- Search Menu
- Sign in through your institution
- Advance Articles
- Supplements
- Author Guidelines
- Reviewer guidelines
- Submission Site
- Open Access
- Self-Archiving Policy
- Why Publish?
- Advertising & Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Paediatrics & Child Health
- About The Canadian Paediatric Society
- Editorial Board
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
Case 1 diagnosis: allergy bullying, clinical pearls.
- < Previous
Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation
- Article contents
- Figures & tables
- Supplementary Data
Lopamudra Das, Michelle GK Ward, Case 1: A 12-year-old girl with food allergies and an acute asthma exacerbation, Paediatrics & Child Health , Volume 19, Issue 2, February 2014, Pages 69–70, https://doi.org/10.1093/pch/19.2.69
- Permissions Icon Permissions
A 12-year-old girl with a history of asthma presented to the emergency department with a three-day history of increased work of breathing, cough and wheezing. She reported no clear trigger for her respiratory symptoms, although she had noted some symptoms of a mild upper respiratory tract infection. With this episode, the patient had been using a short-acting bronchodilator more frequently than she had in the past, without the expected resolution of symptoms.
On the day of presentation, the patient awoke feeling ‘suffocated’ and her mother noted her lips to be blue. In the emergency department, her oxygen saturation was 85% and her respiratory rate was 40 breaths/min. She had significantly increased work of breathing and poor air entry bilaterally to both lung bases, with wheezing in the upper lung zones. She was treated with salbutamol/ipratropium and received intravenous steroids and magnesium sulfate. Her chest x-ray showed hyperinflation and no focal findings.
Her medical history revealed that she was followed by a respirologist for her asthma, had good medication adherence and had not experienced a significant exacerbation for six months. She also had a history of wheezing, dyspnea and pruritis with exposure to peanuts, chickpeas and lentils; she had been prescribed an injectible epinephrine device for this. However, her device had expired at the time of presentation. In the past, her wheezing episodes had been seasonal and related to exposure to grass and pollens; this presentation occurred during the winter. Further history revealed the probable cause of her presentation.
Although reluctant to disclose the information, our patient later revealed that she had been experiencing significant bullying at school, which was primarily related to her food allergies. Three days before her admission, classmates had smeared peanut butter on one of her schoolbooks. She developed pruritis immediately after opening the book and she started wheezing and coughing later that day. This event followed several months of being taunted with peanut products at school. The patient was experiencing low mood and reported new symptoms of anxiety related to school. The review of systems was otherwise negative, with no substance use.
The patient's asthma exacerbation resolved with conventional asthma treatment. Her pulmonary function tests were nonconcerning (forced expiratory volume in 1 s 94% and 99% of predicted) after her recovery. The trigger for her asthma exacerbation was likely multifactorial, related to exposure to the food allergen as well as the upper respiratory infection. A psychologist was consulted to assess the symptoms of anxiety and depression that had occurred as a result of the bullying. During the hospitalization, the medical team contacted the patient's school to provide education on allergy bullying, treatment of severe allergic reactions and its potential for life-threatening reactions with exposure to allergens. The medical team also recommended community resources for further education of students and staff about allergy bullying and its prevention.
Allergy bullying is a form of bullying with potentially severe medical outcomes. In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study [ 1 ]), this bullying is related directly to the food allergy. From a medical perspective, there are little published data regarding allergy bullying, and many health care providers may not be aware of the issue.
Allergy bullying can include teasing a child about their allergy, throwing food at a child, or even forcing them to touch or eat allergenic foods. Most episodes of allergy bullying occur at school, and can include episodes perpetrated by teachers and/or staff ( 2 ).
Allergy bullying can lead to allergic reactions, which may be mild or severe (eg, urticaria, wheezing, anaphylaxis), but may also lead to negative emotional consequences (sadness, depression) ( 2 ) and an overall decrease in quality of life measures ( 1 ). Adolescents commonly resist using medical devices, such as injectible epinephrine devices, and bullying may be a contributing factor for this ( 3 ). Attempting to conceal symptoms in a bullying situation may place children at risk for a worse outcome.
Physicians can play a key role in detecting allergy bullying and its health consequences. In many cases, children have not discussed this issue with their parents ( 1 ). Given the prevalence of bullying, its potential to lead to severe harm, including death, and the lack of awareness of this issue, clinicians should specifically ask about bullying in all children and teens with allergies. Physicians can also work with families and schools to support these children, educate their peers and school staff, and help prevent negative health outcomes from allergy bullying.
Online resources
www.anaphylaxis.ca − A national charity that aims to inform, support, educate and advocate for the needs of individuals and families living with anaphylaxis, and to support and participate in research. This website includes education modules for schools and links to local support groups throughout Canada.
www.whyriskit.ca/pages/en/live/bullying.php − A website for teenagers with food allergies; includes a segment that addresses food bullying.
www.foodallergy.org − Contains numerous resources for children and their families, including a significant discussion on bullying and ways to prevent it.
Allergy bullying is common but is often unrecognized as a factor in clinical presentations of allergic reactions.
Physicians should make a point of asking about bullying in patients with allergies and become familiar with resources for dealing with allergy bullying.
Physicians can play roles as advocates, educators and collaborators with the school system to help make the school environment safer for children with allergies who may be at risk for allergy bullying.
Google Scholar
| Month: | Total Views: |
|---|---|
| February 2017 | 2 |
| June 2017 | 2 |
| November 2017 | 3 |
| December 2017 | 6 |
| January 2018 | 3 |
| February 2018 | 5 |
| March 2018 | 7 |
| April 2018 | 10 |
| May 2018 | 8 |
| June 2018 | 13 |
| July 2018 | 3 |
| August 2018 | 5 |
| September 2018 | 8 |
| October 2018 | 6 |
| November 2018 | 8 |
| December 2018 | 22 |
| January 2019 | 1 |
| February 2019 | 8 |
| March 2019 | 3 |
| April 2019 | 13 |
| May 2019 | 9 |
| June 2019 | 5 |
| July 2019 | 17 |
| August 2019 | 12 |
| September 2019 | 15 |
| October 2019 | 6 |
| November 2019 | 3 |
| December 2019 | 4 |
| January 2020 | 8 |
| February 2020 | 10 |
| March 2020 | 16 |
| April 2020 | 44 |
| May 2020 | 65 |
| June 2020 | 102 |
| July 2020 | 119 |
| August 2020 | 104 |
| September 2020 | 221 |
| October 2020 | 286 |
| November 2020 | 391 |
| December 2020 | 336 |
| January 2021 | 456 |
| February 2021 | 492 |
| March 2021 | 661 |
| April 2021 | 646 |
| May 2021 | 662 |
| June 2021 | 507 |
| July 2021 | 389 |
| August 2021 | 484 |
| September 2021 | 628 |
| October 2021 | 860 |
| November 2021 | 752 |
| December 2021 | 627 |
| January 2022 | 599 |
| February 2022 | 698 |
| March 2022 | 922 |
| April 2022 | 913 |
| May 2022 | 728 |
| June 2022 | 450 |
| July 2022 | 299 |
| August 2022 | 406 |
| September 2022 | 668 |
| October 2022 | 956 |
| November 2022 | 915 |
| December 2022 | 771 |
| January 2023 | 649 |
| February 2023 | 707 |
| March 2023 | 769 |
| April 2023 | 646 |
| May 2023 | 748 |
| June 2023 | 428 |
| July 2023 | 356 |
| August 2023 | 387 |
| September 2023 | 567 |
| October 2023 | 743 |
| November 2023 | 691 |
| December 2023 | 524 |
| January 2024 | 493 |
| February 2024 | 667 |
| March 2024 | 743 |
| April 2024 | 767 |
| May 2024 | 750 |
| June 2024 | 333 |
| July 2024 | 283 |
| August 2024 | 489 |
Email alerts
Citing articles via, looking for your next opportunity.
- About Paediatrics & Child Health
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1918-1485
- Print ISSN 1205-7088
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Case report
- Open access
- Published: 21 February 2018
Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
- Virginia Mirra 1 ,
- Silvia Montella 1 &
- Francesca Santamaria 1
BMC Pediatrics volume 18 , Article number: 73 ( 2018 ) Cite this article
11k Accesses
14 Citations
12 Altmetric
Metrics details
The primary goal of asthma management is to achieve disease control for reducing the risk of future exacerbations and progressive loss of lung function. Asthma not responding to treatment may result in significant morbidity. In many children with uncontrolled symptoms, the diagnosis of asthma may be wrong or adherence to treatment may be poor. It is then crucial to distinguish these cases from the truly “severe therapy-resistant” asthmatics by a proper filtering process. Herein we report on four cases diagnosed as difficult asthma, detail the workup that resulted in the ultimate diagnosis, and provide the process that led to the prescription of omalizumab.
Case presentation
All children had been initially referred because of asthma not responding to long-term treatment with high-dose inhaled steroids, long-acting β 2 -agonists and leukotriene receptor antagonists. Definitive diagnosis was severe asthma. Three out four patients were treated with omalizumab, which improved asthma control and patients’ quality of life. We reviewed the current literature on the diagnostic approach to the disease and on the comorbidities associated with difficult asthma and presented the perspectives on omalizumab treatment in children and adolescents. Based on the evidence from the literature review, we also proposed an algorithm for the diagnosis of pediatric difficult-to-treat and severe asthma.
Conclusions
The management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma. The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism in the management of children and adolescents with atopic severe asthma.
Peer Review reports
Children with poor asthma control have an increased risk of severe exacerbations and progressive loss of lung function, which results in the relevant use of health resources and impaired quality of life (QoL) [ 1 ]. Therefore, the primary goal of asthma management at all ages is to achieve disease control [ 2 , 3 , 4 ].
According to recent international guidelines, patients with uncontrolled asthma require a prolonged maintenance treatment with high-dose inhaled corticosteroids (ICS) in association with a long-acting β 2 -agonist (LABA) plus oral leukotriene receptor antagonist (LTRA) (Table 1 ) [ 5 ].
Nevertheless, in the presence of persistent lack of control, reversible factors such as adherence to treatment or inhalation technique should be first checked for, and diseases that can masquerade as asthma should be promptly excluded. Finally, additional strategies, in particular anti-immunoglobulin E (anti-IgE) treatment (omalizumab), are suggested for patients with moderate or severe allergic asthma that remains uncontrolled in Step 4 [ 5 ].
Herein, we reviewed the demographics, clinical presentation and treatment of four patients with uncontrolled severe asthma from our institution in order to explain why we decided to prescribe omalizumab. We also provided a review of the current literature that focuses on recent advances in the diagnosis of pediatric difficult asthma and the associated comorbidities, and summarizes the perspectives on anti-IgE treatment in children and adolescents.
Case presentations
Table 2 summarizes the clinical characteristics and the triggers/comorbidities of the cases at referral to our Institution. Unfortunately, data on psychological factors, sleep apnea, and hyperventilation syndrome were not available in any case. Clinical, lung function and airway inflammation findings at baseline and after 12 months of follow-up are reported in Table 3 . In the description of our cases, we used the terminology recommended by the ERS/ATS guidelines on severe asthma [ 6 ].
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 11, severe asthma was diagnosed. Sensitization to multiple inhalant allergens (i.e., house dust mites, dog dander, Graminaceae pollen mix, and Parietaria judaica ) and high serum IgE levels (1548 KU/l) were found. Body mass index (BMI) was within normal range. Combined treatment with increasing doses of ICS (fluticasone, up to 1000 μg/day) in association with LABA (salmeterol, 100 μg/day) plus LTRA (montelukast, 5 mg/day) has been administered over 2 years. Nevertheless, persistent symptoms and monthly hospital admissions due to asthma exacerbations despite correct inhaler technique and good adherence were reported. Parents refused to perform any test to exclude gastroesophageal reflux (GER) as comorbidity [ 6 ]. However, an ex-juvantibus 2-month-course with omeprazole was added to asthma treatment [ 7 ], but poor control persisted. Anterior rhinoscopy revealed rhinosinusitis that was treated with nasal steroids for six months [ 8 ], but asthma symptoms were unmodified. Treatment with omalizumab was added at age 12. Reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 2.0 up to 6.7 out of a maximum of 7 points) were documented over the following months. Unfortunately, after one year of treatment, adherence to omalizumab decreased because of family complaints, and eventually parents withdrew their informed consent and discontinued omalizumab. Currently, by age 17, treatment includes inhaled salmeterol/fluticasone (100 μg/500 μg∙day -1 , respectively) plus oral montelukast (10 mg/day). Satisfactory symptom control is reported, with no asthma exacerbations.
A full-term male, who had a recurrent severe preschool wheezing, at 6 years of age developed exercise-induced asthma. At age 10, severe asthma was diagnosed. High serum IgE levels (1300 KU/l) and skin prick tests positive to house dust mites were found. Despite a 3-year treatment with progressively increasing doses of inhaled fluticasone (up to 1000 μg/day) combined with salmeterol (100 μg/day) and oral montelukast (5 mg/day), monthly hospital admissions with systemic steroids use were reported. At age 13, a 24-h esophageal impedance/pH study demonstrated the presence of acid and non-acid GER [ 7 ]. Esomeprazole was added to asthma medications, but with an incomplete clinical benefit for respiratory symptoms. Esomeprazole was withdrawn after 3 months, and parents refused to re-test for GER. As respiratory symptoms persisted uncontrolled despite treatment, severe asthma was definitively diagnosed [ 6 ]. BMI was within the normal range and anterior rhinoscopy excluded rhinosinusitis. Inhaler technique and adherence were good; thus we considered the anti-IgE treatment option [ 9 ]. Subcutaneous omalizumab was started, with fast improvement of both symptoms and QoL score (from 3.9 up to 6.5). Seventeen months later, the dose of ICS had been gradually tapered and oral montelukast definitely discontinued. Currently, at age 14, treatment includes the combined administration of bimonthly subcutaneous omalizumab and of daily inhaled salmeterol/fluticasone (50 μg/100 μg∙day - 1 , respectively). Asthma control is satisfactory and no side effects are reported. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 3, recurrent, severe asthma exacerbations with acute respiratory failure that frequently required intensive care unit (ICU) admission. At age 6, sensitization to multiple perennial inhalant (i.e., house dust mites, dog and cat danders, Alternaria alternata , Graminaceae pollen mix, Artemisia vulgaris , Parietaria judaica , and Olea europaea pollen) and food allergens (i.e., egg, milk, and peanut) was diagnosed. Serum IgE levels were 2219 KU/l. Weight and height were appropriate for age and sex. The patient has been treated over 3 years with a combined scheme of high-dose inhaled fluticasone (up to 1000 μg/day) plus salmeterol (100 μg/day) and oral montelukast (5 mg/day), with correct inhaler technique and good adherence. Despite this, monthly hospital admissions with systemic steroids use were recorded. Rhinosinusitis and GER were excluded on the basis of appropriate testing; thus treatment with omalizumab was started when the patient was 9 years old. At age 11, adherence to treatment is satisfactory, with no side effects. More importantly, reduced hospital admissions for asthma exacerbations, no further need for systemic steroids, and improved QoL score (from 6.4 to 6.8) were reported. Finally, progressive step-down of anti-asthma treatment was started, and at present (by 11.5 years) inhaled fluticasone (200 μg/day) plus bimonthly subcutaneous omalizumab provide good control of symptoms. Omalizumab has been continuously administered for 2.6 years and is still ongoing.
A full-term male had severe preschool wheezing and, since age 4, recurrent, severe asthma exacerbations with frequent hospital admissions. At age 8, multiple perennial inhalants and food sensitization (i.e., house dust mites, dog dander, Graminaceae pollen mix, Olea europaea pollen, tomatoes, beans, shrimps, and peas) and high serum IgE levels (1166 KU/l) were found. The patient has been treated over 5 years with inhaled fluticasone (up to 1000 μg/day) in association with salmeterol (100 μg/day) and oral montelukast (5 mg/day). Despite this, monthly hospital admissions with systemic steroids need were recorded. After checking the inhaler technique and adherence to treatment, comorbidities including obesity, rhinosinusitis and GER were excluded. Omalizumab was proposed, but parents refused it. By 13.6 years, despite a treatment including the association of inhaled salmeterol/fluticasone (100 μg/1000 μg∙day − 1 , respectively) plus oral montelukast (10 mg/day), monthly exacerbations requiring systemic steroids are reported.
