use of the term “problem” user was found across unhealthy commodity industries and was ill defined in all of these. Likewise, research has shown that terms such as “responsible drinking” are often poorly defined and can be used to provide pro industry framings of product harm [ 91 ]. The authors of one high-quality review [ 81 ] highlight key methodological challenges in the climate change/air pollution space, particularly due to the linking of events to climate change, the difficulties in temporal measurements before and after an event and the difficulty of controlling for confounders. Finally, it should also be noted that there is substantial overlap between different industries, even within our review; for example, the food industry is involved in pesticide usage as well as the production of highly processed food products.
Strengths and limitations
This umbrella review is the first to consider the commercial determinants of mental ill health as a primary focus. In drawing together and mapping the evidence both for the unhealthy commodities and wider practices, we can identify where the evidence is strongest and where the evidence gaps are most clear. This makes this review useful for informing development of frameworks of mental health, as well as development of mental health policy.
Our study included both middle, and low-income countries in our search terms and so was not limited just to high income countries.
We made some changes to the review design following pilot searches. Firstly, we established it was not feasible to stratify the results by PROGRESS-Plus due to the number of studies identified, and substantial variations across the evidence base. Following reflection and discussion between the review group about the complexity of obesity-related research, including the confounding role of physical activity, it was agreed that obesity would no longer be included as an outcome. Addiction was also excluded as an outcome due to the large volume of search results returned that were judged not relevant for this review (e.g., focusing on the addictive properties of social media). Following discussion, in the context of this review, addiction seemed to be more of a confounder or a mediator of these relationships. In classing it as an outcome or an exposure, the focus of the review might therefore have shifted to examine whether products were addictive or if addiction itself was associated with mental ill health.
As we limited the included reviews to those that focused specifically on our exposures and outcomes of interest during the search, to avoid a large amount of irrelevant material, we may have excluded some additional findings that were not included in specific reviews. However, we felt that reviews specifically focused on our exposures and outcomes of interest were likely to include most of any relevant evidence. Finally, including only papers in the English language does mean that we could have missed findings from papers published in other languages. We were also unable to comprehensively examine inequalities in commercial determinants of mental ill health. This was due to both the number of papers available and the small number that considered differences in demographic groups. Although wider search terms were included for all industries, this review was also limited to six key industries. It did not include other key and interlinking industries such as the meat and dairy, chemical, beauty, and pharmaceutical industries. Many of these industries have overlapping practices and actors, so separating them can be challenging. For example, although online gambling was included, online gaming was mostly excluded, despite a large overlap between the two and the fact that many online gaming products include gambling elements. Examining the impact of fossil fuels was also particularly challenging – given, for instance, there are clear links with climate change and pollution, but fossil fuels are not the only cause. Finally, we restricted this umbrella review to include only reviews and not primary research papers. This may have led to the exclusion of relevant or new research (that is yet to be included in a review). Throughout the review, discussion with the wider review group aimed to guide these decisions. Overall, this umbrella review is the first that the authors are aware of that maps the evidence base for the ‘commercial determinants’ of mental health. While previous reviews have focused on individual unhealthy commodities (e.g., tobacco products), analysing these commodities within the context of a wider range of industries can lead to a greater understanding of the concept of CDoH, as well as the need to act across various industries and settings to reduce health-harming practices, and improve mental health.
Conclusions
In conclusion, there is strong evidence that smoking, alcohol, and air pollution are associated with mental ill health. The evidence bases for ultra-processed foods, gambling, social media, and climate change are less developed but already include high-quality reviews demonstrating associations between these industries and various negative mental health outcomes. There is a striking lack of research examining the wider actions of corporations on mental health outcomes. Given these findings, commercial determinants should be routinely included within frameworks to examine and improve mental health.
Supporting information
S1 fig. search strategy medline..
Search strategy for our umbrella review using Medline.
https://doi.org/10.1371/journal.pgph.0003605.s001
S2 Fig. Search strategy Embase.
Search strategy for our umbrella review using Embase.
https://doi.org/10.1371/journal.pgph.0003605.s002
S3 Fig. Search strategy PsychInfo.
Search strategy for our umbrella review using PsychInfo.
https://doi.org/10.1371/journal.pgph.0003605.s003
- 1. Mental disorders n.d. https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed January 24, 2024).
- View Article
- PubMed/NCBI
- Google Scholar
- 15. Maani N, Petticrew M, Galea S. The Commercial Determinants of Health. Oxford University Press; 2022.
- 17. N.d. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=320288 (accessed January 24, 2024).
- 19. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org .
- 20. Scottish Intercollegiate Guidelines Network (SIGN). Systematic reviews and meta-analyses. Edinburgh: SIGN; [Online]. Available: https://testing36.scot.nhs.uk . [Accessed 27 January 2023].
- 52. Gambling-related harms evidence review: summary. GOVUK n.d. https://www.gov.uk/government/publications/gambling-related-harms-evidence-review/gambling-related-harms-evidence-review-summary--2 (accessed January 24, 2024).
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Meditation and Mindfulness: Effectiveness and Safety

.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What are meditation and mindfulness?
Meditation has a history that goes back thousands of years, and many meditative techniques began in Eastern traditions. The term “meditation” refers to a variety of practices that focus on mind and body integration and are used to calm the mind and enhance overall well-being. Some types of meditation involve maintaining mental focus on a particular sensation, such as breathing, a sound, a visual image, or a mantra, which is a repeated word or phrase. Other forms of meditation include the practice of mindfulness, which involves maintaining attention or awareness on the present moment without making judgments.
Programs that teach meditation or mindfulness may combine the practices with other activities. For example, mindfulness-based stress reduction is a program that teaches mindful meditation, but it also includes discussion sessions and other strategies to help people apply what they have learned to stressful experiences. Mindfulness-based cognitive therapy integrates mindfulness practices with aspects of cognitive behavioral therapy.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Are meditation and mindfulness practices safe?
Meditation and mindfulness practices usually are considered to have few risks. However, few studies have examined these practices for potentially harmful effects, so it isn’t possible to make definite statements about safety.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} More
A 2020 review examined 83 studies (a total of 6,703 participants) and found that 55 of those studies reported negative experiences related to meditation practices. The researchers concluded that about 8 percent of participants had a negative effect from practicing meditation, which is similar to the percentage reported for psychological therapies. The most commonly reported negative effects were anxiety and depression. In an analysis limited to 3 studies (521 participants) of mindfulness-based stress reduction programs, investigators found that the mindfulness practices were not more harmful than receiving no treatment.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} How popular are meditation and mindfulness?
According to the National Health Interview Survey, an annual nationally representative survey, the percentage of U.S. adults who practiced meditation more than doubled between 2002 and 2022, from 7.5 to 17.3 percent. Of seven complementary health approaches for which data were collected in the 2022 survey, meditation was the most popular, beating out yoga (used by 15.8 percent of adults), chiropractic care (11.0 percent), massage therapy (10.9 percent), guided imagery/progressive muscle relaxation (6.4 percent), acupuncture (2.2 percent), and naturopathy (1.3 percent).
For children aged 4 to 17 years, data are available for 2017; in that year, 5.4 percent of U.S. children used meditation.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Why do people practice mindfulness meditation?
In a 2012 U.S. survey, 1.9 percent of 34,525 adults reported that they had practiced mindfulness meditation in the past 12 months. Among those responders who practiced mindfulness meditation exclusively, 73 percent reported that they meditated for their general wellness and to prevent diseases, and most of them (approximately 92 percent) reported that they meditated to relax or reduce stress. In more than half of the responses, a desire for better sleep was a reason for practicing mindfulness meditation.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What are the health benefits of meditation and mindfulness?
Meditation and mindfulness practices may have a variety of health benefits and may help people improve the quality of their lives. Recent studies have investigated if meditation or mindfulness helps people manage anxiety, stress, depression, pain, or symptoms related to withdrawal from nicotine, alcohol, or opioids.
Other studies have looked at the effects of meditation or mindfulness on weight control or sleep quality.
However, much of the research on these topics has been preliminary or not scientifically rigorous. Because the studies examined many different types of meditation and mindfulness practices, and the effects of those practices are hard to measure, results from the studies have been difficult to analyze and may have been interpreted too optimistically.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Stress, Anxiety, and Depression
- A 2018 NCCIH-supported analysis of 142 groups of participants with diagnosed psychiatric disorders such as anxiety or depression examined mindfulness meditation approaches compared with no treatment and with established evidence-based treatments such as cognitive behavioral therapy and antidepressant medications. The analysis included more than 12,000 participants, and the researchers found that for treating anxiety and depression, mindfulness-based approaches were better than no treatment at all, and they worked as well as the evidence-based therapies.
- A 2021 analysis of 23 studies (1,815 participants) examined mindfulness-based practices used as treatment for adults with diagnosed anxiety disorders. The studies included in the analysis compared the mindfulness-based interventions (alone or in combination with usual treatments) with other treatments such cognitive behavioral therapy, psychoeducation, and relaxation. The analysis showed mixed results for the short-term effectiveness of the different mindfulness-based approaches. Overall, they were more effective than the usual treatments at reducing the severity of anxiety and depression symptoms, but only some types of mindfulness approaches were as effective as cognitive behavioral therapy. However, these results should be interpreted with caution because the risk of bias for all of the studies was unclear. Also, the few studies that followed up with participants for periods longer than 2 months found no long-term effects of the mindfulness-based practices.
- A 2019 analysis of 23 studies that included a total of 1,373 college and university students looked at the effects of yoga, mindfulness, and meditation practices on symptoms of stress, anxiety, and depression. Although the results showed that all the practices had some effect, most of the studies included in the review were of poor quality and had a high risk of bias.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} High Blood Pressure
Few high-quality studies have examined the effects of meditation and mindfulness on blood pressure. According to a 2017 statement from the American Heart Association, the practice of meditation may have a possible benefit, but its specific effects on blood pressure have not been determined.
- A 2020 review of 14 studies (including more than 1,100 participants) examined the effects of mindfulness practices on the blood pressure of people who had health conditions such as hypertension, diabetes, or cancer. The analysis showed that for people with these health conditions, practicing mindfulness-based stress reduction was associated with a significant reduction in blood pressure.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Pain
Studies examining the effects of mindfulness or meditation on acute and chronic pain have produced mixed results.
- A 2020 report by the Agency for Healthcare Research and Quality concluded that mindfulness-based stress reduction was associated with short-term (less than 6 months) improvement in low-back pain but not fibromyalgia pain.
- A 2020 NCCIH-supported analysis of five studies of adults using opioids for acute or chronic pain (with a total of 514 participants) found that meditation practices were strongly associated with pain reduction.
- Acute pain, such as pain from surgery, traumatic injuries, or childbirth, occurs suddenly and lasts only a short time. A 2020 analysis of 19 studies examined the effects of mindfulness-based therapies for acute pain and found no evidence of reduced pain severity. However, the same analysis found some evidence that the therapies could improve a person’s tolerance for pain.
- A 2017 analysis of 30 studies (2,561 participants) found that mindfulness meditation was more effective at decreasing chronic pain than several other forms of treatment. However, the studies examined were of low quality.
- A 2019 comparison of treatments for chronic pain did an overall analysis of 11 studies (697 participants) that evaluated cognitive behavioral therapy, which is the usual psychological intervention for chronic pain; 4 studies (280 participants) that evaluated mindfulness-based stress reduction; and 1 study (341 participants) of both therapies. The comparison found that both approaches were more effective at reducing pain intensity than no treatment, but there was no evidence of any important difference between the two approaches.
- A 2019 review found that mindfulness-based approaches did not reduce the frequency, length, or pain intensity of headaches. However, the authors of this review noted that their results are likely imprecise because only five studies (a total of 185 participants) were included in the analysis, and any conclusions made from the analysis should be considered preliminary.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Insomnia and Sleep Quality
Mindfulness meditation practices may help reduce insomnia and improve sleep quality.
- A 2019 analysis of 18 studies (1,654 total participants) found that mindfulness meditation practices improved sleep quality more than education-based treatments. However, the effects of mindfulness meditation approaches on sleep quality were no different than those of evidence-based treatments such as cognitive behavioral therapy and exercise.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Substance Use Disorder
Several clinical trials have investigated if mindfulness-based approaches such as mindfulness-based relapse prevention (MBRP) might help people recover from substance use disorders. These approaches have been used to help people increase their awareness of the thoughts and feelings that trigger cravings and learn ways to reduce their automatic reactions to those cravings.
- A 2018 review of 37 studies (3,531 total participants) evaluated the effectiveness of several mindfulness-based approaches to substance use disorder treatment and found that they significantly decreased participants’ craving levels. The mindfulness-based practices were slightly better than other therapies at promoting abstinence from substance use.
- A 2017 analysis specifically focused on MBRP examined 9 studies (901 total participants) of this approach. The analysis concluded that MBRP was not more effective at preventing substance use relapses than other treatments such as health education and cognitive behavioral therapy. However, MBRP did slightly reduce cravings and symptoms of withdrawal associated with alcohol use disorders.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Post-Traumatic Stress Disorder
Studies have suggested that meditation and mindfulness may help reduce symptoms of post-traumatic stress disorder (PTSD).
- A 2018 review supported by NCCIH examined the effects of meditation (in 2 studies, 179 total participants) and other mindfulness-based practices (in 6 studies, 332 total participants) on symptoms of PTSD. Study participants included veterans, nurses, and people who experienced interpersonal violence. Six of the eight studies reported that participants had a reduction of PTSD symptoms after receiving some form of mindfulness-based treatment.
- A 2018 clinical trial funded by the U.S. Department of Defense compared the effectiveness of meditation, health education, and prolonged exposure therapy, a widely accepted treatment for PTSD recommended by the American Psychological Association. Prolonged exposure therapy helps people reduce their PTSD symptoms by teaching them to gradually remember traumatic memories, feelings, and situations. The study included 203 veterans with PTSD as a result of their active military service. The results of the study showed that meditation was as effective as prolonged exposure therapy at reducing PTSD symptoms and depression, and it was more effective than PTSD health education. The veterans who used meditation also showed improvement in mood and overall quality of life.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Cancer
Mindfulness-based approaches may improve the mental health of people with cancer.
- A 2019 analysis of 29 studies (3,274 total participants) of mindfulness-based practices showed that use of mindfulness practices among people with cancer significantly reduced psychological distress, fatigue, sleep disturbance, pain, and symptoms of anxiety and depression. However, most of the participants were women with breast cancer, so the effects may not be similar for other populations or other types of cancer.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Weight Control and Eating Behavior
Studies have suggested possible benefits of meditation and mindfulness programs for losing weight and managing eating behaviors.
- A 2017 review of 15 studies (560 total participants) looked at the effects of mindfulness-based practices on the mental and physical health of adults with obesity or who were overweight. The review found that these practices were very effective methods for managing eating behaviors but less effective at helping people lose weight. Mindfulness-based approaches also helped participants manage symptoms of anxiety and depression.
- A 2018 analysis of 19 studies (1,160 total participants) found that mindfulness programs helped people lose weight and manage eating-related behaviors such as binge, emotional, and restrained eating. The results of the analysis showed that treatment programs, such as mindfulness-based stress reduction and mindfulness-based cognitive therapy, that combine formal meditation and mindfulness practices with informal mindfulness exercises were especially effective methods for losing weight and managing eating.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Attention-Deficit Hyperactivity Disorder
Several studies have been done on using meditation and mindfulness practices to improve symptoms of attention-deficit hyperactivity disorder (ADHD). However, the studies have not been of high quality and the results have been mixed, so evidence that meditation or mindfulness approaches will help people manage symptoms of ADHD is not conclusive.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} How do meditation and mindfulness work?
Some research suggests that meditation and mindfulness practices may affect the functioning or structure of the brain. Studies have used various methods of measuring brain activity to look for measurable differences in the brains of people engaged in mindfulness-based practices. Other studies have theorized that training in meditation and mindfulness practices can change brain activity. However, the results of these studies are difficult to interpret, and the practical implications are not clear.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} NCCIH-Funded Research
NCCIH supports a variety of meditation and mindfulness studies, including:
- An evaluation of how the brain responds to the use of mindfulness meditation as part of a combined treatment for migraine pain.
- A study of the effectiveness of mindfulness therapy and medication (buprenorphine) as a treatment for opioid use disorder.
- A study of a mindfulness training program designed to help law enforcement officers improve their mental health by managing stress and increasing resilience.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Tips To Consider
- Don’t use meditation or mindfulness to replace conventional care or as a reason to postpone seeing a health care provider about a medical problem.
- Ask about the training and experience of the instructor of the meditation or mindfulness practice you are considering.
- Take charge of your health—talk with your health care providers about any complementary health approaches you use. Together, you can make shared, well-informed decisions
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} For More Information
Nccih clearinghouse.
The NCCIH Clearinghouse provides information on NCCIH and complementary and integrative health approaches, including publications and searches of Federal databases of scientific and medical literature. The Clearinghouse does not provide medical advice, treatment recommendations, or referrals to practitioners.
Toll-free in the U.S.: 1-888-644-6226
Telecommunications relay service (TRS): 7-1-1
Website: https://www.nccih.nih.gov
Email: [email protected] (link sends email)
Know the Science
NCCIH and the National Institutes of Health (NIH) provide tools to help you understand the basics and terminology of scientific research so you can make well-informed decisions about your health. Know the Science features a variety of materials, including interactive modules, quizzes, and videos, as well as links to informative content from Federal resources designed to help consumers make sense of health information.
Explaining How Research Works (NIH)
Know the Science: How To Make Sense of a Scientific Journal Article
Understanding Clinical Studies (NIH)
A service of the National Library of Medicine, PubMed® contains publication information and (in most cases) brief summaries of articles from scientific and medical journals. For guidance from NCCIH on using PubMed, see How To Find Information About Complementary Health Approaches on PubMed .
Website: https://pubmed.ncbi.nlm.nih.gov/
NIH Clinical Research Trials and You
The National Institutes of Health (NIH) has created a website, NIH Clinical Research Trials and You, to help people learn about clinical trials, why they matter, and how to participate. The site includes questions and answers about clinical trials, guidance on how to find clinical trials through ClinicalTrials.gov and other resources, and stories about the personal experiences of clinical trial participants. Clinical trials are necessary to find better ways to prevent, diagnose, and treat diseases.
Website: https://www.nih.gov/health-information/nih-clinical-research-trials-you
Research Portfolio Online Reporting Tools Expenditures & Results (RePORTER)
RePORTER is a database of information on federally funded scientific and medical research projects being conducted at research institutions.