Discussion and conclusions
Most children and adolescents with asthma respond well to inhaled short-acting beta 2 -agonists (SABA) on demand if symptoms are intermittent, or to low dose controller drugs plus as-needed SABA if the risk of exacerbations increases [ 1 ]. Nevertheless, a proportion of patients is referred to specialists because this strategy is not working and asthma is persistently uncontrolled [ 4 ]. For these children, assessment is primarily aimed at investigating the reasons for poor control. Indeed, when the child is initially referred, before the label of “severe, therapy-resistant asthma” (i.e., not responding to treatment even when factors as exposure to allergens and tobacco smoke have been considered) is assigned, three main categories need to be identified: 1) “not asthma at all”, in which response to treatment is suboptimal because the diagnosis is wrong; 2) “asthma plus ”, when asthma is mild but exacerbated by one or more comorbidities; and 3) “difficult-to-treat asthma”, when asthma is uncontrolled because of potentially reversible factors [ 10 ].
The reported cases highlight some aspects of the disease process that may expand the diagnosis and improve patients’ care. At our institution, the severe asthma program includes a multidisciplinary approach with consultations by gastroenterologists as well as ear, nose and throat experts. Recently, sleep medicine experts joined this multidisciplinary team; thus, unfortunately, sleep-disordered breathing (SDB) could not be excluded at the time of our patients’ assessment. Inhalation technique is periodically evaluated by nurses or doctors in each patient. Unfortunately, in Italy an individual prescription database is not available and thus we cannot assess patients’ use of medication. In two cases, the filtering process eventually identified GER and rhinosinusitis, but poor control of asthma persisted even after comorbidities were treated. In all subjects, inhaler skills, treatment adherence, and environmental exposure to indoor/outdoor allergens as well as to second- and third-hand smoke were excluded as cause of lack of control. Eventually, three out of four patients started anti-IgE treatment; asthma control was obtained and maintenance drugs were progressively reduced. In the case that refused omalizumab therapy, pulmonary function, clinical features and controller treatment including high-dose ICS were unchanged.
Previous studies have highlighted an association between increasing asthma severity in children and reduced QoL [ 11 , 12 , 13 ]. Uncontrolled asthma symptoms not only affect children physically, but can impair them socially, emotionally, and educationally [ 13 ]. In line with previous observations, 3 out 4 of our cases had poor QoL, assessed by a standardized questionnaire [ 14 ]. It is well known that improving QoL in difficult asthma is not an easy task, despite a variety of treatments aimed at achieving control [ 12 ], and much more remains to be done to address the problem. Nevertheless, 2 of our 3 cases showed a remarkable improvement of QoL after one year of treatment with omalizumab.
Reduction in forced expiratory volume in the first second (FEV 1 ) is often used to define childhood asthma severity in treatment guidelines and clinical studies [ 5 , 11 , 15 ]. Nevertheless, children with severe asthma often have a normal FEV 1 that does not improve after bronchodilators, indicating that spirometry may be a poor predictor of asthma severity in childhood [ 6 , 16 , 17 ]. Actually, children with a normal FEV 1 , both before and after β 2 -agonist, may show a bronchodilator response in terms of forced expiratory flow between 25% and 75% (FEF 25–75 ) [ 18 ]. However, the utility of FEF 25–75 in the assessment or treatment of severe asthma is currently unknown. Interestingly, all the reported cases showed normal or slightly reduced values of FEV 1 but severe impairment of FEF 25–75 . Two cases showed a bronchodilator response in terms of FEV 1 (subjects 3 and 4), while 3 patients had a significant increase of FEF 25–75 (cases 1, 3 and 4). Unfortunately, we could not provide the results of bronchodilator response during or after the treatment with omalizumab in any case.
Available literature on the diagnostic approach to difficult asthma in children offers a number of reviews which basically summarize the steps needed to fill the gap between a generic diagnosis of “difficult asthma” and more specific labels (i.e., “severe” asthma, “difficult-to-treat” asthma, or even different diagnoses) [ 3 , 5 , 6 , 8 , 10 , 19 , 20 , 21 ]. So far, few original articles and case reports have been published, probably due to the peculiarity of the issue, which makes retrospective discussion of cases easier than the design of a prospective clinical study [ 4 , 22 , 23 , 24 , 25 , 26 ]. Available knowledge mainly derives from the experience of specialized centers.
The evaluation of a child referred for uncontrolled asthma should start with a careful history focused on typical respiratory symptoms and on the definition of possible triggers. In the “severe asthma” process, it is crucial for clinicians to maintain a high degree of skepticism about the ultimate diagnosis, particularly in the presence of relevant discrepancies between history, physical features and lung function, as many conditions may be misdiagnosed as asthma. In order to simplify this process, herein we propose an algorithm for the diagnosis of difficult-to-treat and severe asthma (Fig. 1 ). Confirmation of the diagnosis through a detailed clinical and laboratory re-evaluation is important because in 12–50% of cases assumed to have severe asthma this might not be the correct diagnosis [ 10 ]. Several documents have indicated the main steps of the process that should be followed in children with uncontrolled asthma [ 3 , 8 , 10 ]. The translation of these procedures into real life practice may deeply change from one subject to another due to the variability of individual patients’ history and clinical features, which will often lead the diagnostic investigations towards the most likely reason for uncontrolled asthma. For children with apparently severe asthma, the first step is to confirm the diagnosis and, before proceeding to broader investigations, to verify that the poor control is not simply determined by poor adherence to treatment, inadequate inhaler skills and/or environmental exposure to triggers. A nurse-led assessment, including a home visit, despite not being applicable in all settings, may be useful for identifying potentially modifiable factors in uncontrolled pediatric asthma [ 27 ].
A practical algorithm for the diagnosis of difficult-to-treat and severe asthma. ICS, inhaled corticosteroids; OCS, oral corticosteroids
A number of comorbidities have been increasingly recognized as factors that may impact asthma clinical expression and control in childhood [ 10 , 28 ]. Children with uncontrolled disease should be investigated for GER, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, obesity, psychological factors, smoke exposure, hormonal influences, and ongoing drugs [ 3 , 6 , 8 , 20 ]. Indeed, the exact role played by comorbidities in pediatric asthma control is still debated [ 28 ]. The most impressive example is GER. Several pediatric documents recommend assessing for GER because reflux may be a contributing factor to problematic or difficult asthma [ 7 , 29 ]. Nevertheless, GER treatment might not be effective for severe asthma [ 30 , 31 ], as confirmed by current cases 1 and 2. There is an established evidence that chronic rhinosinusitis is associated with more severe asthma in children [ 32 , 33 , 34 ]. Therefore, examination of upper airways and ad hoc treatment if rhinosinusitis is evident are recommended in children with severe asthma [ 3 , 8 , 35 ]. However, intranasal steroids for rhinitis resulted in a small reduction of asthma risk in school-aged children [ 36 ], and actual placebo-controlled studies on the effect of treatment of rhinosinusitis on asthma control in children are lacking [ 10 , 37 ].
Dysfunctional breathing, including hyperventilation and vocal cord dysfunction, is associated with poorer asthma control in children [ 8 , 10 , 38 , 39 ]. Unfortunately, there is scarce literature on the effect of its treatment on the control of severe asthma in children [ 40 ]. SDB ranging from primary snoring to obstructive sleep apnea syndrome is very common in children [ 41 ], and an increased prevalence of SDB together with increasing asthma severity has been reported [ 42 ]. Interestingly, GER may also be worsened by recurrent episodes of upper airway obstruction associated with SDB, and this may further trigger bronchial obstruction. Asthma guidelines recommend the assessment of SDB through nocturnal polysomnography in poorly controlled asthmatics, particularly if they are also obese [ 5 ]. There are no studies examining whether pediatric asthma improves after SDB has been treated, for example, with nasal steroids, adenotonsillectomy, continuous positive airway pressure or weight reduction if the child is also obese [ 43 ]. The parallel increase in obesity and asthma suggests that the two conditions are linked and that they can aggravate each other [ 44 , 45 ], even though the exact mechanisms that underlie this association remain unclear [ 46 ]. Indeed, other coexisting comorbidities such as SDB or GER may play a confounding role in the development of the interactions between obesity and the airways [ 47 , 48 ]. Obesity is associated with increased markers of inflammation in serum and adipose tissue and yet decreased airway inflammation in obese people with asthma [ 49 ]. Several interventions, including behavioral and weight reduction programs or bariatric surgery, may result in improved asthma control, quality of life and lung function in adult obese asthmatics [ 50 ]. Although reports of adolescent bariatric surgery demonstrate a significant body weight decrease, this approach is not widely available and there are no published reports on its effect on pediatric severe asthma control [ 51 ]. Finally, although it is still unclear whether food allergy is causative or shares a common pathway with difficult asthma, it might explain the loss of asthma control at least in some children and thus be considered as a comorbid condition [ 10 , 16 , 52 ].
In conclusion, establishing the impact of comorbidities on asthma control may be cumbersome, and an ex-juvantibus treatment is sometimes necessary to assess their role. Comorbid conditions can also worsen each other, and symptoms arising from some of them may mimic asthma [ 6 ]. Although the ability to improve pediatric severe asthma by treating comorbidities remains unconfirmed, they should be treated appropriately [ 9 ].
The vast majority of asthmatic children exhibit a mild or at most a moderate disease that can be fully controlled with low-to-medium dose ICS associated or not with other controllers [ 5 , 6 ]. However, a subset of asthmatics remains difficult-to-treat [ 5 , 6 ]. With the advent of biologics, these severe steroid-dependent asthmatics have alternative options for treatment, as steroid-related adverse events are common in severe asthma [ 53 ]. Omalizumab, an anti-IgE monoclonal antibody, is the only biologic therapy recommended in children with moderate-to-severe asthma by the recent guidelines [ 5 , 6 ]. In Italy, this treatment is fully covered by the National Health System. Therefore, there is no influence by any funding on treatment decisions. It was approved by the US (Food and Drug Administration) in 2003 and by the European Union (European Medicines Agency) in 2005 as an add-on treatment for patients aged > 12 years with severe persistent allergic asthma and who have a positive skin test or in-vitro reactivity to a perennial aeroallergen, FEV 1 < 80% predicted, frequent daytime symptoms or nighttime awakenings, and multiple documented severe asthma exacerbations despite daily ICS plus a LABA [ 54 , 55 ]. In 2009, it also received approval in Europe for treating patients aged 6–12 years. Figure 2 illustrates current indications for treatment with omalizumab in children and adolescents with severe asthma.
Indications for omalizumab in children and adolescents with severe asthma
IgE antibodies, Th 2 -derived cytokines and eosinophils play a major role in the development of chronic airway inflammation in asthmatic subjects [ 56 ]. Once released from plasma cells, IgE binds principally to the high-affinity IgE receptor (FcεRI) on mast cells, triggering different effector responses, including the release of mediators leading to allergic inflammatory reactions [ 56 ]. The activation of the allergic cascade by IgE, under constant allergen stimulation, leads to the establishment of chronic allergic inflammation in the airways of asthmatic patients, with IgE being a key element of the vicious circle that maintains it. Cytokines produced during the late phase and subsequent chronic inflammation stage have been directly associated with the induction of airway remodelling, indirectly implicating IgE in the process [ 56 ]. At present, omalizumab is the only commercially available recombinant humanized anti-IgE monoclonal antibody that specifically binds serum free IgE at its CH 3 domain, in the proximity of the binding site for FcεRI, thus preventing IgE from interacting with its receptor on mast cells, basophils, antigen-presenting cells and other inflammatory cells [ 57 ]. The rapid reduction of free IgE levels leads to a downregulation of the FcεRI expression on inflammatory cells and an interruption of the allergic cascade, which results in the reduction of peripheral and bronchial tissue eosinophilia and of levels of granulocyte macrophage colony stimulating factor, interleukin (IL)-2, IL-4, IL-5, and IL-13 [ 58 ]. Moreover, basophils have a relevant role in the initiation and progression of allergic inflammation, suggesting that they may represent a viable therapeutic target. Indeed, in children with severe asthma, it has been reported that omalizumab therapy is associated with a significant reduction in circulating basophil numbers, a finding that is concurrent with improved clinical outcomes [ 59 ]. This finding supports a mechanistic link between IgE levels and circulating basophil populations, and may provide new insights into one mechanism by which omalizumab improves asthma symptoms.
Several clinical controlled and real-life studies of adults with severe, inadequately controlled allergic asthma have demonstrated the efficacy and safety of omalizumab in reducing asthma-related symptoms, corticosteroid use, exacerbation rates, and healthcare resource utilization, and in improving QoL and lung function [ 60 , 61 , 62 , 63 ]. Fewer studies have been published in children. In two double-blind, randomized, placebo-controlled trials (RCTs) of children aged 6 to 12 years with moderate-to-severe allergic asthma, treatment with omalizumab reduced the requirement for ICS and protected against disease exacerbations, but there was little change in asthma symptom scores or spirometry [ 9 , 64 ]. These findings were confirmed and extended in older children [ 65 , 66 , 67 ].
The results of the ICATA study, a multicenter RCT of 419 inner-city children, adolescents and young adults with persistent allergic asthma, showed that, compared to placebo, omalizumab reduces the number of days with asthma symptoms and the proportion of participants with at least one exacerbation by approximately 25% and 19%, respectively ( p < 0.001), thus reducing the need for asthmatic symptom controllers [ 68 ]. Another multicenter RCT of inner-city children and adolescents showed that the addition of omalizumab to ongoing guidelines-based care before patients return to school reduces fall asthma exacerbations (odds ratio, 0.48), particularly in subjects with a recent exacerbation [ 69 ]. Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases ( p < 0.001), while FEV 1 improved by 4.9% ( p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% ( p < 0.001) [ 70 ]. The same authors also showed that, after two years of treatment, exacerbation rate and healthcare utilisation were further decreased by 83% and 100%, respectively, while level of asthma control, steroid use and lung function remained unchanged [ 71 ].
A systematic review of pediatric RCTs pooled the data of 1381 children and adolescents with moderate-to-severe allergic asthma in order to establish the efficacy of omalizumab as an add-on therapy [ 72 ]. During the stable-steroid phase, omalizumab decreased the number of patients with at least one exacerbation (risk ratio, 0.69; p < 0.001), the mean number of asthma exacerbations per patient (risk ratio, 0.35; p < 0.001), and the asthma symptom score (mean difference, 0.12; p = 0.005) when compared to placebo. During the steroid reduction phase, omalizumab further reduced the number of patients with at least one exacerbation (risk ratio, 0.48; p < 0.001) and the mean number of asthma exacerbations per patient (mean difference, 0.12; p < 0.05).
Given the cost of omalizumab, many authors have argued for the importance of identifying specific asthma populations who will have significant benefit from it [ 68 , 73 , 74 ]. In the ICATA study, baseline predictors of good response to treatment were sensitization and exposure to cockroach allergen, sensitization to house dust mite allergens, a serum IgE level of more than 100 IU per milliliter, a BMI of 25 or more, and a history of at least one unscheduled medical visit in the previous year [ 68 ].
Several studies have assessed the long-term safety of omalizumab in children and adults. A pooled analysis of 67 RCTs conducted over 2 decades on 4254 children and adults treated with omalizumab showed no association between omalizumab treatment and risk of malignancy [ 75 ]. In an RCT evaluating 225 school-aged children, omalizumab was well tolerated, there were no serious adverse events, and the frequency and types of all adverse events were similar to the placebo group [ 9 ]. These results have been further confirmed by a recent systematic review of RCTs that concluded that treatment with omalizumab does not result in increased risk of malignancy or hypersensitivity reactions [ 72 ].
While the rationale for long-term treatment with omalizumab is supported by pharmacokinetic-pharmacodynamic models [ 76 ], the duration of treatment is still under discussion. Results from published studies suggest that omalizumab should be continued for > 1 year [ 77 , 78 ]. In a retrospective study of adults and children with uncontrolled severe asthma treated with omalizumab, the response to treatment was ‘excellent’ in 52.5% of patients, particularly in the subgroup of children aged 6 to 11 years [ 77 ]. After the discontinuation of treatment, loss of asthma control was documented in 69.2% of the patients who had received omalizumab for < 1 year, 59.1% of the subjects treated for 1–2 years, and 46.1% of the cases treated for > 2 years. Time to loss of control was shorter in younger children and longer in patients with an ‘excellent’ response compared with patients with a ‘good’ response. No early loss of control (within 6 months) was observed among patients with > 3.5 years of continuous treatment with omalizumab. Finally, 20% of patients in whom omalizumab was re-prescribed because of loss of control did not respond to the treatment anymore [ 77 ]. Despite these encouraging findings, the impact of omalizumab on the natural history of severe asthma in children deserves to be further investigated by long-term studies that will also define the criteria and timing for discontinuing the treatment.
It is well known that asthma pharmacotherapy is effective in controlling symptoms and bronchial inflammation, but cannot affect the underlying immune response, thus leading to the possibility of symptom reappearance after its discontinuation [ 79 ]. In this scenario, allergen-specific immunotherapy (AIT) has been proposed as the only therapeutic method that can modulate the underlying immune pathophysiology in allergic asthma [ 80 ].
AIT is currently indicated in children and adults with mild-moderate allergic asthma that is completely or partially controlled by pharmacotherapy and with the evidence of a clear relationship between symptoms and exposure to a specific allergen [ 81 , 82 , 83 , 84 ]. However, according to recent guidelines, the efficacy of AIT in asthmatic subjects is limited, and its potential benefits must be weighed against the risk of side effects and the inconvenience and costs of the prolonged therapy [ 5 ]. Moreover, severe or uncontrolled asthma (regardless of its severity) is a major independent risk factor for non-fatal or even fatal adverse reactions, thus representing a contraindication for AIT [ 85 , 86 , 87 ]. Finally, children with severe asthma are often sensitized to multiple allergens, thus making AIT prescription even more complicated [ 88 ].
In subjects with uncontrolled and/or severe allergic asthma, a combination of omalizumab and AIT has been proposed [ 88 ]. Surprisingly, only a few studies have addressed this issue [ 89 , 90 , 91 , 92 ]. However, pre-treatment with omalizumab seems to improve the efficacy and tolerability of subcutaneous AIT in children and adults with severe allergic asthma both during omalizumab treatment and after its discontinuation [ 89 , 91 , 92 ]. Omalizumab has also been successfully used as a supplementary treatment to AIT in order to improve asthma control in children ≥6 years with severe persistent allergic asthma [ 90 ]. Given the scarcity of studies on AIT plus omalizumab in children with severe allergic asthma, further research is warranted to assess risks and benefits of the combined treatment.
Children with severe asthma require a detailed and individualized approach including re-assessment for differential diagnoses, comorbidities and contributory factors, environmental triggers, lung function and inflammation, adherence and response to therapy, and QoL. Treatment of pediatric severe asthma still relies on the maximal optimal use of corticosteroids, bronchodilators and other controllers recommended for moderate-to-severe disease. However, the management of asthma is becoming much more patient-specific, as more and more is learned about the biology behind the development and progression of asthma.
In the current paper, we described the characteristics of four children with severe asthma in whom omalizumab was prescribed. A review of the relevant literature on the topic was also performed. Finally, we provided an algorithm for the diagnosis of difficult-to-treat and severe asthma in children and adolescents, based on the evidence from the literature review. As all algorithms, it is not meant to replace clinical judgment, but it should drive physicians to adopt a systematic approach towards difficult and severe asthma and provide a useful guide to the clinician.
The addition of omalizumab, the first targeted biological treatment approved for asthma, has led to renewed optimism of outcome improvements in patients with allergic severe asthma. As severe asthma is a heterogeneous condition consisting of different phenotypes, the future of asthma management will likely involve phenotypic and potentially even genotypic characterization in selected cases in order to determine appropriate therapy and thus to provide the highest possible benefit, especially if specific responder phenotypes can be identified and selected for this highly specific treatment.
Abbreviations
Anti-immunoglobulin E
Body mass index
IgE receptor
Forced expiratory flow between 25% and 75%
Forced expiratory volume in the first second
Gastroesophageal reflux
Inhaled corticosteroids
Intensive care unit
Interleukin
Long-acting β 2 -agonist
Oral leukotriene receptor antagonist
Quality of life
Randomized controlled trials
Short-acting β 2 -agonists
Sleep-disordered breathing
O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest. 2013;143:511–3.
Article PubMed Google Scholar
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–8.
Article Google Scholar
Hedlin G. Management of severe asthma in childhood-state of the art and novel perspectives. Pediatr Allergy Immunol. 2014;25:111–21.
Konradsen JR, Nordlund B, Lidegran M, Pedroletti C, Grönlund H, van Hage M, et al. Problematic severe asthma: a proposed approach to identifying children who are severely resistant to therapy. Pediatr Allergy Immunol. 2011;22:9–18.
Global Initiative for Asthma Report. Global strategy for asthma management and prevention (updated 2016). https://www.ginasthma.org . Accessed 07 June 2017.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–53.
Article CAS PubMed Google Scholar
Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the north American Society for Pediatric Gastroenterology, Hepatology, and nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–507.
Lødrup Carlsen KC, Hedlin G, Bush A, Wennergren G, de Benedictis FM, De Jongste JC, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37:432–40.
Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics. 2001;108:E36.
Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–5.
Article CAS PubMed PubMed Central Google Scholar
Lang A, Mowinckel P, Sachs-Olsen C, Riiser A, Lunde J, Carlsen KH, et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr Allergy Immunol. 2010;21:945–53.
Nordlund B, Konradsen JR, Pedroletti C, Kull I, Hedlin G. The clinical benefit of evaluating health-related quality-of-life in children with problematic severe asthma. Acta Paediatr. 2011;100:1454–60.
Dean BB, Calimlim BC, Sacco P, Aguilar D, Maykut R, Tinkelman D. Uncontrolled asthma: assessing quality of life and productivity of children and their caregivers using a cross-sectional internet-based survey. Health Qual Life Outcomes. 2010;8:6.
Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46.
British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, 2014. https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline . Accessed 13 Apr 2016.
Montella S, Baraldi E, Cazzato S, Aralla R, Berardi M, Brunetti LM, et al. Severe asthma features in children: a case-control online survey. Ital J Pediatr. 2016;42:9.
Article PubMed PubMed Central Google Scholar
Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25.
Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34.
Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36:196–201.
Fitzpatrick AM, Teague WG. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's severe asthma research program. Pediatr Allergy Immunol Pulmonol. 2010;23:131–8.
Konradsen JR, Caffrey Osvald E, Hedlin G. Update on the current methods for the diagnosis and treatment of severe childhood asthma. Expert Rev Respir Med. 2015;9:769–77.
Lang AM, Konradsen J, Carlsen KH, Sachs-Olsen C, Mowinckel P, Hedlin G, et al. Identifying problematic severe asthma in the individual child—does lung function matter? Acta Paediatr. 2010;99:404–10.
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul WJ. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. Asthma. 2012;49:586–92.
Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma? Pediatrics. 2000;105:354–8.
Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: consider all of the possibilities. Can Respir J. 2000;7:415–8.
Wener RR, Bel EH. Severe refractory asthma: an update. Eur Respir Rev. 2013;22:227–35.
Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–4.
De Groot EP, Kreggemeijer WJ, Brand PL. Getting the basics right resolves most cases of uncontrolled and problematic asthma. Acta Paediatr. 2015;104:916–21.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
American Lung Association Asthma Clinical Research Centers, Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–9.
Article PubMed Central Google Scholar
Writing Committee for the American Lung Association Asthma Clinical Research Centers, Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012;307:373-381.
Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94:895–901.
CAS PubMed Google Scholar
De Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7.
Rotiroti G, Roberts G, Scadding GK. Rhinitis in children: common clinical presentations and differential diagnoses. Pediatr Allergy Immunol. 2015;26:103–10.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA). 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:S8–160.
Deliu M, Belgrave D, Simpson A, Murray CS, Kerry G, Custovic A. Impact of rhinitis on asthma severity in school-age children. Allergy. 2014;69:1515–21.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Weinberger M, Abu-Hasan M. Pseudo-asthma: when cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120:855–64.
De Groot EP, Duiverman EJ, Brand PL. Dysfunctional breathing in children with asthma: a rare but relevant comorbidity. Eur Respir J. 2013;41:1068–73.
Barker NJ, Jones M, O'Connell NE, Everard ML. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013;12:CD010376.
Google Scholar
Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome, American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12.
Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, Weaver DE, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol. 2015;50:1128–36.
Ross KR, Storfer-Isser A, Hart MA, Kibler AM, Rueschman M, Rosen CL, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42.
Santamaria F, Montella S, Greco L, Valerio G, Franzese A, Maniscalco M, et al. Obesity duration is associated to pulmonary function impairment in obese subjects. Obesity (Silver Spring). 2011;19:1623–8.
Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–5.
Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43.
Santamaria F, Montella S, Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes Rev. 2012;13:822–33.
Lang JE, Hossain J, Holbrook JT, Teague WG, Gold BD, Wise RA, et al. Gastro-oesophageal reflux and worse asthma control in obese children: a case of symptom misattribution? Thorax. 2016;71:238–46.
Santamaria F, Montella S, De Stefano S, Sperlì F, Barbarano F, Valerio G. Relationship between exhaled nitric oxide and body mass index in children and adolescents. J Allergy Clin Immunol. 2005;116:1163–4.
Van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70:659–67.
Katzmarzyk PT, Bouchard C. Where is the beef? Waist circumference is more highly correlated with BMI and total body fat than with abdominal visceral fat in children. Int J Obes. 2014;38:753–4.
Article CAS Google Scholar
De Groot EP, Duiverman EJ, Brand PL. Comorbidities of asthma during childhood: possibly important, yet poorly studied. Eur Respir J. 2010;36:671–8.
Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016; https://doi.org/10.1136/thoraxjnl-2015-207630 .
Federal Drug Administration Advisory for Omalizumab. Available at: https://wayback.archive-it.org/7993/20170111075347/ . http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm . Accessed 4 Feb 2018.
European Medicines Agency: assessment report for Xolair. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124 . Accessed 7 June 2017.
Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–96.
Jensen RK, Plum M, Tjerrild L, Jakob T, Spillner E, Andersen GR. Structure of the omalizumab Fab. Acta Crystallogr F Struct Biol Commun. 2015;71:419–26.
Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36.
Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674–7.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
Lai T, Wang S, Xu Z, Zhang C, Zhao Y, Hu Y, Cao C, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep. 2015;5:8191.
Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. “real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2015; https://doi.org/10.1111/all.12815 .
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6.
Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61.
Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004;28:152–7.
Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–70.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136:1476–85.
Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–33.
Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, et al. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–9.
Rodrigo GJ, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26:551–6.
Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9.
Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–8.
Busse W, Buhl R, Fernandez Vidaurre C, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129:983–9.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–10.
Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6.
Busse WW, Trzaskoma B, Omachi TA, Canvin J, Rosen K, Chipps BE, et al. Evaluating Xolair persistency of response after long-term therapy (XPORT). Am J Respir Crit Care Med. 2014;189:A6576.
Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97.
Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–49.
National Heart, Lung, and Blood Institute. Expert panel report 3: Guidelines for the diagnosis and management of asthma—full report 2007. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf . Accessed 4 Feb 2018.
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunolgy. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–85.
Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30.
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13.
Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Tsabouri S, Mavroudi A, Feketea G, Guibas GV. Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pediatr. 2017;5:82.
Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68.
Hedlin G, van Hage M. The role of immunotherapy in the management of childhood asthma. Ther Adv Respir Dis. 2012;6:137–46.
Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25:829–32.
Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39:271–9.
Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9.
Stelmach I, Kaczmarek-Woźniak J, Majak P, Olszowiec-Chlebna M, Jerzynska J. Efficacy and safety of high-doses sublingual immunotherapy in ultra-rush scheme in children allergic to grass pollen. Clin Exp Allergy. 2009;39:401–8.
Download references
Acknowledgements
The authors gratefully thank Dr. Marco Maglione for his contribution in the clinical assessment of the described cases. Medical writing assistance was provided by Stephen Walters on behalf of City Hills Proofreading.
No funding was secured for this study.
Availability of data and materials
All relevant data and materials are published in the manuscript.
Author information
Authors and affiliations.
Department of Translational Medical Sciences, Federico II University, Via Sergio Pansini 5, 80131, Naples, Italy
Virginia Mirra, Silvia Montella & Francesca Santamaria
You can also search for this author in PubMed Google Scholar
Contributions
VM, SM and FS, authors of the current manuscript, declare that they have participated sufficiently in the work to take public responsibility for appropriate portions of the content. VM and SM carried out the initial investigations, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. FS conceptualized and designed the study, and critically reviewed and approved the final manuscript as submitted. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Francesca Santamaria .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee “Carlo Romano”, Federico II University, Naples, Italy. Children’s parents/legal guardians gave informed written consent to participate. The description of our cases adheres to the CARE standards of reporting checklist.
Consent for publication
Children’s parents/legal guardians provided informed written consent for the case report to be published.
Competing interests
The authors declare that they have no competing interests to disclose. Authors have no financial relationships relevant to this article to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Mirra, V., Montella, S. & Santamaria, F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr 18 , 73 (2018). https://doi.org/10.1186/s12887-018-1019-9
Download citation
Received : 24 May 2016
Accepted : 29 January 2018
Published : 21 February 2018
DOI : https://doi.org/10.1186/s12887-018-1019-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Adolescents
- Asthma exacerbations
BMC Pediatrics
ISSN: 1471-2431
- General enquiries: [email protected]
- Campus Directory
- Current Students
- Faculty & Staff