Website: https://reporter.nih.gov
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Key References
- Anheyer D, Leach MJ, Klose P, et al. Mindfulness-based stress reduction for treating chronic headache: a systematic review and meta-analysis . Cephalalgia . 2019;39(4):544-555.
- Black LI, Barnes PM, Clarke TC, Stussman BA, Nahin RL. Use of yoga, meditation, and chiropractors among U.S. children aged 4–17 years . NCHS Data Brief, no 324. Hyattsville, MD: National Center for Health Statistics. 2018.
- Breedvelt JJF, Amanvermez Y, Harrer M, et al. The effects of meditation, yoga, and mindfulness on depression, anxiety, and stress in tertiary education students: a meta-analysis . Frontiers in Psychiatry . 2019;10:193.
- Burke A, Lam CN, Stussman B, et al. Prevalence and patterns of use of mantra, mindfulness and spiritual meditation among adults in the United States . BMC Complementary and Alternative Medicine. 2017;17(1):316.
- Carrière K, Khoury B, Günak MM, et al. Mindfulness‐based interventions for weight loss: a systematic review and meta‐analysis . Obesity Reviews . 2018;19(2):164-177.
- Cavicchioli M, Movalli M, Maffei C. The clinical efficacy of mindfulness-based treatments for alcohol and drugs use disorders: a meta-analytic review of randomized and nonrandomized controlled trials . European Addiction Research . 2018;24(3):137-162.
- Cillessen L, Johannsen M, Speckens AEM, et al. Mindfulness‐based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta‐analysis of randomized controlled trials . Psychooncology . 2019;28(12):2257-2269.
- Creswell JD. Mindfulness interventions . Annual Review of Psychology. 2017;68:491-516.
- Davidson RJ, Kaszniak AW. Conceptual and methodological issues in research on mindfulness and meditation . American Psychologist. 2015;70(7):581-592.
- Farias M, Maraldi E, Wallenkampf KC, et al. Adverse events in meditation practices and meditation-based therapies: a systematic review . Acta Psychiatrica Scandinavica. 2020;142(5):374-393.
- Garland EL, Brintz CE, Hanley AW, et al. Mind-body therapies for opioid-treated pain: a systematic review and meta-analysis . JAMA Internal Medicine . 2020;180(1):91-105.
- Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis . Clinical Psychology Review . 2018;59:52-60.
- Grant S, Colaiaco B, Motala A, et al. Mindfulness-based relapse prevention for substance use disorders: a systematic review and meta-analysis . Journal of Addiction Medicine . 2017;11(5):386-396.
- Haller H, Breilmann P, Schröter M et al. A systematic review and meta‑analysis of acceptance and mindfulness‑based interventions for DSM‑5 anxiety disorders . Scientific Reports . 2021;11(1):20385.
- Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis . Annals of Behavioral Medicine. 2017;51(2):199-213.
- Hirshberg MJ, Goldberg SB, Rosenkranz M, et al. Prevalence of harm in mindfulness-based stress reduction . Psychological Medicine. August 18, 2020. [Epub ahead of print].
- Intarakamhang U, Macaskill A, Prasittichok P. Mindfulness interventions reduce blood pressure in patients with non-communicable diseases: a systematic review and meta-analysis . Heliyon. 2020;6(4):e03834.
- Khoo E-L, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioural therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis . Evidence-Based Mental Health. 2019;22(1):26-35.
- Levine GN, Lange RA, Bairey-Merz CN, et al. Meditation and cardiovascular risk reduction: a scientific statement from the American Heart Association . Journal of the American Heart Association. 2017;6(10):e002218.
- Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial . Lancet Psychiatry . 2018;5(12):975-986.
- Niles BL, Mori DL, Polizzi C, et al. A systematic review of randomized trials of mind-body interventions for PTSD . Journal of Clinical Psychology . 2018;74(9):1485-1508.
- Rogers JM, Ferrari M, Mosely K, et al. Mindfulness-based interventions for adults who are overweight or obese: a meta-analysis of physical and psychological health outcomes . Obesity Reviews . 2017;18(1):51-67.
- Rosenkranz MA, Dunne JD, Davidson RJ. The next generation of mindfulness-based intervention research: what have we learned and where are we headed? Current Opinion in Psychology. 2019;28:179-183.
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials . Annals of the New York Academy of Sciences . 2019;1445(1):5-16.
- Schell LK, Monsef I, Wöckel A, et al. Mindfulness-based stress reduction for women diagnosed with breast cancer. Cochrane Database of Systematic Reviews. 2019;3(3):CD011518. Accessed at cochranelibrary.com on June 3, 2022.
- Semple RJ, Droutman V, Reid BA. Mindfulness goes to school: things learned (so far) from research and real-world experiences . Psychology in the Schools. 2017;54(1):29-52.
- Shires A, Sharpe L, Davies JN, et al. The efficacy of mindfulness-based interventions in acute pain: a systematic review and meta-analysis . Pain . 2020;161(8):1698-1707.
- Van Dam NT, van Vugt MK, Vago DR, et al. Mind the hype: a critical evaluation and prescriptive agenda for research on mindfulness and meditation . Perspectives on Psychological Science. 2018;13(1):36-61.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Other References
- American Academy of Pediatrics Section on Integrative Medicine. Mind-body therapies in children and youth. Pediatrics . 2016;138(3):e20161896.
- Coronado-Montoya S, Levis AW, Kwakkenbos L, et al. Reporting of positive results in randomized controlled trials of mindfulness-based mental health interventions. PLoS One . 2016;11(4):e0153220.
- Dakwar E, Levin FR. The emerging role of meditation in addressing psychiatric illness, with a focus on substance use disorders. Harvard Review of Psychiatry . 2009;17(4):254-267.
- Goyal M, Singh S, Sibinga EMS, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine. 2014;174(3):357-368.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research . Washington, DC: National Academies Press; 2011.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry. 1992;149(7):936-943.
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300(11):1350-1352.
- McKeering P, Hwang Y-S. A systematic review of mindfulness-based school interventions with early adolescents. Mindfulness . 2019;10:593-610.
- Muratori P, Conversano C, Levantini V, et al. Exploring the efficacy of a mindfulness program for boys with attention-deficit hyperactivity disorder and oppositional defiant disorder. Journal of Attention Disorders . 2021;25(11):1544-1553.
- Nahin RL, Rhee A, Stussman B. Use of complementary health approaches overall and for pain management by US adults. JAMA. 2024;331(7):613-615.
- Poissant H, Mendrek A, Talbot N, et al. Behavioral and cognitive impacts of mindfulness-based interventions on adults with attention-deficit hyperactivity disorder: a systematic review. Behavioural Neurology . 2019;2019:5682050.
- Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Comparative Effectiveness Review no. 227. Rockville, MD: Agency for Healthcare Research and Quality; 2020. AHRQ publication no. 20-EHC009.
- Stieger JR, Engel S, Jiang H, et al. Mindfulness improves brain–computer interface performance by increasing control over neural activity in the alpha band. Cerebral Cortex . 2021;31(1):426-438.
- Teasdale JD, Segal ZV, Williams JMG, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology . 2000;68(4):615-623.
- Weng HY, Lewis-Peacock JA, Hecht FM, et al. Focus on the breath: brain decoding reveals internal states of attention during meditation. Frontiers in Human Neuroscience . 2020;14:336.
- Yoshida K, Takeda K, Kasai T, et al. Focused attention meditation training modifies neural activity and attention: longitudinal EEG data in non-meditators. Social Cognitive and Affective Neuroscience . 2020;15(2):215-223.
- Yuan JP, Connolly CG, Henje E, et al. Gray matter changes in adolescents participating in a meditation training. Frontiers in Human Neuroscience . 2020;14:319.
- Zhang J, Díaz-Román A, Cortese S. Meditation-based therapies for attention-deficit/hyperactivity disorder in children, adolescents and adults: a systematic review and meta-analysis. Evidence-Based Mental Health . 2018;21(3):87-94.
Acknowledgments
Thanks to Elizabeth Ginexi, Ph.D., Erin Burke Quinlan, Ph.D., and David Shurtleff, Ph.D., NCCIH, for their review of this 2022 publication.
This publication is not copyrighted and is in the public domain. Duplication is encouraged.
NCCIH has provided this material for your information. It is not intended to substitute for the medical expertise and advice of your health care provider(s). We encourage you to discuss any decisions about treatment or care with your health care provider. The mention of any product, service, or therapy is not an endorsement by NCCIH.
Related Topics
Pain: Considering Complementary Approaches (eBook)
For Consumers
8 Things to Know About Meditation and Mindfulness
For Health Care Providers
Use of Yoga, Meditation, and Chiropractic by Adults and Children
Mind and Body Approaches for Chronic Pain
Meditation - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
Meditation - Randomized Controlled Trials (PubMed®)
Research Results
National Survey Reveals Increased Use of Yoga, Meditation, and Chiropractic Care Among U.S. Adults
National Survey Reveals Increased Use of Yoga and Meditation Among U.S. Children
Mindfulness-Based Stress Reduction, Cognitive-Behavioral Therapy Shown To Be Cost Effective for Chronic Low-Back Pain
- Open access
- Published: 30 August 2024
Academic burnout syndrome among medical students in Serbia: prevalence of high risk and determinants
- Irena Ilic 1 &
- Milena Ilic 2
BMC Medical Education volume 24 , Article number: 948 ( 2024 ) Cite this article
Metrics details
Although burnout syndrome has been described in numerous research studies, the data on burnout syndrome among medical students in developing countries is scanty. This study aimed to determine the prevalence of high-risk for burnout syndrome and its associated factors among university undergraduate medical students in Serbia.