Asthma Case Study
Asthma affects about 6.1 million children in the US under 18 years of age, making it one of the most common chronic childhood disorders (American Lung Association, 2021). Asthma occurs as a result of a stimulus which can range from allergens, cigarette smoke, changes in temperature, stress, or exercise. In this case we’ll experience an asthma attack and subsequent treatment with 16-year-old Ben Mason.
Module 9: Asthma
Review structure and functions of the respiratory system...
Asthma - Page 1

Ben was struggling to breathe when he reached the ER...
Asthma - Page 2
Ben was also given an additional breathing treatment...
Asthma - Page 3

Case Summary
Summary of the Case
Asthma - Summary

Answers to Case Questions
Asthma - Answers

Professionals
Health Professionals Introduced in Case
Asthma - Professionals
Additional Links
Asthma - Links
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Topic collections
- BMJ Journals
You are here
- Volume 6, Issue 1
- Diagnosis and management of asthma in children
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Joanne Martin 1 , 2 , 3 ,
- Jennifer Townshend 4 ,
- http://orcid.org/0000-0003-4591-8299 Malcolm Brodlie 1 , 4
- 1 Translational and Clinical Research Institute , Newcastle University , Newcastle upon Tyne , UK
- 2 Northern Foundation School , Health Education England North East , Newcastle upon Tyne , UK
- 3 James Cook University Hospital , South Tees NHS Foundation Trust , Middlesbrough , UK
- 4 Paediatric Respiratory Medicine , Great North Children's Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust , Newcastle upon Tyne , UK
- Correspondence to Dr Malcolm Brodlie; malcolm.brodlie{at}newcastle.ac.uk
Asthma is the the most common chronic respiratory condition of childhood worldwide, with around 14% of children and young people affected. Despite the high prevalence, paediatric asthma outcomes are inadequate, and there are several avoidable deaths each year. Characteristic asthma features include wheeze, shortness of breath and cough, which are typically triggered by a number of possible stimuli. There are several diagnostic challenges, and as a result, both overdiagnosis and underdiagnosis of paediatric asthma remain problematic.
Effective asthma management involves a holistic approach addressing both pharmacological and non-pharmacological management, as well as education and self-management aspects. Working in partnership with children and families is key in promoting good outcomes. Education on how to take treatment effectively, trigger avoidance, modifiable risk factors and actions to take during acute attacks via personalised asthma action plans is essential.
This review aimed to provide an overview of good clinical practice in the diagnosis and management of paediatric asthma. We discuss the current diagnostic challenges and predictors of life-threatening attacks. Additionally, we outline the similarities and differences in global paediatric asthma guidelines and highlight potential future developments in care. It is hoped that this review will be useful for healthcare providers working in a range of child health settings.
- therapeutics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjpo-2021-001277
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Key messages
Paediatric asthma outcomes are poor and many deaths are preventable.
Diagnosing asthma in childhood can be challenging, and the diagnosis should be reviewed during follow-up to ensure it is correct.
Asthma attacks should be viewed as never events. Postattack reviews are essential to optimise maintenance therapy and prevent future attacks.
Education is key to improving asthma outcomes.
Personalised asthma action plans are essential, and a significant number of children with asthma do not have one.
Introduction
Asthma is a chronic respiratory disease characterised by episodes of wheeze, cough, and shortness of breath. Around 14% of children worldwide have a diagnosis of asthma, making it the most common chronic respiratory disease of childhood. 1
Poor asthma control is associated with a number of negative effects on children and families. For example, they are more likely to be absent from school, have additional educational needs and have lower educational attainment. 2 Caregivers also experience missed work days and financial challenges as a result. 3 Some children will experience severe symptoms and life-threatening attacks. 4
Taking the UK as an example, paediatric asthma outcomes are poor overall with considerable associated morbidity and high rates of emergency hospital admissions, and most pertinently, there are several preventable deaths each year. 5 Alarmingly, the National Review of Asthma Deaths (NRAD) found that in almost all paediatric cases, there were a number of significant avoidable contributing factors and that these deaths may have been preventable. 6
There are several factors that make the diagnosis and management of asthma in children challenging. The aim of this review was to explore these issues and highlight good clinical practice in the diagnosis and management of paediatric asthma.
Presentation of asthma
Children with asthma typically present with a symptom triad of wheeze, shortness of breath and cough. However, ‘asthma’ is an umbrella term used to describe this collection of symptoms and, when present, should prompt practitioners to ask, ‘What type of asthma is this?’ There are a number of asthma subtypes that present and respond to treatment differently. Identification of the features of asthma and modifiable or treatable traits should only be the start of the diagnostic journey. 7 Asthma symptoms are normally intermittent in nature and may not be present at the time of clinical review, making the diagnosis challenging in some cases. 8 Additionally, disease phenotypes are not fixed and may evolve over time, necessitating ongoing review of symptoms and treatment. 9
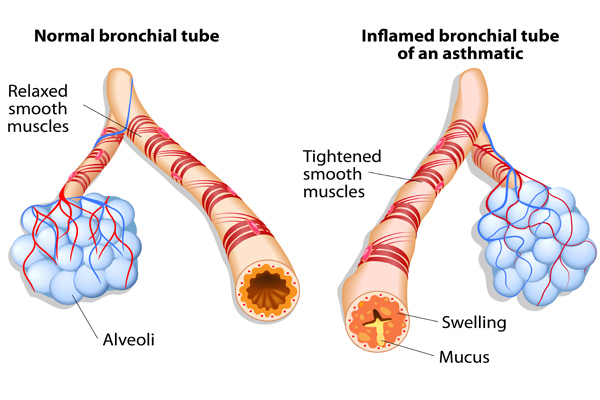
Wheeze is a key feature of asthma and, if not present, a diagnosis of asthma in a child is unlikely. Wheeze is an expiratory high-pitched whistle that occurs as a result of inflammation and narrowing of the small airways. Parental understanding of wheeze varies, and clarifying what is meant when it is reported is key in making an accurate diagnosis. 10
The prevalence of ‘preschool wheeze’ is an additional challenge when diagnosing asthma in young children. In the first few years of life, many children will experience wheeze, but not all will go on to develop true asthma. The diagnosis of asthma should therefore be reviewed routinely to identify true asthma and alter treatment where necessary. 11 Favourable response to an appropriate trial of asthma treatment is an important confirmatory piece of diagnostic evidence.
Clinical examination may be normal in children and adolescents with asthma if they present during asymptomatic periods. During acute attacks, use of accessory muscles of respiration and widespread wheeze may be present. 12 Chest hyperinflation may be identified in acute and chronic disease settings.
Asthma triggers
Asthma attacks commonly occur following exposure to one or several triggers. Viral respiratory infections remain the leading cause, 13 but there are a number of other known triggers ( box 1 ), including aeroallergens, secondhand smoke exposure, or changes in ambient air temperature or humidity. Identification and documentation of specific asthma triggers should be part of routine care. Education on trigger recognition and avoidance is essential.
Common asthma triggers
Viral respiratory tract infections 6
Exercise 6 59
Weather changes in temperature and humidity 6 59
Domestic pollutants (eg, pests, mould and dust mites) 6
Environmental pollutants (eg, air pollution) 6
Secondhand smoke exposure 13 59
Pets and animals 13
Strong odours 13
Anxiety or strong emotions 59
Drugs (eg, non-steroidal anti-inflammatory drugs and beta blockers) 59
Gastro-oesophageal reflux 59
Risk factors for asthma
There are a number of risk factors that should be explored in the history of children who present with features of asthma. In symptomatic children, a personal or family history of atopic features, including asthma, eczema or rhinitis, supports a diagnosis of asthma. Some additional risk factors are outlined in box 2 . Education on modifiable risk factors, for example, exposure to secondhand smoke or air pollution and obesity, should be delivered routinely during consultations and asthma reviews. A range of social determinants that are linked to poverty impact on outcomes and the health of children with asthma. 14
Asthma risk factors
Personal or family history of atopy: eczema, allergic rhinitis or nasal polyposis 60
Family history of asthma 60
Exposure to secondhand smoke 60
Preterm birth 21
Low birth weight 61
Poor housing quality/mould and dampness 6
Air pollution 63
Paediatric asthma phenotypes
Asthma is a heterogeneous disease in which there are several phenotypes and underlying endotypes. Phenotypes are subtypes of asthma that share clinical characteristics such as symptom triggers, atopic features, disease severity and response to treatment. Endotypes are subtypes of asthma that are characterised by similar underlying biological mechanisms. 15
Key endotypes include ‘type 2-high’ and ‘type 2-low’ asthma. 16 Identifying asthma phenotypes and endotypes can facilitate targeted treatment based on the pathophysiology occurring in a specific individual. 17 For example, allergic or eosinophilic asthma that frequently starts in childhood is type 2-high and is characterised by eosinophilic airway inflammation, raised IgE and fractional exhaled nitric oxide (FeNO) levels. 15 Typically, type 2-high asthma responds well to inhaled corticosteroid (ICS) treatment. 7 A number of biologic agents can be used in the management of asthma, under specialist supervision, and their use varies on asthma endotypes (table 8). 18
Differential diagnoses and diagnostic uncertainty
Misdiagnosis of asthma remains a major problem with rates of both underdiagnosis and overdiagnosis being high. 19 Overdiagnosis is problematic as it exposes children to unnecessary side effects of medications and runs the risk of trivialising asthma. 7
There are several conditions that may be associated with chronic cough, wheeze and/or shortness of breath in children and therefore present similarly to asthma ( table 1 ). Due to the difficulties with diagnosis, especially in young children where objective testing is not possible, the diagnosis of asthma should be reviewed at each clinical presentation and interaction.
- View inline
Asthma differentials and clues in medical history
Diagnosing asthma in children
There is no single ‘gold-standard’ test that can be used to accurately diagnose asthma. In practice, a diagnosis should be made based on characteristic symptom patterns, evidence of variability in airflow limitation in the presence of airway inflammation, likelihood of alternative diagnoses and response to treatment. Getting the diagnosis correct is key for optimal management of paediatric asthma.
Lung function tests can be used to aid the diagnosis of asthma in children over the age of 5 years. Peak expiratory flow (PEF) and spirometry are commonly used to assess airflow obstruction and reversibility. PEF can be used to detect diurnal variation, which is a typical feature of asthma. The Global Initiative for Asthma (GINA) specifically recommends the use of either PEF or spirometry in the diagnosis of asthma in children over 5 years. 20 Once a child is old enough to reliably perform lung function testing, it is recommended that this be undertaken if the diagnosis of asthma has not been previously confirmed. In children under 5, lung function testing is rarely practical outside a research setting. This makes diagnosis in this age group additionally challenging. 21 Guidelines vary between countries and regions with regard to diagnostic criteria. An overview of the similarities and differences between these guidelines is displayed in table 2 . Lung function testing is frequently used to monitor progress of children with asthma as part of their care. Objective testing should be repeated if there is poor response to treatment or diagnostic uncertainty.
Summary of paediatric asthma national guidelines: focusing on diagnosis
FeNO is used to detect and quantify eosinophilic airway inflammation with levels elevated in those with eosinophilic asthma. 22 Once staff are trained, and provided equipment is available, FeNO is a practically useful test that is quick to perform in school-aged children. The exact positioning of FeNO testing varies between guidelines worldwide ( table 2 ). FeNO monitoring may also be useful in titrating dosage of ICS in those with an established diagnosis of asthma. 23
Allergy testing (skin prick testing or measurement of specific IgE levels) is not routinely carried out in the diagnostic process; however, it is recommended in a number of clinical guidelines and may identify individual triggers. 24–27
There are several aspects that make paediatric asthma diagnosis challenging. Most diagnoses are made in primary care where there is often limited access to objective testing at present. Despite guideline recommendations, objective testing is frequently only available in secondary or tertiary care settings where equipment and trained staff are available. The COVID-19 pandemic has served to exacerbate these issues and increase backlogs. Various solutions have been proposed, including community diagnostic hubs. 28 In some healthcare systems, the cost of undergoing objective testing is a cause of health inequalities.
Additionally, the symptom onset for most cases of paediatric asthma occurs before the age of 3 years 29 when lung function testing cannot be used to aid diagnosis. In this age group, response to an asthma treatment trial is useful to aid diagnostic decision making and is recommended in a number of national guidelines. 27 30–32
Management of asthma in children
The management of asthma is multifactorial, and to optimise disease control, a number of pharmacological, non-pharmacological and self-management aspects need to be considered.
Pharmacological management
The pharmacological management of asthma involves two key components: maintenance and reliever therapies. Maintenance therapies are the mainstay of asthma management, and the treatment aim is that no reliever therapies are required. Use of reliever therapy suggests asthma control is poor.
An overview of maintenance and reliever therapies is outlined in tables 3 and 4 , respectively. A stepwise approach to asthma management is encouraged, and pharmacological management varies on age, symptom control and the national guideline used. An overview of management approach in a number of national guidelines is summarised in table 5 .
Maintenance therapies
Reliever therapies
Summary of paediatric asthma national guidelines: focusing on management
Biologic agents used in the management of asthma
GINA guidelines recommend dual ICS and short-acting beta-2 agonist (SABA) therapy to children over the age of 5. 20 SABA monotherapy was previously the main management starting point; however, compared with combined treatment, SABA monotherapy has been shown to be associated with asthma mortality. 33 SABA monotherapy is now only recommended by GINA for use in children aged 5 or less. 20 As seen in table 5 , GINA recommends symptom-driven ICS use, compared with daily ICS use, as initial therapy in children over 6 years of age. In comparison to daily ICS use, symptom-driven use has demonstrated a similar exacerbation risk and reduces the risk of ICS adverse effects. 34
Single maintenance and reliever therapy (SMART) inhalers are combined inhalers offering both maintenance and reliever therapy in those with asthma. These inhalers contain a number of maintenance and reliever therapies in different combinations. The use of these inhalers have been shown to reduce the risk of asthma attacks and emergency department (ED) admissions, 35 improve lung function and decrease the need for reliever therapy. 36 There is limited evidence in the effectiveness of SMART inhalers in children, but children over 12 years may be prescribed a SMART inhaler, which acts as both a maintenance and reliever therapy, if symptoms are not well controlled. 37
There are a number of biologic agents ( table 6 ) that may be used in the management of paediatric asthma. These are endotype-specific, targeted therapies that should be used only under the supervision of specialists. Their availability and cost vary between countries and different healthcare systems. Detailed appraisal of the evidence base for their use is provided in the individual management guidelines and has been recently reviewed. 17
Non-pharmacological management
Non-pharmacological aspects of asthma management include providing education on modifiable risk factors and comorbidities to caregivers and conducting annual asthma reviews to assess control and future risk.
Education is key to improving caregiver and child understanding of asthma and its management. Clear information regarding modifiable risk factors, such as smoke exposure, domestic pollutants and obesity, should be given. Short-term educational interventions aimed to improve self-management have been shown to increase medication adherence, 38 improve symptom control and reduce mortality. 39
All young people with asthma should have asthma reviews at least annually. These reviews should focus on current symptom control and management, previous attacks, triggers, modifiable risk factors and personal asthma action plans (PAAPs). Asthma reviews are opportunities to assess child and caregiver understanding of asthma and provide education, if necessary. Annual asthma reviews are also opportunities to assess inhaler technique (including spacer use) and provide education on this if necessary. Poor inhaler technique is common in young people with asthma 40 and associated with poor disease control. 41
Taking time to understand the perceptions of young people and their caregivers in relation to their asthma diagnosis and management is important, and exploring such perceptions may enhance engagement during consultations, subsequently improving outcomes for young people. 42
Self-management
Self-management aspects of paediatric asthma management include asthma education and PAAPs. PAAPs are written documents that are given to young people and/or caregivers that advise them on day-to-day asthma management and what to do in the event of an attack. 43 Action plans should be created with patient/caregiver input, shared with relevant individuals (eg, school teachers) and should be reviewed and updated regularly. PAAPs have been shown to reduce ED attendance and missed school days and to increase caregiver confidence when managing attacks. 44 The 2018 Annual Asthma Survey found that over 50% of children with asthma in the UK had no PAAP, and around 20% of caregivers did not seek medical advice during acute asthma attacks, highlighting large gaps in education. 45
Diet and exercise are additional important self-management aspects within paediatric asthma care. A number of short-term exercise interventions have demonstrated improvements in lung function and symptom control. 46 Healthy eating interventions can help reduce body mass index and improve the quality of life of both young people and their caregivers. 47
Withdrawing management/stepping down
Asthma control should be reviewed at every medical contact. When asthma symptoms are well controlled on pharmacological therapy, stopping or stepping down medication should be considered to protect young people from unnecessary adverse effects.
The GINA 2021 guidelines advise that clinicians should consider stepping down asthma management to the lowest effective treatment regimen when good symptom control has been achieved for at least 3 months. 20 When stepping down treatment, an individualised risk–benefit approach should be taken with focus on the child’s medical history, including frequency of oral corticosteroid use, frequency of asthma attacks, and previous intensive or high-dependency care admissions. 48
When to refer to a specialist
Most paediatric asthma cases are diagnosed in primary care without the input of general paediatricians or paediatric respiratory physicians. 6 However, a number of children with asthma may need to be referred to specialists for diagnostic or management input. Common indications for specialist referral include no or poor response to asthma treatments, inconclusive objective testing, poor symptom control with appropriate treatment, frequent oral corticosteroid use or the occurrence of a severe asthma attack. 20 27 30 31 49 50 A key element of specialist care is a multidisciplinary team consisting of a number of professionals, including specialist nurses, psychologists, physiologists and pharmacists.
Healthcare professionals must consider any safeguarding implications at all paediatric asthma reviews as part of delivering holistic care. Unexplained or frequent ‘do not attend’ appointments or suspicion of poor medical management at home should be flagged and acted on locally.
Predictors of life-threatening attacks
The following features have been shown to increase the likelihood of future severe attacks, and particular attention should be given to these factors during asthma reviews:
Previous attack. The strongest risk factor for a future asthma attack is a personal history of a previous attack. One large systematic review and meta-analysis found that children with a recent history of ED attendance with an asthma attack were up to 5.8 times more likely to have another ED attendance and up to three times more likely to be admitted to the hospital with a future asthma attack. 51
Frequent SABA use and prescription requests. Frequent use of SABA reliever therapy suggests poor control of asthma symptoms. If asthma symptoms are well controlled, no more than two SABA inhalers should be required annually. 52 The UK NRAD found that excess SABA prescription and use were prominent in individuals who died of asthma attacks. For those with data available, around 40% had been prescribed 12 or more SABA inhalers in the 12 months before death. 6
Postattack review
Asthma attacks should be viewed as never events. It is essential that a postattack review is conducted to review asthma maintenance treatment, as this is likely to be suboptimal. Failure to review patients post attack, and to alter treatment where appropriate, is likely to predispose to future attacks, which could be life-threatening. Management of the current attack should be reviewed to ensure treatment is appropriate and symptoms are resolving. Some individuals may require additional courses of oral corticosteroids to settle symptoms. 7
Current NICE quality standards (UK) state that all individuals hospitalised with an asthma attack should receive a follow-up review in primary care within two working days of discharge, 49 to review maintenance management and ensure resolution of symptoms. However, the 2018 National Asthma Survey completed in the UK found that 64% of respondents had no primary care follow-up post attack, and most patients were not aware that this was required. 45
Salbutamol weaning
Salbutamol weaning plans are commonly used by a number of healthcare organisations following discharge after an asthma attack. These plans direct caregivers to provide regular SABA therapy, often in a reducing regime, in the days following discharge. There have been a number of concerns raised with regard to these plans with some believing that providing regular SABA therapy may potentially mask deterioration and could delay care givers seeking medical advice. 53 Healthcare professionals should enquire about salbutamol weaning plans during postattack reviews and urge caregivers to seek medical advice if they have concerns or the effects of SABA are not lasting the 4 hours of duration.
Future developments in care
The management of paediatric asthma is changing over time with, just as two examples, developments in technology and service structure:
Technology. The growing use of technology in asthma care has huge potential to improve clinical outcomes. Smartphone applications can be used to provide medication reminders to users, and this has been shown to increase ICS adherence. 54 Applications can also be used to provide educational content to young people and caregivers, 55 as well as store PAAPs. 56 ‘Smart’ inhalers, not to be confused with SMART inhalers, are devices that can provide audio reminders to users and record when they are used. One paediatric study found that the use of smart inhalers increased treatment adherence to 84%, compared with 30% in the control group. 57
Diagnostic hubs. In the UK, regional diagnostic hubs for asthma care have been recommended in NHS England’s Long Term Plan. 58 Implementation of diagnostic hubs is hoped to result in earlier and more accurate asthma diagnoses by improving access to objective testing and specialised interpretation. Hubs are designed to improve asthma outcomes by enabling most appropriate treatment initiation and monitoring. There is currently no evidence in the literature of the clinical effects of diagnostic hubs being used in the management of paediatric asthma.
Conclusions
Paediatric asthma outcomes are currently poor and many deaths are preventable. The aim should be to avoid asthma attacks occurring with appropriate maintenance therapy, and they should be viewed as never events. In order to improve outcomes, accurate diagnosis and management are essential. Good asthma care extends beyond providing medication and should include education, as well as supported self-management advice. The use of PAAPs remains limited and a significant number of young people with asthma do not have one. Postattack asthma reviews are a key opportunity to review maintenance medication and current symptom control.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Fleming M ,
- Fitton CA ,
- Steiner MFC , et al
- Nichols M ,
- Treiber F , et al
- FitzGerald JM ,
- Barnes PJ ,
- Chipps BE , et al
- Fleming L ,
- Warner JO , et al
- Physicians RCo
- McCormack MC ,
- Kuruvilla ME ,
- Silverman M ,
- Strippoli M-PF , et al
- Fainardi V ,
- Santoro A ,
- Caffarelli C
- Townshend J ,
- Gautier C ,
- Matsui EC ,
- McCormack MC , et al
- Castagnoli R ,
- Brambilla I , et al
- Brusselle GG ,
- Koppelman GH
- McGregor MC ,
- Krings JG ,
- Nair P , et al
- Kavanagh J ,
- Jackson DJ ,
- Lugtenberg MJ ,
- Smets E , et al
- Shaw DE , et al
- Petsky HL ,
- Plaza Moral V ,
- Alonso Mostaza S ,
- Alvarez Rodríguez C , et al
- Papadopoulos NG ,
- Arakawa H ,
- Carlsen K-H , et al
- Ebisawa M , et al
- Sylvester KP ,
- Rutter MA , et al
- Devonshire AL ,
- Australia TNAC
- Zealand AaRFN
- Mitchell P , et al
- Benayoun S , et al
- Muneswarao J ,
- Hassali MA ,
- Ibrahim B , et al
- Scicchitano R ,
- Aalbers R ,
- Ukena D , et al
- Sobieraj DM ,
- Nguyen E , et al
- Boutopoulou B ,
- Koumpagioti D ,
- Matziou V , et al
- Guevara JP ,
- Grum CM , et al
- Gillette C ,
- Rockich-Winston N ,
- Kuhn JA , et al
- Capanoglu M ,
- Dibek Misirlioglu E ,
- Toyran M , et al
- Henderson J , et al
- Ducharme FM ,
- Lakupoch K ,
- Manuyakorn W ,
- Preutthipan A , et al
- Wanrooij VHM ,
- Willeboordse M ,
- Dompeling E , et al
- Kallenbach JM ,
- Frankel AH ,
- Lapinsky SE , et al
- (NICE) NIfHaCE
- Dermot Nolan DM
- Ardura-Garcia C ,
- Stolbrink M ,
- Zaidi S , et al
- Ekström M ,
- Hasvold P , et al
- Mosnaim G ,
- Martin M , et al
- Burbank AJ ,
- Hewes M , et al
- Stewart AW ,
- Harrison J , et al
- Tarasidis GS ,
- Bai M-J , et al
- Ettinger AS ,
- Pedersen CB ,
- Thygesen M , et al
- Ullmann N ,
- Di Marco A , et al
- Rugg-Gunn CE ,
- Sellick V , et al
- Society SAT
- Doroudchi A ,
- Pathria M ,
- Morgan WJ ,
- Gergen PJ , et al
- Garcia E , et al
- Ortiz B , et al
- Korenblat PE
- Chanez P , et al
- Pavord ID , et al
- Godor D , et al
- Ortega HG ,
- Pavord ID ,
- Howarth P , et al
- Gibson PG , et al
- Geng B , et al
- Wechsler M ,
- Virchow JC ,
- Brusselle GG , et al
- Kolbeck R ,
- Kozhich A ,
- Koike M , et al
- Harrison TW ,
- Menzella F , et al
JT and MB contributed equally.
Contributors All authors conceived the ideas for the article. JM wrote the first draft that was then commented on by JT and MB.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MB received investigator-led research grants from Pfizer and Roche Diagnostics; speaker fees paid to Newcastle University from Novartis, Roche Diagnostics and TEVA; and travel expenses to educational meetings Boehringer Ingelheim and Vertex Pharmaceuticals.
Provenance and peer review Commissioned; externally peer reviewed.
Read the full text or download the PDF:
Sustainment of a Successful Pediatric Asthma Management Program Case Study

Executive Summary
In 2016, Children’s Hospital of Orange County (CHOC) was awarded the HIMSS Davies Award. One of the award-winning case studies focused on using embedded, evidence-based care guidelines to control pediatric asthma and an alert system that triggered when patients were about to be discharged before a home management plan was created. CHOC drove down the average length of stay for pediatric asthma patients from 2.14 days to 1.72 days. Pediatric asthma readmissions within 30 days also fell from an average of 1.7 per quarter to 0.7 per quarter. To sustain and expand on this work, in 2017 the organization decided to re-focus on the pediatric condition registries which had first been installed in 2015. It organized and stabilized the number of primary care patients who received an pediatric asthma action plan (AAP) and asthma control test (ACT) resulting in lower emergency room visits and inpatient hospital admissions. The process re-design was driven by analytical data which was reviewed on a monthly basis for transparency into how providers were performing against their value measurements. The utilization of a population health platform empowered the team to take disparate sources of data (different electronic health records, claims, labs, pharmacy data, etc., and transform the data in to meaningful, useful information and integrate the required documentation into the EHR. In total, CHOC built six ambulatory guidelines of care into their EMR, one of which was asthma, leading to the overall improvement in the ambulatory care of pediatric asthmatics. The results included:
- In December 2019, ~59% of primary care patients received the pediatric asthma action plan—up from 28.89% in April 2017.
- In December 2019, 57% of primary care patients received the asthma control test—up from 29.61% in April 2017.
- Patients being seen in the emergency department (ED) for asthma decreased, as well as the readmission rate within seven days of discharge per 100 discharges decreased from 1.84 to 0.3.
The key difference in this case study as compared to the one in 2016 is the work to improve the ambulatory care aspect, as well as continued work in the Breathmobile™ which is the only mobile asthma clinic serving preschool and school-aged kids in Orange County.
Lessons learned include:
The primary and most important take away is to first manage the care of the patients who show up for treatment—and then create an ambulatory plan of care that prevents them from requiring emergent care or hospital admission.
- When choosing registries for implementation, determine which diseases are most prominent in your population.
- Identify which of these diseases lead to increased morbidity, mortality or overall healthcare utilization.
- Understand which of these diseases have well established care guidelines.
- Target disease groups that have willing physicians to partner with on disease registry development.
- Patient and family education, access to care, overcoming social determinants of health issues, improving the use of the Asthma Control Test and the Asthma Action Plan are key to preventing pediatric asthma ED visits and readmissions.
- Engaging the community, schools, and resources like the Breathmobile help to prevent ED asthma visits.
- Care managers and school nurses also play a huge role in asthma management.
Defining the Clinical Problem and Pre-Implementation Performance
CHOC Medical Group is composed of 54 pediatricians and pediatric nurse practitioners who practice in five clinical sites in Orange County, California. Two sites are on the campus of the Children’s Hospital in the city of Orange, and three sites are in surrounding communities (Santa Ana and Garden Grove) where they support a multicultural population which is entirely Medicaid (MediCal) funded.
The population that CHOC Medical Group serves struggles with barriers such as language discordance, transportation, food and housing insecurity, and various cultural beliefs related to their healthcare. Given the proximity to the U.S./Mexico border, there is a large Latin American population who struggle with immigration issues and in some cases delay/avoid contact with the medical system.
Finally, given CHOCH’s affiliation with the children’s hospital, which is a free-standing tertiary/quaternary children’s hospital, CHOCH primary care clinics support a large volume of hospital graduates with a high degree of complex medical conditions. Preventing emergency room visits and hospital admissions is key to claiming “successful” management of this population. The medical group was struggling to maintain incremental increases in the percentage of pediatric asthmatic patients who had an updated annual AAP and ACT. This was an initiative that the organization and Practice Transformation Network (Southwest PTN—CHOC Children’s and Rady Children’s) had embraced as part of the chronic disease management goal of the Population Health Division’s engagement in the Transforming Clinical Practice Initiative Award CHOC received from the Centers for Medicare and Medicaid Services. CHOC’s subject matter experts identified a wide range of provider/panel completion rates and, as a collective, the group was averaging in the low 30% range. Difficulties with this stemmed from insufficient time for providers to address asthma during routine sick visits and well visits, ineffective capture of patients in the EMR Chronic Disease Registries, inconsistent standards of work for the care team, and variable approaches to compliance from provider to provider and clinic to clinic.
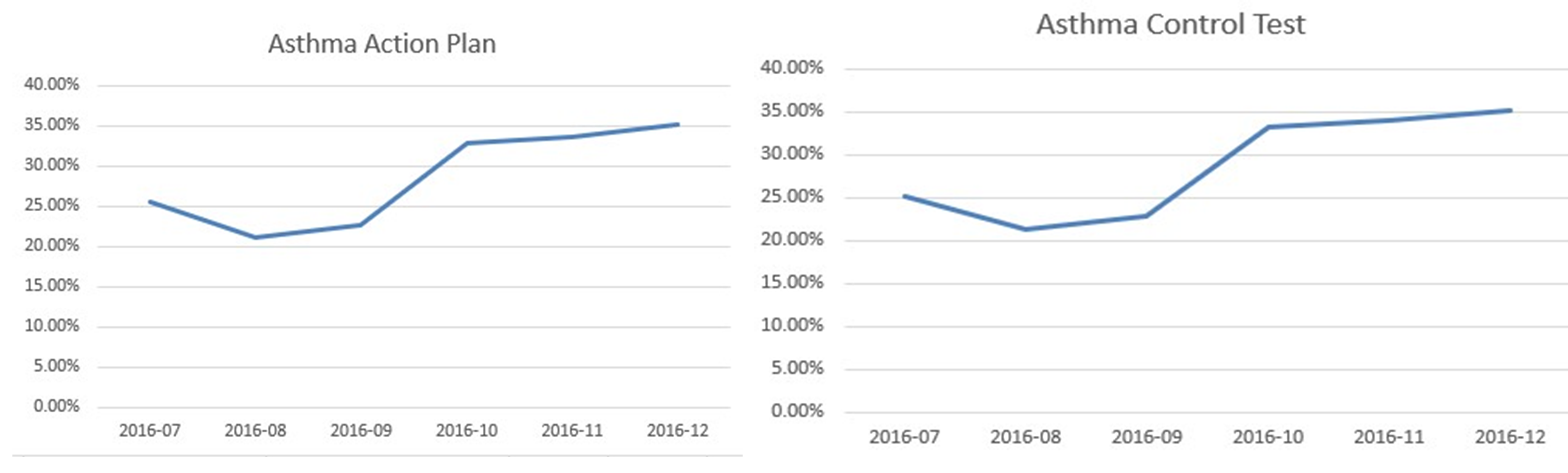
The numerators are respectively the patients who received the AAP and ACT, and patients seen in the ED with the diagnosis of asthma. The denominator is the number of patients in the asthma registry with the diagnosis of asthma.
The American Academy of Pediatrics, the Centers for Disease Control, and the National Institutes of Health (NIH) all support the utilization of an Asthma Action Plan and care guidelines specific to pediatric asthma management. Following these guidelines, the chief medical information officer (CMIO) led the asthma registry development with input from an expert panel consisting of asthma experts in pulmonary and allergy, primary care physicians, practice staff, and other pertinent providers. The common goal was to improve compliance and decrease the ED visits and hospital admissions.
The ultimate target to date is to meet and exceed a 54% compliance rate—the benchmark from the American Academy of Pediatrics and Mayo Clinic is 43%.
The organization is currently continuing these efforts and at the time of this writing is working toward The Joint Commission (TJC) Disease Specific Care Pediatric Asthma Certification. This certification will help ensure our program meets clinical performance standards and targeted metrics as well as other compliance standards from TJC.
Designing and Implementing Model Practices and Governance
The decision to purchase a population health platform to augment other sources of data such as claims data and the enterprise data warehouse was a turning point in improving our workflows and outcomes. The ability to join the platform supplier as a development partner for pediatric content provided an opportunity to focus on the specific needs of the pediatric population. The purchase of the population health platform was a collaborative decision between the information systems department leadership (the chief medical information officer and the chief information officer at the time), the head of CHOC Health Alliance (independent physician association or IPA) and director over the CHOC population health program, and the vice president of strategic planning, along with other ambulatory and senior leaders. The population health platform, HealtheIntent™ was chosen after a formal RFP process involving several vendors. None of the other population health platforms CHOC evaluated had pediatric content, and the HealtheIntent provider had a long history with our organization of working with CHOC to develop pediatric applications, leading to the choice of HealtheIntent. The multidisciplinary team driving pediatric asthma care is referenced below.
Care guidelines supported by the population health management tool which include measures from the asthma registry were embedded in the standard primary care workflow. Processes were developed to create a uniform and consistent standard of work regardless of provider or location and to identify patients with asthma requiring an asthma AAP and ACT.
Learning resources included:
- HealtheRegistries® Help Page
- User Guides for Providers, Office Staff, and Personnel
- Ex: Pamphlets—Ideal for scattered audiences or as a supplement educational piece
- Ex: Hospital Sessions—Ideal for large group trainings
- Ex: Individual Clinic Visits—Ideal for onboarding new physicians
Enabling Clinical Transformation Through Information and Technology
The foundational component of the CHOC Children’s Population Health program is the creation of a single patient-centered plan of care that can be used by the entire care team across the complete care continuum. Having the actionable data visible at the point of care helps to ensure compliance and outcomes are constantly at the forefront of patient care. The primary office staff prep the asthma charts prior to the visit, the care team medical assistants, licensed vocational nurses, mid-levels, physicians, care managers, school nurses, and Breathmobile staff all have access to the same clinical data.
To develop the condition-specific pediatric registries, the team conducted research to determine which diseases were most prevalent in CHOC’s population and which of those diseases led to the most frequent ED visits and hospitalizations. The pediatric asthma registry was created because of the volume and impact this population of patients brings to CHOC. Approximately 13% of CHOC’s capitated, (value-based per member, per month payment system), population has a diagnosis of asthma, and it is the most frequent cause of visits to the ED as 30% of children with asthma visit the ED every year. Asthma is also one of CHOC’s most frequent causes of hospitalization and 5% of children with asthma are hospitalized every year, while 40% miss five or more school days every year from asthma exacerbations. Asthma severity is classified as mild intermittent, moderate, and severe persistent, per NIH guidelines. The team’s recommended care includes assessing an ACT at least once a year and annually updating an AAP specific for that patient. The asthma registry focusses on these two and other measures like appropriate controller meds being given as prescribed.
Operationalizing the registries is another key to our success. Prior to the patient visit, the primary office staff have already identified key elements which may need to be addressed and can intervene to raise awareness to the appropriate care team member, including the patient’s family. Running in the background of the EHR is technology that goes beyond just the claims data, and pulls in disease specific clinical data. This data platform allows specific care plan and treatment modalities to be pushed to the provider’s workflow at the point of care. This population health platform is a cloud-based, programmable platform that is vendor-agnostic, which means it can receive data from any EHR, existing health IT system and other data sources, but also pharmacy, eligibility, laboratory and other sources of relevant information. In building the disease specific registries, the first step was to consider the data needs, and then to prioritize the development of the tools. The steps include acquiring the data, normalizing and data transformation, creating the disease specific algorithms, and building specific tools to push the information into the workflow at the point of care as shown in the interface diagram.
Based on the figures below, you can see a patient who has met all measures, compared to a patient who has some measures that have not been achieved.
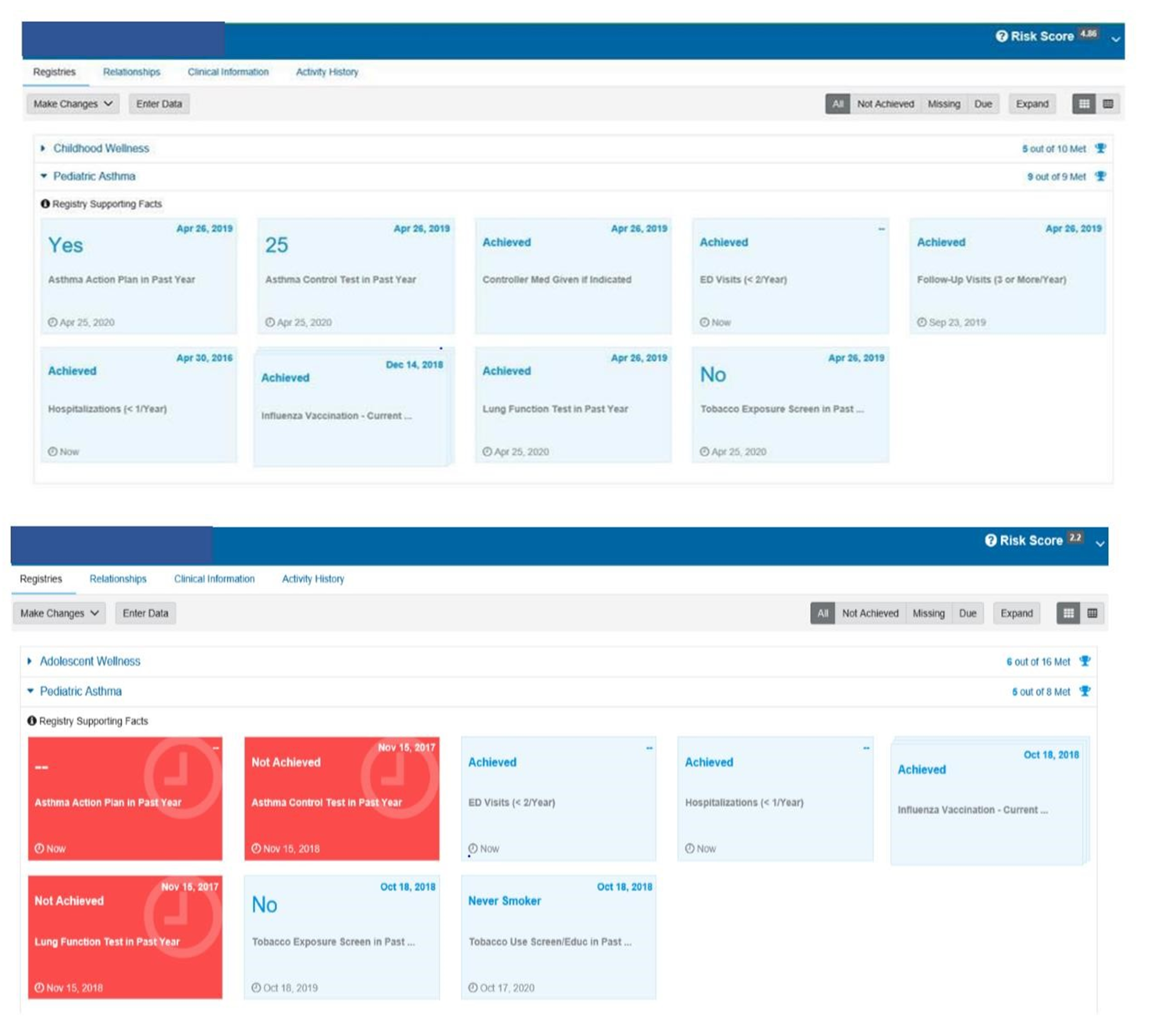
The provider’s view is a push of the registry requirements to the point of care within the workflow.
The primary care team participated in a LEAN Kaizen event to gain an understanding of and improve upon the current state of asthma management. Care team education was based on a train-the-trainer format and materials included job aids, classroom time, online videos, tutorials, and elbow to elbow support during the rollout.
Following the importance of the design and implementation of the asthma registry, is access to care managers who are front and center receiving alerts, providing availability for telehealth visits, and working with the care team to keep everyone updated on their asthma population.
The organization is dedicated to keeping the pediatric asthma patient healthy, managing the disease holistically, and preventing exacerbations. CHOC is ranked as one of the top pulmonology programs in the nation by U.S. News & World Report. CHOC offers patient and family-centered care and comprehensive inpatient and outpatient management for children with asthma. The organization offers comprehensive asthma education by a certified asthma educator for families to learn how to manage and control asthma symptoms. CHOC proudly offer the Breathmobile, the only mobile asthma clinic serving preschool and school-aged kids in Orange County. CHOC also provides the only high-risk asthma clinic in the region dedicated to treating life-threatening asthma. The Breathmobile is another key to the success in reducing the ED visits and hospitalization. In 2019 alone, this team helped to reduce ED visits, hospitalizations, and missed school days.