An observational, analytical, cross-sectional study was conducted using the Maslach Burnout Inventory-Student Survey and a survey on associated factors. The research was carried out from February to March 2014. Multivariate logistic regression was used to determine the adjusted odds ratio (Odds Ratio, OR) with 95% Confidence Interval (95%CI) in order to identify independent correlates of high risk of burnout syndrome.
A high risk for burnout syndrome was noted in 15.0% of respondents; that is, 114/760 medical students suffered from high emotional exhaustion, high cynicism, and low academic efficiency. According to the results of multivariate logistic regression, the main significant independent correlates of high risk for burnout syndrome in medical students were: age of students (for aged 22–24: adjusted OR = 5.64, 95%CI = 2.58–12.34, p < 0.001 for aged ≥ 25: adjusted OR = 5.65, 95%CI = 2.08–15.39, p = 0.001) with p for trend < 0.001, higher frequency of alcohol consumption (for habit 1–2 times a week: adjusted OR = 2.01, 95%CI = 1.01–4.03, p = 0.048) with p for trend = 0.025, and use of sedatives (adjusted OR = 3.44, 95%CI = 1.31–9.04; p = 0.012).
The present study identified several factors associated with burnout syndrome in Serbian medical students. Some factors associated with the high risk of burnout syndrome in medical students are modifiable. It is important to carry out similar research on burnout syndrome in the future, especially using longitudinal studies, in order to evaluate the associations found in this cross-sectional study.
Peer Review reports
Introduction
Burnout syndrome in students is a psychological term for long-term emotional exhaustion, a gradual decline in interest in studies, and a decrease in academic efficiency, which occurs due to prolonged stress during studies [ 1 ]. Burnout syndrome is specific in particular for occupations characterized by working with people in emotionally demanding situations and represents a triad of symptoms that include emotional exhaustion, cynicism and reduced professional efficiency [ 2 ]. Emotional exhaustion refers to an individual’s assessment that his emotional strength has been exhausted beyond limits. Depersonalization (cynicism) refers to developing a callous and cynical attitude towards the people who receive the service/patients and a negative attitude towards studying. The feeling of reduced personal achievement refers to a negative self-assessment of competencies and achievements in studies [ 3 , 4 , 5 ].
Previous research shows that medical students are in study groups with very stressful studies. As such, they may be at an increased risk for burnout syndrome [ 6 ]. The highly competitive medical faculty entrance exam, along with difficult transitions from preclinical to clinical training, the reality of dealing with critically ill patients with a poor prognosis are traditional stressor patterns in the life of most medical students [ 7 ]. For medical students, developing excellent skills and fulfilling the requirements to be good experts is a challenge. One of the sequelae of efforts accompanying medical education is academic burnout or burnout syndrome [ 8 ]. Studies have shown that the occurrence of at least one symptom of burnout syndrome in medical students can cause negative effects that not only interfere with teaching/learning, but also cause sleepiness, emotional instability, serious professional and personal consequences, including lack of professionalism (e.g., altruism or self-control, and serious thoughts of dropping out of studies) [ 9 , 10 , 11 , 12 , 13 , 14 ]. Quantitative studies of the burnout syndrome have been possible due to the development of reliable and valid instruments [15…]. Although other instruments for the assessment of burnout syndrome in medical students have appeared in the meantime, the Maslach Burnout Inventory - Student Survey (MBI-SS) remains the “gold standard” for burnout assessment [ 3 ].
Some recent systematic literature reviews and meta-analyses showed that about one out of two medical students worldwide have burnout syndrome [ 15 , 16 , 17 ]. The authors noted a slightly higher prevalence of burnout syndrome among medical students in countries in Oceania and the Middle East than in other areas. On the other hand, some authors indicated that the pooled prevalence of burnout syndrome among medical students in low- and middle-income countries was 12.1% [ 18 ]. Overall, the prevalence of burnout syndrome in medical students has shown a wide range from 2 to 76% [ 19 , 20 , 21 , 22 ]. The authors reported marked heterogeneity in the results between countries of different economic statuses, with different applied research instruments, cutoff criteria for burnout syndrome, etc. [ 18 , 19 , 22 , 23 ]. Several studies of the prevalence of burnout syndrome in medical students have been conducted in Serbia [ 24 , 25 , 26 ], but only one applied the validated MBI-SS questionnaire in the research [ 27 ].
A variety of demographic factors correlate with burnout syndrome in medical students, including age, sex, whether the student came from an urban or rural setting, marital status, and the number of children, although the findings were not consistent [ 8 , 16 , 19 , 28 ]. In addition, burnout levels were associated with medical students’ maternal education [ 29 ]. Numerous studies identified that senior students experience high levels of burnout, with third and fourth-year students reporting significantly higher scores than younger students [ 30 ]. Some authors reported a statistically significant positive correlation between burnout syndrome and a lower grade point average [ 31 ]. The use of recreational drugs (like marijuana and tramadol) was noted in medical students who had higher burnout scores and reported the presence of a chronic illness and alcohol consumption [ 22 , 32 ]. This study aimed to examine the prevalence of high risk for burnout and identify factors associated with high risk for burnout in medical students.
The study was carried out at the Faculty of Medical Sciences of the University of Kragujevac, Serbia. The Faculty of Medical Sciences in Kragujevac is the youngest state medical faculty in Serbia, founded in 1977. The study program at integrated academic medical studies is organized through blocks (semesters), and medical studies consist of 12 blocks. Teaching by courses is realized through lectures, exercises, other forms of teaching and professional practice lasting six years. The teaching process is carried out using interactive programs oriented towards students, conditions are provided for greater engagement of students during lectures, with constant checking of their knowledge. A relatively small number of students are enrolled in medical studies (only 96 students in recent years), in order to reach the set quality standards of the program. In order to obtain the professional title of the doctor of medicine, a student must pass 35 compulsory and 6 optional courses (out of 15 offered), complete professional practice and defend a diploma (final) thesis. The research was carried out from February to March 2014, and the survey was conducted in the amphitheater and lecture halls at the Faculty of Medical Sciences in Kragujevac. Before the start of the survey, medical students were given a presentation on “burnout syndrome”, with detailed information about this research. All medical students enrolled in the academic year were invited to participate in the study through a direct personal verbal approach by the researcher. Then the students were given a questionnaire, which contained written information about all details of the research, a form for voluntary informed consent, and a questionnaire. Data collection was carried out during the regular curriculum, while students attended routine activities (theoretical activities), i.e. outside the evaluation period of the curriculum. The researcher (the first author of this paper) was present all the time during the survey, to whom the students could ask questions or turn to for help in order to resolve any doubts related to the survey. Only limited demographic data was collected in this survey, to ensure the anonymity of respondents and to encourage participation and honest responses to the survey.
Study design
An epidemiological study was conducted using a cross-sectional design to assess the prevalence of high-risk for burnout and its associated factors in medical students (STROBE checklist – Additional file 1).
Study population
The research included all medical students at the Faculty of Medical Sciences of the University of Kragujevac (including both regular and repeat-year students) from all six years of study.
Study sample
All students enrolled in all six academic years of undergraduate medical studies at the Faculty of Medical Sciences in Kragujevac were invited to participate in the survey through a direct personal verbal address by the researcher. Data collection was carried out during the regular curriculum while students attended routine activities (theoretical activities), i.e., outside of the evaluation period of the curriculum. The criteria for the inclusion of subjects in the study were that they are of age 18 or over, that they attend regular classes, that they have been given voluntary written consent to participate in the study. The criteria for excluding subjects from the study were age 18 age under 18, absence from regular classes, or the existence of any other objective reason that prevents or hinders participation in the study.
In the study, a convenient sample of 760 participants (out of a total of 836 students) voluntarily filled out the questionnaire (response rate = 90.9%).
Ethical considerations
This study is a part of research approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (Ref. No.: 01-1176). All participants provided informed written voluntary consent prior to taking part. All participants provided consent for publication.
Questionnaires were distributed during the class to all medical students attending the class, along with a cover letter providing information about the study and a written consent form to participate in the research. All respondents were informed that the researchers will be exclusively responsible for the data obtained in this study, who are therefore also responsible for the privacy of the respondents. For participation in this observational epidemiological study, the subjects were not financially or in any other way compensated. Medical students faced no consequences for refusing to participate in the study. Also, study participants did not have any conveniences due to participating in the study compared to students who did not participate in the study. Also, all participants were informed that they could withdraw from the study at any stage without any consequences. The study was not sponsored, and the researchers received no compensation for participating in the study’s realization.
Data collection
Data collection was carried out in the classrooms of the Faculty of Medical Sciences. Before the start of the study, i.e., the survey, a notice was sent out to the heads of departments requesting their cooperation in the research, in terms of using a few minutes of the lecture class in the auditorium to explain the research to the students and administer the questionnaires. Also, the protocol and objectives of the study were described in the letter, and the anonymity of the participants was emphasized.