Improving Adherence to the Standard of Care
A foundational component of CHOC Children’s Population Health program is the creation of a single patient-centered plan of care that can be used by the entire care team across the complete care continuum, including schools and the Breathmobile. The foundation of this care sits upon the population health platform which also supplies the data for the system review. The utilization of the population health platform empowers the team to take disparate sources of data (different electronic health records, claims, labs, pharmacy data, etc.) and transform the data in to meaningful, useful information and integrate the required documentation into the EHR. The results included (at top performance):
- In December 2019, ~59% of primary care patients received the asthma action plan—up from 28.89% in April 2017.
- Patients being seen in the ED for asthma deceased as well as the readmission rate within seven days of discharge per 100 patients decreased from 1.84 to 0.3. The numerators are respectively the patients who received the AAP and ACT, patients seen in the ED with the diagnosis of asthma. The denominator is the number of patients in the asthma registry with the diagnosis of asthma. The adherence to the standard of care in the use of the registry, the ACT, and the AAP, along with clinically driven evidenced-based processes which are managed by real-time analytics and standing meetings to address compliance has proven effective in driving outcomes and enhancing utilization of the tools.
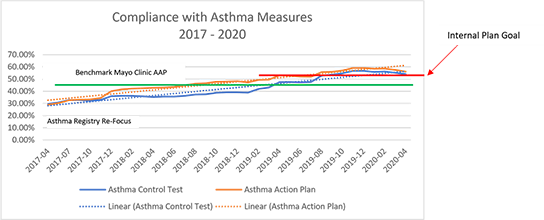
Improving Patient Outcomes
The organization implemented pediatric condition registries with an initial focus on pediatric asthma to be used by primary care providers. The organization increased and stabilized the number of primary care patients who received an AAP and ACT. This resulted in lower ED visits, seven-day readmissions, and overall readmissions for asthma. CHOC achieved these results by using analytical data on a monthly basis for transparency into how providers were performing against their value measures. The numerators are respectively the patients who received the AAP and ACT, patients seen in the ED with the diagnosis of asthma, and patients readmitted after seven days. The denominator is the number of patients in the asthma registry with the diagnosis of asthma.
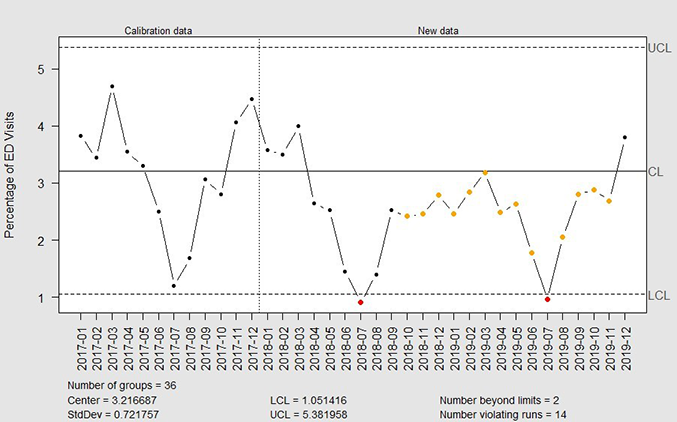
Accountability and Driving Resilient Care Redesign
For a pediatric healthcare system, effective management of the pediatric asthma population improves the well-being of the patients and their families, reduces emergency department visits and improves overall health outcomes. With this goal in mind, physicians, IT leaders, and care management teams at Children’s Hospital of Orange County leveraged a population health registry to improve the health of pediatric asthma patients. As part of this registry, the CHOC Children’s team worked together to research and define measures that were appropriate for the care of pediatric asthma patients. Ten measures were defined and built into the population health asthma registry. Of these, two were selected for a focus on improvement—patients with an asthma control test completed in the past year and patients with an asthma action plan completed in the past year. Key factors for effectively using the measures and the patient registry was making the status of those measures available in real time in the clinical workflow and incorporating the registry data into the standard work of every patient encounter. For example, if a child who has asthma visits with a physician at a primary care clinic and that patient has not had an asthma control test in the past year, that information is front facing to the providers and the care teams. In addition, to track improvements, baseline data was documented, and dashboards were built to provide information on the completion of measures in real time. Since the care team can identify gaps in the service and respond proactively to close those gaps, the organization can maximize the ability to see patients more efficiently.
Along with the push of information directly to the point of care, CHOC also utilizes analytics to measure the compliance of registry and care guideline utilization. Each physician group and each physician are held accountable for care compliance. The organization can focus on local variations in care to reduce frequent ED visits and hospital admissions. CHOC is able to track improvements in compliance with registry metrics over time in reports, dashboards, and scorecards. A re-focus on the asthma registries in 2017 led to improved compliance and improved outcomes.
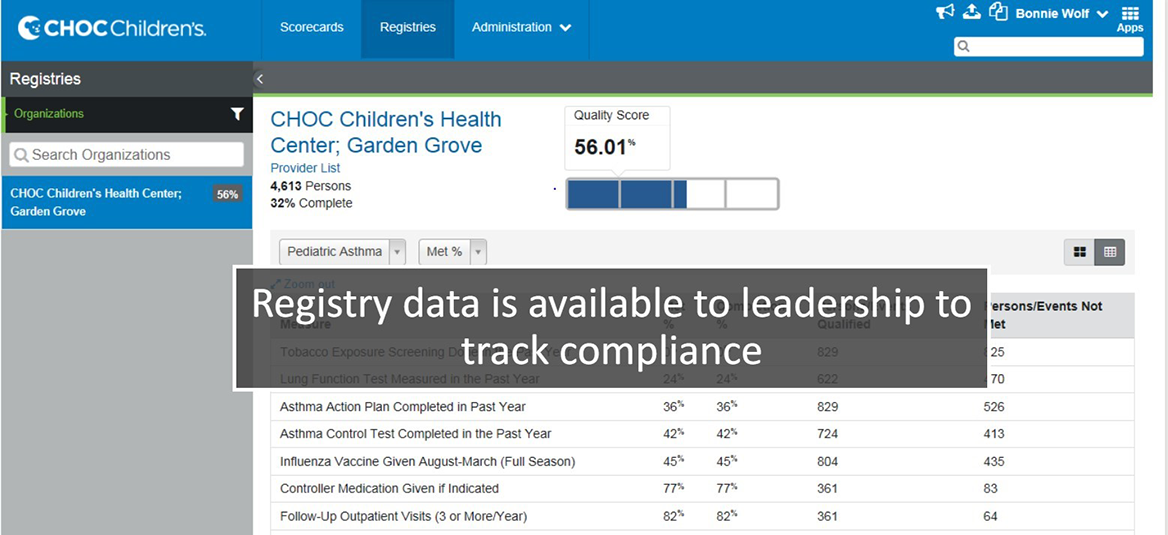
The views and opinions expressed in this content or by commenters are those of the author and do not necessarily reflect the official policy or position of HIMSS or its affiliates.
HIMSS Davies Awards
The HIMSS Davies Award showcases the thoughtful application of health information and technology to substantially improve clinical care delivery, patient outcomes and population health.
Begin Your Path to a Davies Award

Episode 79 – Management of Acute Pediatric Asthma Exacerbations

In this EM Cases episode on Pediatric Asthma we discuss risk stratification (including the PASS and PRAM scores), indications for CXR, the value of blood gases, MDIs with spacer vs nebulizers for salbutamol and ipatropium bromide, the best way to give corticosteroids, the value of inhaled steroids, the importance of early administration of magnesium sulphate in the sickest kids, and the controversies around the use of ketamine, heliox, high flow nasal cannuala oxygen, NIPPV, epinephrine and IV salbutamol in severe asthma exacerbations. So, with the multinational and extensive experience of Dr. Dennis Scolnik , the clinical fellowship Program Director at The Hospital for Sick Children in Toronto and Dr. Sanjay Mehta , multiple award winning educator who you might remember from his fantastic work on our Pediatric Orthopedics episode, we’ll help you become more comfortable the next time you are faced with a child with asthma who is crashing in your ED…
Podcast: Play in new window | Download (Duration: 1:09:19 — 63.5MB)
Subscribe: Apple Podcasts
Written Summary and blog post written by Anton Helman, April 2016
Cite this podcast as: Scolnik, D, Mehta, S, Helman, A. Management of Acute Pediatric Asthma Exacerbations. Emergency Medicine Cases. April, 2016. https://emergencymedicinecases.com/pediatric-asthma/. Accessed [date].
Pediatric Asthma Severity Indicators on History
- life-threatening exacerbations
- admissions to ICU
- deterioration while already on systemic steroids
- using more than 2 canisters of short acting B-agonist per month
- cardiopulmonary and psychiatric comorbidities
Its important to realize that a lack of risk factors does not necessarily confer a lack of risk, so even if a patient has none of these risk factors, they can still be at risk for deterioration from their asthma.
Reliable Validated Measures of Pediatric Asthma Severity
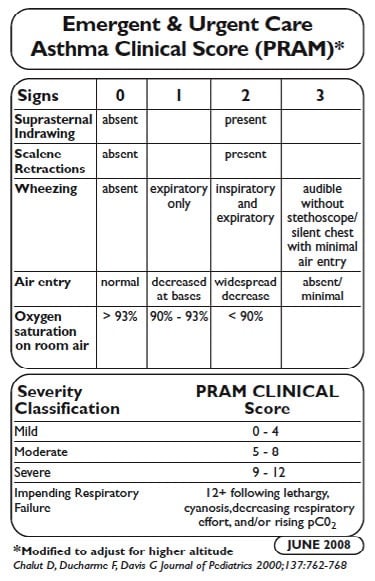

PASS Score for Pediatric Asthma Severity
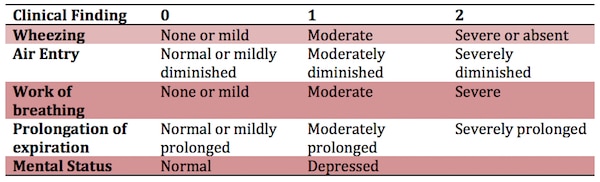
VBG in Pediatric Asthma
A PaCO2 >42 is indicative but not diagnostic of a severe exacerbation A PaCO2 >50 is a risk factor for impending respiratory failure Metabolic Acidosis is an indicator of impending arrest!
A VBG is seldom indicated unless the child has no clinical improvement with maximal therapy. The timing of the VBG is important: It may be most useful as a baseline after ED treatment in a patient going to the ICU.
Don’t forget the classic teaching: A ‘normal’ Hg partial pressure of CO2 in a patient with extreme tachypnea and retractions could indicate impaired ventilation and impending respiratory failure.
Indications for CXR in presumed Pediatric Asthma
The rate of CXR use in kids with asthma increased significantly from the mid 90’s to around 2010. Although it’s not unreasonable for first time wheezers to get a baseline CXR, it’s important to realize that an unsuspected diagnosis made on the basis of a CXR in an acutely wheezing child is rare, even if the child has never wheezed before.
In fact there are no set of predictors in the literature that can accurately identify children likely to have abnormalities on CXR.
Nonetheless, some situations that might warrant a CXR in a child with a wheeze, are focal chest findings, fever, subcutaneous emphysema or a history of choking.
MDI vs Nebs vs IV B-agonists in Pediatric Asthma
Compared with nebulized treatments, metered-dose inhaler (MDI) with a spacer use has been shown to be equally effective for children of all ages with a wide range of illness severity and by multiple outcome measures. Among children 1 to 4 years old, using a MDI with a spacer was associated with a greater reduction in wheezing and a lower hospitalization rate in one study.
Furthermore, a recent cost analysis determined that the use of MDIs to treat children with mild to moderate asthma exacerbations in the ED could yield significant cost savings compared with nebulized treatments. MDIs with a spacer should not be used in patients with impending respiratory failure and it can be difficult to coordinate breathing with administration of the inhaler for patients less than 1 year old.
IV beta-agonists have not been shown to be superior to inhaled beta-agonists. IV beta-agonists should be considered in those who are unable to tolerate nebulized or MDI treatments.
For < 15 kg: Salbutamol MDI 4 puffs or 2.5mg nebulized in 2-3ml NS x3 back to back (continuously) For > 15kg: Salbutamol MDI 8 puffs or 5mg nebulized in 2-3ml NS x3 back to back (continuously)
A Cochrane review found that those treated with continuously nebulized bronchodilators had lower rates of hospitalization, greater improvements in pulmonary function test results, and similar rates of adverse events compared with those treated intermittently. Continuous treatment allows greater compliance with the goal of delivering the equivalent of three intermittent bronchodilator treatments in the first hour of care. In addition, this method will result in less respiratory therapy time and costs; it has been shown to be safe, and it may benefit the sickest patients the most.
B-agonists with Ipatropium Bromide are more effective than B-agonists alone in Pediatric Asthma
In a systematic review and meta-analysis comparing the use of beta-agonists plus anticholinergics with beta-agonists alone, combination therapy was associated with significantly lower hospitalization rates and improvements in asthma scores and pulmonary function test results.
So multiple doses of ipatropium bromide added to beta-agonists are indicated for kids with moderate to severe asthma exacerbations. However, there are no clinical trials supporting ipratropium use beyond the first hour or first 3 doses in children.
Ipatromium Bromide dosing: MDI 4 puffs (80mcg) or 250mcg nebulized
Single dose Dexamethasone is the preferred oral corticosteroid for Pediatric Asthma
A study out of the Annals of EM entitled “ A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department ”, showed that a single dose of dexamethasone dosed at 0.3mg/kg orally compared to prednisolone dosed at 1mg/kg for 3 days in 245 children with known asthma had equivalent PRAM scores at day 4. This is consistent with 3 previous RCTs, the largest of which dosed dexamethasone at 0.6mg/kg po.
So, it is reasonable to give one dose of dexamethasone at 0.3-0.6mg/kg po just prior to starting bronchodilators to all but the sickest of kids who present to the ED with an asthma exacerbation, obviating the need for an outpatient prescription.
It’s also worth noting that dexamethasone is associated with less vomiting compared to prednisolone as well.
Update 2022: A prospective, randomized, single center randomized clinical trial of 318 patients 2 to 20 years of age presenting to a pediatric ED with mild to moderate asthma exacerbation found no difference in the rate of return visits for continued or worsened symptoms, days to symptom resolution, missed school days, or vomiting between patient randomized to 1 or 2 doses of dexamethasone. Abstract
Inhaled corticosteroids
While there’s no evidence that the use of inhaled steroids in the ED are beneficial there is evidence that they decrease relapse rates in the outpatient setting.
The maximum dose of inhaled steroid is the equivalent of Fluticasone (Flovent in Canada) of 100 micrograms 2 puffs twice daily for a maximum of 200 micrograms per day. There is evidence based on observational data from the Canadian Paediatric Surveillance Program that higher doses may lead to adrenal suppression and in some cases adrenal shock.
Discharge Criteria for Pediatric Asthma
Discharge criteria from the ED include:
- Needing beta-agonists less often than q4 h after 4 to 8 h of conventional treatment
- A reading of SpO2 94% on room air
- Minimal or no signs of respiratory distress
- Improved air entry
Discharge Instructions for Pediatric Asthma
- Prepare a written asthma action plan with medications and signs to look out for that would necessitate a return to the ED
- Continue to use a short-acting beta-agonists such as salbutamol until exacerbation resolves and then as needed, with directions to see a health care professional if therapy is needed more often than every 4h.
- For all but the mildest of asthma patients seen in the ED, a prescription for 3 weeks of inhaled streroid such as fluticasone 50 micrograms, 2 puffs twice daily.
- Review techniques for using inhaled asthma medications as well as for cleaning/maintaining the inhaler device. Parents must understand that they need to use the MDI spacer and that the mask fits properly, to use the B-agonist BEFORE the inhanled steroid and to wash the mouth out after the steroid inhaler to prevent thrush.
- Encourage follow-up with the patient’s primary care physician or a local asthma clinic to review asthma control, environmental history and symptom recognition.
Peak expiratory flow should NOT be relied upon solely as a measure of severity or as a sole determinate for discharge.
IV Magnesium Sulphate in Pediatric Asthma
A meta-analyses suggests that use of magnesium sulphate results in improved outcomes for both adults and children, improving respiratory function and decreasing hospital admissions. IV magnesium sulphate may be considered in cases of moderate and severe asthma with incomplete response to conventional.
IV magmesium sulphate 40mg/kg should be given EARLY to patients with severe asthma who do not substantially improve after the first 6o minutes of bronchodilator and steroid therapy.
The most common adverse effect is hypotension; this may be avoided by infusion of the dose over 20 minutes and giving a fluid bolus prior to or during the magnesium infusion.
If there is a delay in obtaining an IV, magnesium sulphate can be given IO or inhaled via nebulizer.
What about Nebulized Magnesium Sulphate?
The RCT entitled MAGNETIC trial in 2013 of about 500 children showed that MgSO4 2.5mL of 250mmol/L solution q20mins x 3 added to the salbutamol and ipratropium bromide nebulizer in the first hour for kids with acute severe asthma, significantly improved asthma severity scores without any increase in adverse events.
Pediatric Asthma Therapy with Equivocal or Mixed Evidence that may be indicated when all else has failed
Up to 26% of children intubated due to asthma suffer complications including pneumothorax, impaired venous return, and cardiovascular collapse because of increased intrathoracic pressure. Mechanical ventilation during an asthma exacerbation is associated with an increased risk of death and should be considered as a last resort and in conjunction with the support of a paediatric ICU specialist.
IM Epinephrine – time tested but no good evidence
IM epinephrine at the same doses used in anaphylaxis (0.01mg/kg of 1:1000, max 0.3mg given in the anterolateral thigh) has been used for decades in children with severe asthma, however there have been no robust RCTs to support it’s use.
Heliox – reserve for the ICU
According to the Canadian Pediatric Society Guidelines for Managing the Patient with Acute Asthma Exacerbation , using a helium-oxygen gas mixture should be reserved for children in the ICU setting with severe asthma exacerbation who have failed to improve despite maximized therapy.
Ketamine to avoid intubation – 3 Mixed Studies
A limited case series has reported the effectiveness of a bolus (2 mg/kg) followed by a continuous infusion (2 to 3 mg/kg/h) of ketamine in children with severe asthma who were approaching respiratory failure. In this study, the use of ketamine resulted in prompt improvement and avoided the need for endotracheal intubation. This is an appealing use of ketamine, because it may allow one to avoid the hazards of endotracheal intubation and mechanical ventilation in the patient with asthma.
A randomized control trial showed no improvement in pulmonary index scores with the administration of ketamine to patients with moderate to severe asthma. Patients were randomized to 0.2mg/kg ketamine bolus followed by 0.5mg/kg/h for 2 hours vs placebo.Pulmonary index scores were measured throughout the 2 hours and no difference was found.
In a 2001 prospective, observational, single-arm pilot study in two pediatric EDs over three months, the effect of IV ketamine added to standard therapy in status asthmaticus wasevaluated. Initiation of ketamine in patients with severe asthma was associated with clinical improvement. Side effects were easily managed with treatment or discontinuation of ketamine.
The take home message is that more convincing evidence is required before ketamine can be recommended for routine treatment of severe pediatric asthma to avoid intubation.
Ketamine, however, is safe at dissociative dosages, and is a reasonable option when all others measures have failed.
BiPAP – the pediatric literature isn’t quite as impressive as the adult literature
A few case reports and observational studies of the use of BiPAP in pediatric asthma show some promise. The one RCT of only 20 patients does show a benefit in clinical asthma scores, respiratory rate, and supplemental oxygen need. While intuitively sensible, there is no evidence that NIPPV prevents the need for intubation in children with status asthmaticus.
Similar to other rescue measures, NIPPV can be considered when all others measures have failed in hopes of avoiding endotracheal intubation.
High Flow Nasal Cannula – gaining popularity
Another way of providing a bit of noninvasive positive pressure that seems to becoming popular among the pedatricians is high flow nasal cannula oxygen. The evidence is conflicting for this, and most studies were done in kids with bronchiolitis rather than asthma.
One study from Pediatric Emergency Care in 2012 showed that the use of high flow nasal oxygen reduced the need for intubation in pediatric acute respiratory failure, but there was no change in mortality or ICU length of stay.
However, a Cochrane review in 2014 based on 11 studies concluded that no evidence could be found to allow determination of the safety or effectiveness of HFNC therapy in children.
The latest study out of Emergency Medicine Journal concluded that HFNC may have a role, but about 1/3 of patients required BiPAP or intubation.
IV salbutamol – may improve recovery time and length of stay
Two RCTs showed a more rapid recovery time and earlier discharge from hospital when IV salbutamol was compared to nebulized ipratopium bromide in one of the studies, and compared to continuous salbutamol nebs in another. When using IV salbutamol, be on the lookout for excessive tachycardia, low DBP and rising lactate. Start at 1mcg/kg/min infusion and titrate to 5mcg/kg/min.
The decision to intubate should be based on clinical judgement as opposed to any single vital sign or blood gas result. Some variables to consider for intubation are worsening hypercapnea, patient exhaustion and changes in mental status.
Putting it all together for Severe Pediatric Asthma Exacerbation: A Step-wise Approach
*note that the blue indicates evidence-based treatment while the red indicates therapies that are reasonable to try when all else has failed but do not have strong evidence for benefit
Put the child on the cardiac monitor | Obtain IV access and draw blood work including electrolytes and a VBG (with particular attention to the K) | Call your RT and pediatric intensivist early | Continuous salbutamol nebulizers with the first 3 including ipratropium bromide | IV steroids: methylprednisolone 1mg/kg or hydrocortisone 5mg/kg (if dexamethasone 0.3mg/kg, max 10mg was not given prior to starting nebs) | IV NS 20mL/kg bolus (preferably before the MgSO4) | IV Magnesium Sulphate 40mg/kg to a maximum of 2g over 20 mins (in the first hour if possible) | Consider epinephrine 0.01mg/kg IM and nebulized MgSO4 (especially if you are having trouble obtaining IV access) | Consider BiPaP or high flow nasal oxygen | Consider IV salbutamol 1-5mcg/kg/min (beware tachycardia, low DBP, rising lactate) | Consider subdissociative dose ketamine | Consider Heliox

Quote of the Month
“Knowledge is not only power; it is happiness,
and being taught is the intellectual analog of being loved.”
– Isaac Asimov
For more on Paediatric Emergencies download our free interactice eBook EM Cases Digest Vol. 2 Pediatric Emergencies
Dr. Helman, Dr. Mehta and Dr. Scolnik have no conflicts of interest to declare
Key References
Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med 2004;11(1):10-8.
Belessis Y, Dixon S, Thomsen A, et al. Risk factors for an intensive care unit admission in children with asthma. Pediatr Pulmonol 2004;37(3):201-9.
Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): A responsive index of acute asthma severity. J Pediatr 2000;137(6):762-8.
Birken CS, Parkin PC, Macarthur C. Asthma severity scores for preschoolers displayed weaknesses in reliability, validity, and responsiveness. J Clin Epidemiol 2004;57(11):1177-81.
Gershel JC, et al: The usefulness of chest radiographs in first asthma attacks. Engl J Med 309: 336, 1983.
Castro-Rodriguez JA, Rodrigo GJ: Beta-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: A systematic review with meta-analysis. J Pediatr 2004; 145:172.
Camargo CA, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003;(4):CD001115.
Randolph C. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;134 Suppl 3:S178-9.
Cronin JJ, Mccoy S, Kennedy U, et al. A Randomized Trial of Single-Dose Oral Dexamethasone Versus Multidose Prednisolone for Acute Exacerbations of Asthma in Children Who Attend the Emergency Department. Ann Emerg Med. 2015; 134:432.
Rodrigo GJ, Castro-Rodriguez JA: Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax 2005; 60:740.)
Goldbloom E, Ahmet A. Adrenal suppression: An under-recognized complication of a common therapy. Paediatr Child Health. 2010;15(7):411-2. Full PDF
Rowe BH, et al: Intravenous magnesium sulfate treatment for acute asthma in the emergency department: A systematic review of the literature. Ann Emerg Med 2000; 36:181. Full PDF
Cheuk DK, Chau TC, Lee SL: A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child 2005; 90:74.
Powell CV, Kolamunnage-dona R, Lowe J, et al. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol Assess. 2013;17(45):v-vi, 1-216.
O Ortiz-Alvarez, A Mikrogianakis; Managing the Patient with an acute asthma exacerbation. Canadian Paediatric Society,Paediatr Child Health 2012;17(5):251-5
Denmark TK, Crane HA, Brown L: Ketamine to avoid mechanical ventilation in severe pediatric asthma. J Emerg Med 30: 163, 2006.
Allen JY, Macia CG. The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Ann Emerg Med. 2005;46(1):43-50.
Petrillo TM, Fortenberry JD, Linzer JF, Simon HK. Emergency department use of ketamine in pediatric status asthmaticus. J Asthma. 2001; 38(8):657-664.
Basnet S, Mander G, Andoh J, Klaska H, Verhulst S, Koirala J. Safety, efficacy, and tolerability of early initiation of noninvasive positive pressure ventilation in pediatric patients admitted with status asthmaticus: a pilot study. Pediatr Crit Care Med. 2012;13(4):393-8.
Wing R, James C, Maranda LS, Armsby CC. Use of high-flow nasal cannula support in the emergency department reduces the need for intubation in pediatric acute respiratory insufficiency. Pediatr Emerg Care. 2012;28(11):1117-23.
Mayfield S, Jauncey-cooke J, Hough JL, Schibler A, Gibbons K, Bogossian F. High-flow nasal cannula therapy for respiratory support in children. Cochrane Database Syst Rev. 2014;3:CD009850.
Long E, Babl FE, Duke T. Is there a role for humidified heated high-flow nasal cannula therapy in paediatric emergency departments?. Emerg Med J. 2016.
Browne GJ, Trieu L, Van asperen P. Randomized, double-blind, placebo-controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Crit Care Med. 2002;30(2):448-53.
Browne GJ, Penna AS, Phung X, Soo M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;349(9048):301-5.
Additional FOAMed Resources for Pediatric Asthma
Evidence-based slide set on ED pediatric asthma management from CAEP 2015 Conference
Mechanical Ventilation in Severe Asthma on Pediatric EM Morsels
Asthma and The Vent on PEM ED podcast
Best Bets review on Ketamine in Severe Asthma
St. Emlyns on Why don’t we just use dexamethasone?
Ryan Radecki on Early Administration of Steroids in Pediatric Asthma
For more EM Cases content on Pediatric Emergencies download our free eBook,
EM Cases Digest Vol. 2 Pediatric Emergencies here .

About the Author: Anton Helman
Recent Posts

Ep 197 Acute Heart Failure Risk Stratification and Disposition

EM Quick Hits 58 – HIV PEP and PrEP, PREOXI Trial, Blast Crisis, Nitrous Oxide Poisoning, Vasopressors in Trauma