Medical students who met the criteria for inclusion in the study were asked to fill out the epidemiological questionnaire and the MBI-SS questionnaire. Respondents had 15 min (± 5 min, depending on the respondents’ cooperation) to complete the questionnaire. The respondents filled in the questionnaires independently during the class.
In this research, we collected only limited demographic data to ensure the anonymity of the respondents and to encourage participation and honest answers to the questions in the questionnaire. All medical students (from the first to the sixth year of studies) were invited to participate in this study on the first day of lectures when students were present at the beginning of the semester according to their schedules and classrooms.
Characteristics of medical students are categorized and included age (years: ≤ 21 / 22–24 / ≥ 25), gender (male / female), place of residence (Urban / Rural), completed secondary school (Grammar school / Medical school), marital status (With partner / Without partner), having children (No / Yes), housing (In own home / With parents / As subtenants / In student dormitory), study financing (State-sponsored / Self-funded), study year (1st / 2nd / 3rd / 4th / 5th / 6th), repeat-year students (No / Yes), length of study (years: ≤ 6 / > 6), cigarette smoking (Never / Ever), smoking status (Non-smokers / Former smokers / Current smokers), Yes / No for sports / recreational activity / positive personal medical history / use of sedatives / use of psychoactive substances, training (Preclinical / Clinical). Medical students were considered as smokers if they regularly smoked at least one cigarette per day for one year, and were classified as current smokers (if they had smoked at least one cigarette every day for the last 12 months), and as former smokers (if at least one year passed since smoking cessation).
The survey included questions about the habit of alcohol consumption (alcohol consumption related to the regular intake of any amounts of the beverages during medical studies, while those who consumed were then asked about the frequency (1–2 times a year / 1–2 times a month / 1–2 times a week / Every day), types of alcoholic beverages (Beer / Wine / Spirits / Mixed), Binge drinking). Binge drinking was defined as the consumption of 5 + standard drinks for men and 4 + standard drinks for women on one occasion at least once a month during the last year preceding of this survey. In Serbia, a “standard” drink is any drink that contains about 13 g of “pure” alcohol, whereby it was noted in the survey that a standard bottle of beer, as well as a glass of wine and a shot of spirits represented measures of consumption.
The study included 760 out of 836 medical students who met the participation criteria. Absence from regular classes was the main reason for exclusion from the study (45 students) (Fig. 1 ). After being informed about the research, out of the total number of medical students who met the criteria for inclusion (791 students), 12 refused to participate. The reason for not accepting or refusing to participate in the survey was most often a lack of interest in the study. After signing the voluntary informed consent to participate in the study (779 students), some subjects did not return the questionnaire or did not complete the questionnaire during recruitment for the study, or the questionnaires were not completely filled out (19 students in total). For this reason, the data of these respondents were excluded from the analysis. In our survey, the response rate was 90.9%. The research flow diagram is shown in Fig. 1 .

Research flow diagram
Statistical analysis
Descriptive and analytical statistics were used for the data analysis. The categorical variables were presented as proportions (percentages). The dependent variable was the level of risk for burnout in medical students. Confounding variables are socio-demographic characteristics (age, gender, place of residence, marital status, completed high school, etc.), academic performance (year of study, length of study), way of financing studies, housing, habits (cigarette smoking, alcohol use), positive personal medical history (presence of any chronic diseases), sport, recreational activity.
Univariate and multivariate logistic regression analyses were used to evaluate variables that could correlate with burnout syndrome. Univariate logistic regression was used to determine the crude odds ratio (Odds Ratio, OR) with 95% Confidence Interval (95%CI) in order to assess the association between burnout syndrome and selected characteristics of the study participants. Multivariate logistic regression analysis was used to determine the adjusted OR with 95%CI to identify independent correlates of the high risk of burnout syndrome.
Adjusting was done for all variables associated with the burnout syndrome in the univariate analysis models with values of p < 0.50. In addition, the definitive model of the multivariate analysis included other variables that, according to the literature data, were associated with the occurrence of burnout syndrome. The Hosmer-Lemeshow goodness-of-fit test and the Cox and Snell, and Nagelkerke values assessed the goodness of fit of the regression models. Among the observed variables, multicollinearity was investigated. The test for linear trend was based on a logistic regression model. For all independent factors, the level of significance was p < 0.05. All statistical analyses were performed using the IBM SPSS Statistics version 20.0 (SPSS, Chicago, USA).
The analysis was divided into two parts, with the following tasks: (1) to determine the prevalence of burnout syndrome and (2) to determine the associated factors of burnout syndrome among medical students.
Of the 760 medical students who filled out the questionnaire, there were 269 (35.4%) men and 491 (64.6%) women (Table 1 ). The majority of students were between the ages of 22 and 24 (327; 43.0%), 256 (33.7%) students were 25 or older, while 177 (23.3%) were 21 or younger. The average age of the respondents was 23.7 ± 2.7 years (with a range of 19–36). The largest number of respondents (over 90%) indicated the city as their place of permanent residence. More than half of the students (65.5%) graduated from medical school.
A high risk for burnout syndrome was noted in 15.0% of respondents, that is, 114 medical students suffered from high emotional exhaustion, high cynicism, and low academic efficiency (Table 1 ).
In medical students, the risk of high levels of burnout syndrome was significantly lower in females than in males ( p = 0.024) (Table 2 ). A higher risk for burnout syndrome was recorded in older age groups of medical students, with statistical significance for the trend ( p = 0.002). Compared with first-year students, a higher risk of burnout syndrome is more often reported among students in higher studies, with statistical significance for the trend ( p < 0.001. Repeat-year students ( p = 0.016) and students with a longer duration of studies ( p = 0.038) often showed a high risk for burnout syndrome. The habits of drinking 1–2 times a week and every day were significantly more common in medical students who had a high risk of burnout syndrome (with significance for trend p = 0.001). The use of certain types of alcoholic beverages and binge drinking were not associated with high risk of burnout syndrome. The use of sedatives was linked to a high risk of burnout in medical students ( p = 0.007). The use of psychoactive substances was associated with a high risk of burnout in medical students ( p = 0.014).
The following variables entered the final model of the multivariate logistic regression analysis (i.e., variables that were significantly associated with a high risk for burnout syndrome in medical students at the p < 0.50 level in the univariate logistic regression analysis model): gender, age, length of study, re-enrollment year, frequency of alcohol consumption, use of sedatives and use of psychoactive substances (Table 3 ). Due to collinearity with age, the variable - study year was excluded from the definitive model. According to the results of multivariate logistic regression, the main significant independent correlates of high risk for burnout syndrome in medical students were: age of students (for 22–24 aged: adjusted OR = 5.64, 95%CI = 2.58–12.34, p < 0.001 for aged ≥ 25: adjusted OR = 5.65, 95%CI = 2.08–15.39, p = 0.001) with p for trend < 0.001 higher frequency of alcohol consumption (for habit 1–2 times a week: adjusted OR = 2.01, 95%CI = 1.01–4.03, p = 0.048) with p for trend = 0.025) and use of sedatives (adjusted OR = 3.44, 95%CI = 1.31–9.04; p = 0.012).
This study is one of the first attempts to assess the prevalence of high-risk of burnout syndrome among medical students in Serbia, as well as to analyze the role of demographic characteristics and academic performance of students in burnout. A high risk for burnout syndrome in medical students was noted in 15.0% of respondents. The main significant independent correlates of high risk for burnout syndrome in medical students were age, higher frequency of alcohol consumption, and use of sedatives.
A comprehensive review and meta-analysis of literature published in countries around the world reported different prevalence rates of burnout syndrome in medical students, with an overall range of 7.0–75.2% [ 19 ]. A recent meta-analysis suggests that one in two medical students worldwide suffers from burnout: the prevalence of burnout (covering 17,431 medical students) was 44.2% (8060 students suffered from burnout) [ 15 ]. The prevalence of burnout was higher in countries in Oceania (55.9%) and the Middle East (53.7%) than in North America − 45.8%, Asia − 40.6%, Europe − 27.5%, South and Central America – 26.0%. The high risk for burnout syndrome in this study was recorded in 15.0% of medical students. Compared to medical students in Kragujevac, a lower prevalence of burnout was recorded in medical students of two universities in Brazil (10.3% and 14.9%) [ 20 , 36 ] and preclinical medical students in Spain (14.8%) [ 37 ], while higher prevalence was found in medical students in Great Britain (26.7%) [ 38 ], Ethiopia (34.0%) [ 39 ], Pakistan (30.6%) [ 9 ], as well as in the USA, India, Malaysia, and Saudi Arabia (45–70%) [ 21 , 30 , 40 , 41 , 42 ]. Some possible reasons for differences in the incidence of burnout among medical students include differences in culture, socioeconomic status, and study population. Also, some studies included only third- and fourth-year medical students, while our study included students from all six years of studies. In addition, different burnout assessment questionnaires and sample sizes may contribute to differences in the prevalence of burnout syndrome. Comparison of the results of this study with data in the literature may be difficult for a number of reasons, including the use of different measuring instruments and different threshold values for the assessment of burnout syndrome, the use of different criteria for defining burnout syndrome, significant variability in medical school curricula between universities, etc. [ 43 ]. A definition of burnout syndrome that includes a high score on both the emotional exhaustion and depersonalization subscales and a low personal achievement score (according to the MBI questionnaire) may result in an underestimation of burnout [ 44 , 45 , 46 ]. Using a definition of burnout syndrome that includes high scores on the emotional exhaustion subscale or the depersonalization subscale but not a low score on the personal accomplishment subscale [ 21 , 47 ] may result in overestimating the frequency of burnout syndrome [ 48 ].
In the present study, the age of medical students was significantly associated with burnout syndrome. Some studies found that older medical students were more likely to have burnout [ 29 ], in contrast to other studies that found no association between age and burnout [ 49 ]. Similarly to our study, studies in the US [ 21 ] and Pakistan [ 9 ] showed that the high risk for burnout syndrome was significantly more often noted in senior medical students than in the youngest students. But, these findings must be interpreted with caution. The possible explanation for that is that the increase in age is associated with higher academic years. Besides, an increase in age is intertwined with experience (either in studies or social), so the question of a secondary association with burnout syndrome is always raised.
In this study, a factor that was independently associated with a high risk of developing burnout in medical students was frequent alcohol consumption. To date, only a few studies have reported the association between burnout and alcohol consumption in medical students [ 21 , 50 ]. Similar results were reported in a study in the USA, where the frequency of risky alcohol use decreased in older medical students, and the frequency of burnout syndrome increased [ 21 ]. Among UK medical students, higher alcohol intake was significantly associated with higher personal achievement scores [ 38 ]. Findings that burnout can result from alcohol consumption suggest that the association between alcohol consumption and stress is not unidirectional [ 50 ]. Namely, some studies have shown that certain people can use alcohol as a method of coping with stress [ 51 , 52 ]. On the other hand, a UK study found that young drinkers have a “hedonic” approach to excessive alcohol consumption, suggesting that medical students may be drinking for pleasure rather than coping with stress and burnout [ 53 ]. According to the National Health Research Study in Serbia in 2013 [ 54 ], about 1.3% of the population aged 15 to 34 consumed alcohol daily in the previous 12 months. Drinking alcoholic beverages in Serbia is a socially acceptable behavior (as part of traditions and customs), which results in a high prevalence of alcohol use, especially among young people.
In this study, a factor that was independently associated with a high risk of developing burnout syndrome in medical students was the use of sedatives. In a study in Brazil [ 55 ], about 12% of medical students in all years of study used anxiolytics. In a multicenter study in France [ 56 ], in a population of medical students, it was found that first-year students consumed 1.5 times more anxiolytics compared to second-year students: the authors of the study relate this result to the pressure that first-year students have to pass their first exams, resulting in higher rates of mood and anxiety disorders. Among medical students in Cameroon, a significant interaction between various predictors (chronic disease, alcohol consumption, and burnout syndrome) and the outcome - recreational drug use [ 22 ] was noted. The question is whether the use of drugs (antidepressants, anxiolytics, sedatives) can be an indicator of the existence of these primary pathologies, thereby making the occurrence of burnout symptoms more likely. It is uncertain whether the use of sedatives directly causes students to burnout more or whether students who are already underachieving and experiencing high levels of stress turn to recreational drug use as a source of comfort [ 22 ]. As education about drug abuse, as well as alcohol and illicit substances, is part of the core curriculum of medical schools [ 57 , 58 ], this association should be investigated in future studies.
It is difficult to compare the results of this study with similar studies in the world for a number of reasons: different research designs used, application of other questionnaires, use of non-validated questionnaires, variations in the concept and construct of burnout syndrome (with consequent assessment of burnout syndrome as a one-dimensional, two-dimensional or three-dimensional concept), different response rates, differences in the studied populations (in terms of age structure, gender, etc.), studies of all or only selected years of studies, study curriculum, length of studies. A better understanding of the correlates of burnout syndrome in medical students is key to determining solutions to prevent burnout. It is important to carry out similar studies of burnout syndrome in the future, especially using longitudinal studies, in order to confirm the association found in prevalence studies.
The results of this study provide practical implications for students, educators and organization of medical school programs. Namely, identification of factors associated with burnout syndrome, i.e. higher frequency of alcohol consumption and use of sedatives, gives guidance for interventions aimed at preventing alcohol and sedatives use and abuse among medical students, through providing sufficient information about these issues during the studies and conducting training for helping medical students identify and provide peer support to persons at risk of concerning use of these substances. Further on, learning about student burnout should be embedded in curriculums of medical schools in order to raise awareness about this issue and reduce fear about unsuccess in medical studies [ 59 ]. The classes, practical exercises and exams should be organized in a way that enables a balance between the studies and private life. Finally, services aimed at providing student support should exist and deliver both individual and group interventions intended to educate medical students about coping strategies, increasing resilience, stress management and encouragement of a healthy lifestyle [ 60 , 61 ].
Respondents were included in this research through recruitment at one medical faculty, whereby all respondents had the same chance to participate in the study. Consequently, the sample was not selected, which suggests the study sample is representative of the entire population of medical students at the University of Kragujevac. Also, the response rate was high (90.9%). Our research used the validated Serbian version of the MBI-SS questionnaire [ 27 ].
However, this study has several limitations. In addition to the known shortcomings of the cross-sectional study design (such as “ecological fallacy”), a limitation of this study is the use of a self-report questionnaire. Although the principle of anonymity was applied during the survey, the existence of information bias cannot be ruled out with certainty because, although the privacy of all information was guaranteed, there is always the possibility that some respondents did not want to reveal the symptoms of burnout. The limitation of this study can be - the limitation of non-response (non-response bias). Although the response rate is high, there may still be response bias, as individuals suffering from burnout may not have been in class at the time of data collection or, may have chosen not to participate in the study, or may have been more or less likely to respond to the survey. Also, a potential source of response bias can be the phenomenon of social desirability, that is, giving socially desirable answers, rather than true answers, in circumstances where sometimes the presence of classmates, teachers and the overall classroom environment can have a significant impact on responses. In this study, the impact of social desirability bias is reduced by using a survey that relies on self-reports, the survey being anonymous and not containing socially unacceptable questions, as well as by the high response rate. Also, since the study was conducted at one faculty, the results may only be representative for some medical students in Serbia. But, although this study was carried out at one medical faculty, the results of the study can be generalized to a considerable extent, since a sample without selection bias was provided in the study, the sample was unselected, all respondents had the same chance to participate in the study, and a high response rate was achieved (response rate was 90.9%). Further, this study did not provide data on other potential predictors of burnout syndrome (such as socioeconomic status, family history, etc.) that could influence the occurrence of burnout syndrome in medical students. Even with these limitations, this study offers an assessment of burnout syndrome among medical students. Since this is a cross-sectional study, the correlations found may not be actual causative factors, and these findings should be verified in future research.
Conclusions
The prevalence of burnout syndrome in Serbian medical students was estimated to be 15.0%. Burnout syndrome among medical students in Serbia showed a high correlation with the age of students, a higher frequency of alcohol consumption, and the use of sedatives. Those at-risk medical students should be identified to provide adequate support.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Schaufeli WB, Buunk BP, Burnout. An overview of 25 years of research in theorizing. In: Schabracq MJ, Winnubst JAM, Cooper CL, editors. The handbook of work and health psychology. Chichester, UK: Wiley; 2003. pp. 383–425.
Google Scholar
Maslach C, Jakson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99–113.
Article Google Scholar
Schaufeli WB, Martinez I, Marques Pinto A, Salanova M, Bakker AB. Burnout and engagement in university students: a cross-national study. J Cross Cult Psychol. 2002;33(5):464–841.
Dyrbye L, Shanafelt T. A narrative review on burnout experienced by medical students and residents. Med Educ. 2016;50(1):132–49.
Pagnin D, de Queiroz V, Carvalho YT, Dutra AS, Amaral MB, Queiroz TT. The relation between burnout and sleep disorders in medical students. Acad Psychiatry. 2014;38(4):438–44.
Fares J, Saadeddin Z, Al Tabosh H, et al. Extracurricular activities associated with stress and burnout in preclinical medical students. J Epidemiol Glob Health. 2016;6(3):177–85.
Schaufeli WB. Burnout: a short Socio-Cultural History. In: Neckel S, Schaffner AK, Wagner G, editors. Burnout, fatigue, exhaustion: an interdisciplinary perspective on a modern affliction. London, UK: Palgrave Macmillan/Springer Nature; 2017. pp. 105–27.
Chapter Google Scholar
Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15(2):103–11.
Muzafar Y, Khan HH, Ashraf H, et al. Burnout and its Associated factors in medical students of Lahore, Pakistan. Cureus. 2015;7(11):e390.
Lyndon MP, Henning MA, Alyami H, et al. Burnout, quality of life, motivation, and academic achievement among medical students: a person-oriented approach. Perspect Med Educ. 2017;6(2):108–14.
Arora A, Kannan S, Gowri S, Choudhary S, Sudarasanan S, Khosla PP. Substance abuse amongst the medical graduate students in a developing country. Indian J Med Res. 2016;143(1):101–3.
Mian A, Kim D, Chen D, Ward WL. Medical Student and Resident Burnout: a review of Causes, effects, and Prevention. J Fam Med Dis Prev. 2018;4:094.
Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334–41.
Dyrbye LN, Thomas MR, Power DV, et al. Burnout and serious thoughts of dropping out of medical school: a multi-institutional study. Acad Med. 2010;85(1):94–102.
Frajerman A, Morvan Y, Krebs MO, Gorwood P, Chaumette B. Burnout in medical students before residency: a systematic review and meta-analysis. Eur Psychiatry. 2019;55:36–42.
Almutairi H, Alsubaiei A, Abduljawad S, et al. Prevalence of burnout in medical students: a systematic review and meta-analysis. Int J Soc Psychiatry. 2022;68(6):1157–70.
Li Y, Cao L, Mo C, Tan D, Mai T, Zhang Z. Prevalence of burnout in medical students in China: a meta-analysis of observational studies. Med (Baltim). 2021;100(26):e26329.
Kaggwa MM, Kajjimu J, Sserunkuma J, et al. Prevalence of burnout among university students in low- and middle-income countries: a systematic review and meta-analysis. PLoS ONE. 2021;16(8):e0256402. https://doi.org/10.1371/journal.pone.0256402 .
Erschens R, Keifenheim KE, Herrmann-Werner A, et al. Professional burnout among medical students: systematic literature review and meta-analysis. Med Teach. 2019;41(2):172–83.
Costa EF, Santos SA, Santos AT, Melo EV, Andrade TM. Burnout syndrome and associated factors among medical students: a cross-sectional study. Clin (Sao Paulo). 2012;67(6):573–80.
Dyrbye LN, Thomas MR, Shanafelt TD. Systematic review of depression, anxiety, and other indicators of psychological distress among U.S. and Canadian medical students. Acad Med. 2006;81(4):354–73.
Mbanga CM, Efie DT, Aroke D, Njim T. Prevalence and predictors of recreational drug use among medical and nursing students in Cameroon: a cross sectional analysis. BMC Res Notes. 2018;11(1):515.
Youssef FF. Medical student stress, Burnout and Depression in Trinidad and Tobago. Acad Psychiatry. 2016;40(1):69–75.
Todorovic J, Terzic-Supic Z, Divjak J, et al. Validation of the study burnout inventory and the Copenhagen Burnout Inventory for the use among medical students. Int J Occup Med Environ Health. 2021;34(6):737–45.
Backović DV, Zivojinović JI, Maksimović J, Maksimović M. Gender differences in academic stress and burnout among medical students in final years of education. Psychiatr Danub. 2012;24(2):175–81.
Ilić Živojinović J, Backović D, Belojević G, Valčić O, Soldatović I, Janković J. Predictors of burnout among Belgrade veterinary students: a cross-sectional study. PLoS ONE. 2020;15(3):e0230685.
Ilic M, Todorovic Z, Jovanovic M, Ilic I. Burnout syndrome among medical students at one University in Serbia: validity and reliability of the Maslach Burnout Inventory-Student Survey. Behav Med. 2017;43(4):323–8.
Ishak W, Nikravesh R, Lederer S, Perry R, Ogunyemi D, Bernstein C. Burnout in medical students: a systematic review. Clin Teach. 2013;10(4):242–5.
Wang Q, Wang L, Shi M, et al. Empathy, burnout, life satisfaction, correlations and associated socio-demographic factors among Chinese undergraduate medical students: an exploratory cross-sectional study. BMC Med Educ. 2019;19(1):341.
Chunming WM, Harrison R, MacIntyre R, Travaglia J, Balasooriya C. Burnout in medical students: a systematic review of experiences in Chinese medical schools. BMC Med Educ. 2017;17(1):217. https://doi.org/10.1186/s12909-017-1064-3 .
Shadid A, Shadid AM, Shadid A, et al. Stress, Burnout, and Associated Risk factors in medical students. Cureus. 2020;12(1):e6633. https://doi.org/10.7759/cureus.6633 .
Njim T, Makebe H, Toukam L, et al. Burnout syndrome amongst medical students in Cameroon: a cross-sectional analysis of the determinants in preclinical and clinical students. Psychiatry J. 2019;2019:4157574. https://doi.org/10.1155/2019/4157574 .
Schutte N, Toppinen S, Kalimo R, Schaufeli WB. The factorial validity of the Maslach Burnout Inventory-General Survey (MBI-GS) across occupational groups and nations. J Occup Organ Psychol. 2000;73(1):53–66. https://doi.org/10.1348/096317900166877 .
Maslach C, Leiter MP, Schaufeli WB. Measuring burnout. In: Cooper CL, Cartwright S, editors. The Oxford Handbook of Organizational Well-Being. Oxford, UK: Oxford University Press; 2009. pp. 86–108.
Ilic I, Zivanovic Macuzic I, Kocic S, Ilic M. High risk of burnout in medical students in Serbia, by gender: a cross-sectional study. PLoS ONE. 2021;16(8):e0256446. https://doi.org/10.1371/journal.pone.0256446 .
Almeida GC, Souza HR, Almeida PC, Almeida BC, Almeida GH. The prevalence of burnout syndrome in medical students. Arch Clin Psychiatry (São Paulo). 2016;43(1):6–10.
Galán F, Sanmartín A, Polo J, Giner L. Burnout risk in medical students in Spain using the Maslach Burnout Inventory-Student Survey. Int Arch Occup Environ Health. 2011;84(4):453–9.
Cecil J, McHale C, Hart J, Laidlaw A. Behaviour and burnout in medical students. Med Educ Online. 2014;19:25209. https://doi.org/10.3402/meo.v19.25209 .
Haile YG, Senkute AL, Alemu BT, Bedane DM, Kebede KB. Prevalence and associated factors of burnout among Debre Berhan University medical students: a cross-sectional study. BMC Med Educ. 2019;19(1):413. https://doi.org/10.1186/s12909-019-1864-8 .
West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306(9):952–60.
Bera T, Biswas NM, Mandal A, et al. Burn out among medical students-a study across three medical colleges in eastern India. Ind Med Gaz. 2013;9:356–9.
Chin RWA, Chua YY, Chu MN, et al. Prevalence of burnout among Universiti Sains Malaysia Medical Students. Educ Med J. 2016;8(3):61–74.
Prins JT, Gazendam-Donofrio SM, Tubben BJ, van der Heijden FM, van de Wiel HB, Hoekstra-Weebers JE. Burnout in medical residents: a review. Med Educ. 2007;41(8):788–800.
Guthrie E, Black D, Shaw C, Hamilton J, Creed F, Tomenson B. Psychological stress in medical students: a comparison of two very different courses. Stress Med. 1997;13:179–84.
Guthrie E, Black D, Bagalkote H, Shaw C, Campbell M, Creed F. Psychological stress and burnout in medical students: a five-year prospective longitudinal study. J R Soc Med. 1998;91(5):237–43.
Santen SA, Holt DB, Kemp JD, Hemphill RR. Burnout in medical students: examining the prevalence and associated factors. South Med J. 2010;103(8):758–63.
Willcock SM, Daly MG, Tennant CC, Allard BJ. Burnout and psychiatric morbidity in new medical graduates. Med J Aust. 2004;181(7):357–60.
Brenninkmeijer V, VanYperen N. How to conduct research on burnout: advantages and disadvantages of a unidimensional approach in burnout research. Occup Environ Med. 2003;60(Suppl 1):i16–20.
Almalki SA, Almojali AI, Alothman AS, Masuadi EM, Alaqeel MK. Burnout and its association with extracurricular activities among medical students in Saudi Arabia. Int J Med Educ. 2017;8:144–50.
Pickard M, Bates L, Dorian M, Greig H, Saint D. Alcohol and drug use in second-year medical students at the University of Leeds. Med Educ. 2000;34(2):148–50.
Williams A, Clark D. Alcohol consumption in university students: the role of reasons for drinking, coping strategies, expectancies, and personality traits. Addict Behav. 1998;23(3):371–8.
Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101(1):139–52.
Szmigin I, Griffin C, Mistral W, Bengry-Howell A, Weale L, Hackley C. Re-framing ‘binge drinking’ as calculated hedonism: empirical evidence from the UK. Int J Drug Policy. 2008;19(5):359–66.
Ministry of Health of Serbia, Institute of Public Health of Serbia. National Health Survey 2013 — Key findings. Ministry of Health of Serbia, Belgrade. 2014. https://www.batut.org.rs/download/publikacije/2013SerbiaHealthSurvey.pdf (accessed December 2023).
Petroianu A, Reis DC, Cunha BD, Souza DM. Prevalence of alcohol, tobacco and psychotropic drug use among medical students at the Universidade Federal de Minas Gerais. Rev Assoc Med Bras (1992). 2010;56(5):568 – 71.
Fond G, Bourbon A, Boucekine M, et al. First-year French medical students consume antidepressants and anxiolytics while second-years consume non-medical drugs. J Affect Disord. 2020;265:71–6.
Moffat KJ, McConnachie A, Ross S, Morrison JM. First year medical student stress and coping in a problem-based learning medical curriculum. Med Educ. 2004;38(5):482–91.
Newbury-Birch D, Walshaw D, Kamali F. Drink and drugs: from medical students to doctors. Drug Alcohol Depend. 2001;64(3):265–70.
Prendergast M, Cardoso Pinto AM, Harvey CJ, Muir E. Burnout in early year medical students: experiences, drivers and the perceived value of a reflection-based intervention. BMC Med Educ. 2024;24(1):7. https://doi.org/10.1186/s12909-023-04948-0 .
Walsh AL, Lehmann S, Zabinski J, et al. Interventions to prevent and reduce Burnout among Undergraduate and Graduate Medical Education Trainees: a systematic review. Acad Psychiatry. 2019;43(4):386–95.
Williams D, Tricomi G, Gupta J, Janise A. Efficacy of burnout interventions in the medical education pipeline. Acad Psychiatry. 2015;39(1):47–54.
Download references
Acknowledgements
This study is conducted as the part of project No 175042 supported by Ministry of Education, Science and Technological development, Republic of Serbia, 2011-2022. We would like to thank the leadership of the Faculty of Medical Sciences Kragujevac for their support and the medical students for their participation in the study. The authors thank Milena Jovanovic and Zeljko Todorovic, who partially helped in data collection and data entry.
This research received no external funding.
Author information
Authors and affiliations.
Faculty of Medicine, University of Belgrade, Belgrade, Serbia
Department of Epidemiology, Faculty of Medical Sciences, University of Kragujevac, S. Markovica 69, Kragujevac, 34000, Serbia
Milena Ilic
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, II and MI; methodology, II and MI; validation, II and MI; formal analysis, II and MI; investigation, II and MI; resources, II and MI; data curation, II and MI; writing-original draft preparation, II; writing-review and editing, II and MI; visualization, II and MI; supervision, MI; project administration. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Correspondence to Milena Ilic .
Ethics declarations
Ethics approval and consent to participate.
This study is a part of research approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (Ref. No.: 01-1176). All participants provided informed written voluntary consent prior to taking part.
Consent for publication
All participants provided consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ilic, I., Ilic, M. Academic burnout syndrome among medical students in Serbia: prevalence of high risk and determinants. BMC Med Educ 24 , 948 (2024). https://doi.org/10.1186/s12909-024-05937-7
Download citation
Received : 20 March 2024
Accepted : 21 August 2024
Published : 30 August 2024
DOI : https://doi.org/10.1186/s12909-024-05937-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Burnout syndrome
- Risk factors
- Medical students
- Cross-sectional study
BMC Medical Education
ISSN: 1472-6920
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Abstract. Alcohol is a major contributor to global disease and a leading cause of preventable death, causing approximately 88,000 deaths annually in the United States alone. Alcohol use disorder is one of the most common psychiatric disorders, with nearly one-third of U.S. adults experiencing alcohol use disorder at some point during their lives.
Alcohol consumption has been identified as an important risk factor for illness, disability, and mortality (Rehm et al. 2009b).In fact, in the last comparative risk assessment conducted by the World Health Organization (WHO), the detrimental impact of alcohol consumption on the global burden of disease and injury was surpassed only by unsafe sex and childhood underweight status but exceeded ...
The main purpose of this review article is to enable any person reading this article to get a comprehensive insight into the effects of alcohol on the various systems of the human body, and for the same, many recognized research articles published in numerous well-acknowledged journals across the globe are reviewed.
Alcohol has a much longer history than any of the other intoxicants or stimulants. It is so intertwined in so many cultures, we invented reasons to justify its use. Alcohol has been condemned in all ancient literature and scripts. Bard commented that," Alcohol provokes the desire but takes away the performance".
Alcohol Research: Current Reviews (ARCR) ARCR, a peer-reviewed scientific journal published by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health, marks its 50th anniversary in 2024. Explore our "News & Notes" webpage for more on this historic accomplishment.
Alcohol, Clinical and Experimental Research. Alcohol, Clinical and Experimental Research is a multi-disciplinary journal providing direct access to the most significant and current research findings on the nature and management of alcoholism and alcohol-related disorders. Increase your chance of being published through our unaccepted manuscript ...
Discover the Alcohol and Alcoholism articles that your peers and the public are talking about, and the news stories they have been ... and Alcoholism welcomes submissions, publishing papers on the biomedical, psychological, and sociological aspects of alcoholism and alcohol research. To gain more information please see the Instructions to ...
The most common disease categories that are entirely or partly caused by alcohol consumption include infectious diseases, cancer, diabetes, neuropsychiatric diseases (including alcohol use disorders), cardiovascular disease, liver and pancreas disease, and unintentional and intentional injury. Knowledge of these disease risks has helped in the ...
Excessive alcohol use is the cause of an ongoing public health crisis, and accounts for ~5% of global disease burden. A minority of people with recreational alcohol use develop alcohol addiction ...
Alcohol is regularly consumed throughout most of the world, including by nearly half the U.S. population age 12 or older. Heavy drinking, which is also common, contributes to multiple adverse medical, psychiatric, and social outcomes and more than 140,000 deaths annually in the United States. It is the major risk factor for alcohol use disorder (AUD), whose current U.S. prevalence is 11% ...
Alcohol use disorders consist of disorders characterised by compulsive heavy alcohol use and loss of control over alcohol intake. Alcohol use disorders are some of the most prevalent mental disorders globally, especially in high-income and upper-middle-income countries; and are associated with high mortality and burden of disease, mainly due to medical consequences, such as liver cirrhosis or ...
The morbidity and mortality associated with alcohol are largely due to the high rates of alcohol use disorder in the population. Alcohol use disorder is defined in the Diagnostic and Statistical Manual for Mental Disorders, 5th edition (DSM-5) as a pattern of alcohol consumption, leading to problems associated with 2 or more of 11 potential symptoms of alcohol use disorder (see Table 1 for ...
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain ...
Alcohol Research Resource (R24 and R28) Awards. Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner. Current and potential alcohol research investigators and trainees are encouraged to subscribe to our ...
To celebrate the anniversary, NIAAA hosted a 2-day symposium, "Alcohol Across the Lifespan: 50 Years of Evidence-Based Diagnosis, Prevention, and Treatment Research," devoted to key topics within the field of alcohol research. This article is based on Dr. Sinha's presentation at the event.
History of Neurobiological Studies in Alcohol Research. Looking at publications from the early 1970s, one is struck by the lack of research on alcohol's actions on the brain. However, closer consideration shows that there also was a lack of neurobiology research in general; moreover, most of the techniques critical to modern neuroscience were ...
Globally, alcohol use is the seventh leading risk factor for both deaths and DALYs (disability-adjusted life years). 1 In India, per-capita consumption of alcohol has increased over time, along with the risk of its public health impact. 2 Besides, the treatment gap for alcohol use disorders (AUDs) is 86%. 3 The relapse rates among untreated patients with AUDs are high. 4 Although the relapse ...
Alcohol as a mediator. Inspired by the Actor Network Theory (ANT), we draw attention to how nonhuman objects - in this case alcohol - act on users, engage in practices, and operate in networks (assemblages) (Latour, Citation 2005, p. 68).The actor-network refers to the relations between human and non-human actors (Latour, Citation 1994), and in the context of this study, the relations ...
Find current medical research and in-depth information on alcoholism, symptoms and treatment of alcoholism as well as clinical depression. Expand your understanding of alcohol's effect on the ...
Alcohol use disorder is characterized by an inability to control drinking, a preoccupation with alcohol and continued use despite negative consequences. According to the World Health Organization, 400 million people, or 7% of the world's population ages 15 and older, had an alcohol use disorder in 2019. Yet the primary prescribed medications ...
Recent research has focused on areas such as the genetics of addiction, links between excessive alcohol use and mental health and other disorders, harm to long-term brain health that can be caused by adolescent alcohol use, and the effects of prenatal alcohol exposure, among others. "We want everyone from pharmacists and nurses to addiction ...
Moreover, not all patients benefit from Alcohol-ApBM, and previous research did not identify reliable pretreatment predictors of Alcohol-ApBM effectiveness. Therefore, the current study focused on learning processes during the Alcohol-ApBM training itself. Specifically, we examined whether changes in approach-avoidance tendencies over the ...
Michael Golding receives funding through a Medical Research Grant from the W. M. Keck Foundation and a research grant from the NIH through the National Institute on Alcohol Abuse and Alcoholism ...
There are important limitations to research on alcohol consumption. Most rely on self-reporting, do not analyze binge drinking, do not assess alcohol consumption over a lifetime, or do not account for the fact that some study subjects may reduce their alcohol consumption due to alcohol-related health problems. Still, this new research is among ...
There are no advance articles currently available. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
That research has paved the way for the development and application of new methods and therapies and will continue to influence treatment practice in the future. This article reviews the origins of alcoholism treatment and major studies of behavioral therapies and medications for treating alcohol dependence.
Alcohol companies that once alienated women with ads suffused with sexist imagery are now marketing directly and more effectively to women, according to a 2020 report by investment research and brokerage firm Bernstein Research. Diageo, one of the world's largest spirit conglomerates, ...
Introduction. Factors that determine mental health are both complex and interrelated [].Globally, around 1 in 8 people are living with a diagnosed mental health disorder [] - although this is likely an underestimate of the true proportion of people living with mental ill health.Existing research shows a focus on individual susceptibility and life experiences such as childhood trauma.
In a 2012 U.S. survey, 1.9 percent of 34,525 adults reported that they had practiced mindfulness meditation in the past 12 months. Among those responders who practiced mindfulness meditation exclusively, 73 percent reported that they meditated for their general wellness and to prevent diseases, and most of them (approximately 92 percent) reported that they meditated to relax or reduce stress.
Although burnout syndrome has been described in numerous research studies, the data on burnout syndrome among medical students in developing countries is scanty. This study aimed to determine the prevalence of high-risk for burnout syndrome and its associated factors among university undergraduate medical students in Serbia. An observational, analytical, cross-sectional study was conducted ...