Compassionate Care to Improve Patient Outcomes and Your Career from EMU 2024
[…] Helman goes through an evidence based approach to management of asthma exacerbations in children. […]
Simple but covers all the basics, enjoyable read through Thank you
Leave A Comment Cancel reply
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals
You are here
- Volume 11, Issue 7
- Management of asthma in childhood: study protocol of a systematic evidence update by the Paediatric Asthma in Real Life (PeARL) Think Tank
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-4675-9616 Alexander G Mathioudakis 1 , 2 , 3 ,
- Michael Miligkos 4 ,
- Cristina Boccabella 5 ,
- Gioulinta S Alimani 3 , 6 ,
- Adnan Custovic 7 ,
- A Deschildre 8 ,
- Francine Monique Ducharme 9 ,
- Omer Kalayci 10 ,
- Clare Murray 1 , 2 ,
- Antonio Nieto Garcia 11 ,
- Wanda Phipatanakul 12 ,
- David Price 13 , 14 ,
- Aziz Sheikh 15 ,
- Ioana Octavia Agache 16 ,
- Leonard Bacharier 17 ,
- http://orcid.org/0000-0001-5639-0528 Apostolos Beloukas 6 , 18 ,
- Andrew Bentley 2 , 19 ,
- Matteo Bonini 5 , 20 ,
- Jose A Castro-Rodriguez 21 ,
- Giuseppe De Carlo 22 ,
- Timothy Craig 23 ,
- Zuzana Diamant 24 , 25 , 26 ,
- Wojciech Feleszko 27 ,
- Tim Felton 1 , 2 ,
- James E Gern 28 ,
- Jonathan Grigg 29 ,
- Gunilla Hedlin 30 ,
- Elham M Hossny 31 ,
- Despo Ierodiakonou 32 ,
- Tuomas Jartti 33 ,
- Alan Kaplan 34 ,
- Robert F Lemanske 28 ,
- Peter N Le Souëf 35 ,
- Mika J Mäkelä 36 ,
- Georgios A Mathioudakis 3 ,
- Paolo Matricardi 37 ,
- Marina Mitrogiorgou 38 ,
- Mario Morais-Almeida 39 ,
- Karthik Nagaraju 40 ,
- Effie Papageorgiou 6 ,
- Helena Pité 39 , 41 , 42 ,
- Paulo M C Pitrez 43 ,
- Petr Pohunek 44 ,
- Graham Roberts 45 , 46 , 47 ,
- Ioanna Tsiligianni 32 ,
- Stephen Turner 48 ,
- Susanne Vijverberg 49 ,
- Tonya A Winders 50 ,
- http://orcid.org/0000-0001-5939-812X Gary WK Wong 51 ,
- Paraskevi Xepapadaki 52 ,
- Heather J Zar 53 , 54 ,
- http://orcid.org/0000-0002-4448-3468 Nikolaos G Papadopoulos 1 , 52
- 1 Division of Infection, Immunity and Respiratory Medicine , The University of Manchester , Manchester , UK
- 2 North West Lung Centre, Manchester University NHS Foundation Trust , Manchester , UK
- 3 Athens Breath Centre , Athens , Greece
- 4 First Department of Pediatrics, "Aghia Sofia" Children's Hospital , University of Athens , Athens , Attica , Greece
- 5 Department of Cardiovascular and Thoracic Sciences , Catholic University of the Sacred Heart , Milano , Lombardia , Italy
- 6 Department of Biomedical Sciences , University of West Attica , Egaleo , Attica , Greece
- 7 Department of Paediatrics , Imperial College London , London , UK
- 8 Unité de Pneumologie et Allergologie Pédiatriques, Hôpital Jeanne de Flandre , CHU Lille , Lille , Hauts-de-France , France
- 9 Pediatrics , University of Montreal , Montreal , Quebec , Canada
- 10 Pediatric Allergy and Asthma Unit , Hacettepe Universitesi , Ankara , Turkey
- 11 Pulmonology and Allergy Unity , La Fe University and Polytechnic Hospital , Valencia , Comunidad Valenciana , Spain
- 12 Pediatric Allergy and Immunology , Children's Hospital Boston , Boston , Massachusetts , USA
- 13 Centre of Academic Primary Care , University of Aberdeen , Aberdeen , UK
- 14 Observational and Pragmatic Research Institute , Singapore
- 15 Asthma UK Centre for Applied Research, Usher Institute of Population Health Sciences and Informatics , The University of Edinburgh , Edinburgh , UK
- 16 Allergy and Clinical Immunology , Transylvania University , Brasov , Romania
- 17 Department of Allergy, Immunology, and Pulmonary Medicine , University of Washington , Seattle , Washington , USA
- 18 Institute of Infection and Global Health , University of Liverpool , Liverpool , UK
- 19 Acute Intensive Care Unit , University Hospital of South Manchester NHS Foundation Trust , Manchester , Greater Manchester , UK
- 20 National Heart and Lung Institute (NHLI) , Imperial College London , London , UK
- 21 Department of Pediatrics , Pontifical Universidad Catolica de Chile , Santiago , Chile
- 22 Allergy and Airway Diseases Patient's Associations , European Federation of Pharmaceutical Industries and Associations , Brussels , Belgium
- 23 Allergy, Asthma and Immunology , Penn State University , Hershey , Pennsylvania , USA
- 24 Department of Respiratory Medicine and Allergology, Institute for Clinical Science , Skane University Hospital Lund Hematological Clinic , Lund , Skåne , Sweden
- 25 Department of Respiratory Medicine , First Faculty of Medicine, Charles University and Thomayer Hospital , Prague , Czech Republic
- 26 Department of Clinical Pharmacy & Pharmacology , University of Groningen, University Medical Center of Groningen and QPS-NL , Groningen , Netherlands
- 27 Department of Pediatric Pulmonology and Allergy , Medical University of Warsaw , Warszawa , Poland
- 28 Department of Pediatrics and Medicine , University of Wisconsin School of Medicine and Public Health , Madison , Wisconsin , USA
- 29 Centre for Genomics and Child Health, Blizard Institute , Queen Mary University of London , London , UK
- 30 Department of Women's and Children's Health , Karolinska Institute , Stockholm , Stockholm , Sweden
- 31 Pediatric Allergy and Immunology Unit , Ain Shams University , Cairo , Egypt
- 32 Department of Social Medicine, Faculty of Medicine , University of Crete , Rethimno , Greece
- 33 Department of Paediatrics , University of Turku , Turku , Finland
- 34 Family Physician, Airways Group of Canada , University of Toronto , Toronto , Ontario , Canada
- 35 School of Paediatrics and Child Health , University of Western Australia , Perth , Western Australia , Australia
- 36 Department of Allergy , University of Helsinki , Helsinki , Uusimaa , Finland
- 37 Department of Pediatric Pulmonology, Immunology and Intensive Care Medicine , Charité - University Medicine , Berlin , Germany
- 38 Third Department of Paediatrics , National and Kapodistrian University of Athens School of Health Sciences , Athens , Greece
- 39 Allergy Center , Hospital CUF Descobertas , Lisboa , Portugal
- 40 Allergy & Asthma , VN , Chennai , India
- 41 Allergy Center , CUF Infante Santo Hospital , Lisbon , Portugal
- 42 Chronic Diseases Research Center (CEDOC) , NOVA Medical School / Faculdade de Ciências Médicas, Universidade NOVA de Lisboa , Lisbon , Portugal
- 43 Laboratory of Respiratory Physiology, Infant Center , School of Medicine, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) , Porto Alegre , Brazil
- 44 Paediatric Department , Motol University Hospital , Praha , Czech Republic
- 45 The David Hide Asthma and Allergy Research Centre , St Mary's Hospital , Newport Isle of Wight , UK
- 46 Faculty of Medicine, Clinical and Experimental Sciences and Human Development in Health Academic Units , University of Southampton , Southampton , UK
- 47 NIHR Biomedical Research Centre , University Hospital Southampton NHS Foundation Trust , Southampton , UK
- 48 Department of Child Health , University of Aberdeen , Aberdeen , Aberdeen , UK
- 49 Department of Respiratory Medicine and Department of Pediatric Pulmonology , University of Amsterdam , Amsterdam , Netherlands
- 50 Allergy & Asthma , Global Patient Platform , Virginia , Virginia , USA
- 51 Department of Paediatrics, Faculty of Medicine , The Chinese University of Hong Kong , Sha Tin , Hong Kong
- 52 Allergy Department, 2nd Paediatric Clinic , National and Kapodistrian University of Athens , Athens , Attica , Greece
- 53 Department of Paediatrics and Child Health, Red Cross War Memorial Children’s Hospital , University of Cape Town , Rondebosch , Western Cape , South Africa
- 54 Unit on Child and Adolescent Health , Medical Reaserch Council , Cape Town , South Africa
- Correspondence to Professor Nikolaos G Papadopoulos; ngpallergy{at}gmail.com
Introduction Clinical recommendations for childhood asthma are often based on data extrapolated from studies conducted in adults, despite significant differences in mechanisms and response to treatments. The Paediatric Asthma in Real Life (PeARL) Think Tank aspires to develop recommendations based on the best available evidence from studies in children. An overview of systematic reviews (SRs) on paediatric asthma maintenance management and an SR of treatments for acute asthma attacks in children, requiring an emergency presentation with/without hospital admission will be conducted.
Methods and analysis Standard methodology recommended by Cochrane will be followed. Maintenance pharmacotherapy of childhood asthma will be evaluated in an overview of SRs published after 2005 and including clinical trials or real-life studies. For evaluating pharmacotherapy of acute asthma attacks leading to an emergency presentation with/without hospital admission, we opted to conduct de novo synthesis in the absence of adequate up-to-date published SRs. For the SR of acute asthma pharmacotherapy, we will consider eligible SRs, clinical trials or real-life studies without time restrictions. Our evidence updates will be based on broad searches of Pubmed/Medline and the Cochrane Library. We will use A MeaSurement Tool to Assess systematic Reviews, V.2, Cochrane risk of bias 2 and REal Life EVidence AssessmeNt Tool to evaluate the methodological quality of SRs, controlled clinical trials and real-life studies, respectively.
Next, we will further assess interventions for acute severe asthma attacks with positive clinical results in meta-analyses. We will include both controlled clinical trials and observational studies and will assess their quality using the previously mentioned tools. We will employ random effect models for conducting meta-analyses, and Grading of Recommendations Assessment, Development and Evaluation methodology to assess certainty in the body of evidence.
Ethics and dissemination Ethics approval is not required for SRs. Our findings will be published in peer reviewed journals and will inform clinical recommendations being developed by the PeARL Think Tank.
PROSPERO registration numbers CRD42020132990, CRD42020171624.
- paediatrics
- paediatric thoracic medicine
- thoracic medicine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2020-048338
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
Broad evidence syntheses on the management of childhood asthma, with a focus on the differential treatment response according to age and disease phenotypes could reveal clinically exploitable information, that will be used in the development of clinical and research recommendations by Paediatric Asthma in Real Life.
A rigorous methodology that includes thorough evaluation of the literature, appropriate evaluation of the methodological quality of individual studies and—when appropriate—of the body of evidence, and presentation of overall effect estimates.
A prospectively published protocol increases the transparency and allowed for peer-review of the methodology used.
A potential limitation of the overview of systematic reviews (SRs) is that the feasibility of conducting the planned subgroup analyses will depend on whether relevant data have been captured in existing SRs.
Introduction
Having a global prevalence that is anticipated to exceed 400 million children by the year 2025, childhood asthma represents a huge health and socioeconomic burden to patients, their families and the society. 1–3 Despite its diverging mechanisms, triggers, outcomes and response to treatment, childhood asthma is often still approached as an extension of adult asthma. 4 It is underaddressed in clinical guidelines, likely due to unclear diagnosis, limited availability of safety, efficacy and effectiveness data in this population. Clinical recommendations are to a large extent informed by data extrapolated from clinical studies conducted in adults. 2–5
Numerous challenges complicate conducting interventional research studies in children with asthma. Besides the lack of consensus on its definition and diagnostic criteria, childhood asthma is highly heterogeneous and our understanding of different paediatric asthma phenotypes is still limited or contradictory. 6 This is further emphasised by significant variability in disease progression, outcomes and treatment response in children with different phenotypes or ages5, 7 potentially complicating interpretation of trials’ findings. In addition, there are regulatory and ethical constraints in conducting interventional research in children. 8 9 However, this results in the administration of treatments that have not been adequately evaluated in relevant (paediatric) populations, that is, evidently suboptimal.
Paediatric Asthma in Real Life (PeARL), an international Think Tank focusing on paediatric asthma, was initiated in the context of the respiratory effectiveness group, to address this evidence deficit. In a recent international, multistakeholder survey, we have identified and prioritised unmet needs on paediatric asthma. 10 A need for systematic evidence updates focusing on the management of asthma in different age groups emerged. Herein, we present the protocol for a series of systematic evidence updates aiming to summarise direct evidence from clinical studies in children with asthma, evaluating the safety and clinical effectiveness of pharmacological interventions for maintenance management and for the treatment of acute severe asthma attacks, defined as those leading to an emergency presentation with/without hospital admission, in different age groups. Our work will be used to inform clinical recommendations being developed by the PeARL Think Tank. Therefore, we need solid evidence on the efficacy on safety of various interventions. It is considered crucial to incorporate evidence derived from real-life observational studies, which may carry a lower strength of evidence than randomised controlled trials (RCTs), but are available in higher abundance and provide a better representation of clinical practice in real life, where for example, treatment compliance or inhaler technique may be problematic.
Methods and analysis
We will conduct two systematic evidence updates, based on protocols prospectively registered in the PROSPERO register (CRD42020132990, 11 CRD42020171624 12 ). The first will evaluate the safety and clinical effectiveness of pharmacological maintenance treatments for childhood asthma, while the other will focus on the pharmacotherapy of acute severe asthma attacks, defined as those requiring a hospital admission or emergency presentation. We will use standard methodology recommended by the Cochrane Collaboration 13 and will follow the Preferred Reported Items for Systematic Reviews and Meta-Analyses statement. 14
Preliminary searches revealed several RCTs evaluating maintenance pharmacotherapy of childhood asthma, which have already been summarised in high-quality systematic reviews (SRs), some conducted by the Cochrane Collaboration. We identified >40 up-to-date SRs evaluating inhaled corticosteroids (ICS), long-acting beta-2 agonists (LABA), long-acting muscarinic antagonists (LAMA), leukotriene receptor antagonists (LTRA) or biologic therapies, as first line or add-on treatment for asthma in children. As a result, we opted to produce an overview of existing SRs of clinical trials and real-life studies. 15 .
We found less up-to-date SRs on the management of acute severe asthma attacks in children, mainly focusing on short-acting beta-2 agonists (SABA), short acting muscarinic antagonists, oral corticosteroids, aminophylline and magnesium that were recently summarised in a Cochrane Overview of SRs. 16 However, when evaluating the literature, we identified several other pharmacological interventions that are tested in small trials or real-life studies, and while they may show promising early results, they have not been assessed further or introduced in clinical practice guidelines. 17–23 For this reason, we will conduct de novo synthesis of comparative clinical studies of any design aiming to identify any pharmacological intervention that has been tested for acute severe asthma attacks, followed by focused meta-analyses of promising interventions not covered by existing high-quality SRs or clinical practice guidelines.
Overview of SRs evaluating maintenance pharmacotherapy for paediatric asthma
Eligibility criteria.
Eligible studies will comprise SRs and meta-analyses of controlled clinical trials or of real-life studies evaluating maintenance treatments that are broadly used in clinical practice for asthma or recurrent wheeze in children and adolescents, aged up to 18 years. More specifically, we will include SRs comparing any combination of ICS, LABA, LAMA, LTRA, biological therapies (namely omalizumab, mepolizumab, reslizumab, benralizumab or dupilumab), or placebo as monotherapy or add-on maintenance therapy for paediatric asthma. We will accept SRs and meta-analyses evaluating any molecule of the above-mentioned categories, administered at any dose and for a duration of at least 6 weeks. SRs comparing asthma maintenance treatment both in children and adults will be included provided that paediatric data are presented separately. We will only include SRs published between 2005 and December 2020 and reported in the English language. Older SRs are probably outdated and will only be considered in the absence of high-quality, newer SRs.
Outcome measures
The primary outcomes of this overview will be the number of acute attacks requiring the administration of oral corticosteroids or an emergency visit, and the number of acute attacks requiring hospitalisation. Secondary outcomes will include lung function measures, acute attacks irrespective of the severity, symptom scores (including symptom free and rescue medication free days), asthma control, asthma-specific quality of life scores, use of rescue medications, withdrawal rates (overall, due to lack of efficacy or adverse events), adverse events and serious adverse events.
Search strategy and study selection
The electronic databases of Medline/PubMed and Cochrane Library will be systematically searched, using appropriate controlled vocabulary and free search terms to identify relevant SRs (terms describing: childhood asthma, LABA, LAMA, LTRA, ICS, biologics, SRs, detailed search strategy is available in online supplemental appendix ). Databases will be searched from 2006 onwards. Titles and abstracts of all identified manuscripts, and the full texts of potentially relevant manuscripts, will be screened by two investigators independently. We will report the reasons of exclusion of studies that will be excluded after full-text review. Disagreement will be resolved through discussion or adjudication by a third investigator, when necessary.
Supplemental material
Data abstraction.
For each of the included SRs, one investigator will extract the full reference and study identifiers, references of the included trials evaluating paediatric populations, eligibility criteria, predefined outcomes, number and baseline characteristics of the participants and details on the outcomes of interest. A second investigator will cross-check for validity.
Risk of bias assessment
A MeaSurement Tool to Assess systematic Reviews, V.2 (AMSTAR 2) tool will be used to evaluate the methodological quality of all included SRs. 24 25 The AMSTAR 2 tool evaluates 16 domains, focusing on the methodological design, interpretation and potential risk of bias involved in the conduct of a SR. It is considered by the AMSTAR 2 team that seven domains could critically affect the validity of the review, while the remaining domains describe non-critical weaknesses. Critical flaws for an SR include (1) lack of prospective protocol registration, (2) inadequate literature searches, (3) lack of justification of excluding individual studies, (4) of risk of bias evaluation or (5) of risk of bias consideration in interpreting the results, (6) of assessment of presence and likely impact of publication bias and (7) inadequate methodology for conducting meta-analysis. We will consider the results of an SR of high quality, if there is only one or none non-critical weakness, and of moderate quality, if there are more than one non-critical weaknesses. If there are one or more critical weaknesses, then we will consider the confidence low or very low, respectively. Two of the SRs will evaluate the risk of bias independently and disagreement will be resolved through discussion, or adjudication by a third reviewer.
Qualitative synthesis
We will summarise descriptively or in a tabulated format the characteristics of the included SRs and outcomes of interest. When several SRs evaluate the same intervention, we will compare their eligibility criteria, included studies and methodological quality as evaluated by the AMSTAR-2 tool, as well as the pertinent subgroup analyses that are presented. We will present in detail the results of the SR that is most recent, more complete and of high methodological quality. If no single SR fulfil these criteria, we will present in detail more than one SRs. From the remaining SRs, we will present pertinent additional information that may include, such as details about additional outcomes, or additional subgroups.
We will specifically report on the differential effectiveness of the interventions across different maintenance treatment steps (severity), age groups or paediatric asthma phenotypes.
SR of clinical studies evaluating the management of acute severe asthma attacks
Over the past decades, several interventions have been tested for the management of acute severe asthma attacks, such as ketamine or macrolide antibiotics. 17–23 Despite promising early findings, some of these interventions were not further tested in robust, prospective controlled clinical trials. This may partially be due to challenges in conducting experimental clinical studies in children, as previously discussed, particularly during acute, life-threatening conditions.
To identify all evaluated treatments, a two-stage approach will be followed. First, a broad search strategy will be used to identify all pharmacological interventions that have been tested as potential treatments for acute severe asthma attacks. Next, medications that showed positive clinical results, but are not yet thoroughly evaluated in clinical studies and meta-analyses and are therefore not recommended by international asthma guidelines (such as the National Institute for Health and Care Excellence asthma guidelines, the British Thoracic Society and Scottish Intercollegiate Guidelines Network asthma guidelines, the National Asthma Education and Prevention Programme or the Global Strategy for Asthma Management and Prevention document), will be selected and further evaluated in individual meta-analyses. The aim will be to identify novel interventions that could be recommended for use in clinical practice, or might require further evaluation in clinical research studies, to confirm their safety and effectiveness profiles.
Medline/PubMed and the Cochrane Library will be searched, using a broad search strategy, aimed to identify any clinical research studies evaluating the management of acute severe asthma attacks (detailed search strategy is available in online supplemental appendix ).
Any study evaluating pharmacological treatments for acute severe asthma attacks in children and adolescents (<18 years of age) will be included. Any comparative clinical research study, including experimental and observational studies, as well as SRs of such studies will be considered eligible for inclusion. We will only include studies published until May 2021 and reported in the English language, without time restrictions.
Eligible studies will be grouped according to the drug category they evaluate and will be presented narratively. Study design, characteristics and outcomes of interest will be reported descriptively or in a tabulated format. Outcomes of interest are the same for this broad SR and individual medication meta-analyses and are detailed in the next section.
Individual medication meta-analyses
These meta-analyses will further evaluate the safety and clinical effectiveness of individual medications that were assessed by the initial broad SR and were found to be of potential clinical value for the treatment of acute severe asthma attacks. In contrast to most preceding SRs and meta-analyses, we will include data from observational comparative effectiveness (real-life) studies, as well as controlled clinical trials.
For each meta-analysis, eligible studies will comprise controlled clinical trials and observational comparative effectiveness studies comparing the index medication with placebo, no treatment or any active control, as an add-on treatment for acute severe asthma attacks. Index medication will be defined based on the pharmacological action, meaning that molecules targeting the same pharmacological target (eg, salbutamol and terbutaline, both being SABA) will be grouped. Only studies evaluating the management of acute severe asthma attacks, defined as those requiring a hospital admission or emergency presentation, in children and adolescents, aged between 1 and 18 years of age will be included. Studies evaluating both children and adults will be included, provided that paediatric data are reported separately or that we will be able to access these data after requesting them from the investigators. We will only include observational studies that meet the primary criteria of the REal Life EVidence AssessmeNt Tool (RELEVANT) tool (see risk of bias). We will include studies published until May 2021 and reported in the English language.
The primary outcome measures will be (1) treatment success or treatment failure rate evaluated at any time point, within 2 weeks from presentation, (2) serious adverse events and (3) need for asthma related hospitalisation evaluated at any tim epoint within 2 weeks from presentation. Treatment success will be defined as a complete resolution of the symptoms, or an improvement in the clinical signs, symptoms and/or laboratory findings that fulfils specific criteria or thresholds prespecified by the study team. Treatment failure will be defined as a significant deterioration of the patients’ clinical conditions that fulfils specific criteria prespecified by the study team. For example, treatment failure may be defined as the need for paediatric intensive care unit admission, ventilation or death. The definitions of treatment success and treatment failure vary significantly across clinical studies evaluating the management of acute asthma in children; for this reason, meta-analyses will only be conducted in cases they are considered meaningful by the investigators. Need for asthma-related hospitalisation will not be relevant for studies only evaluating hospitalised participants. Secondary outcomes will include (1) mortality, (2) duration of asthma-related hospitalisation, (3) need for intensive care unit admission, (4) duration of intensive care unit stay, (5) re-exacerbation rate, (6) rehospitalisation rate and (7) adverse events. All outcomes will be evaluated at a maximum follow-up of 6 months, as longer-term outcomes are less likely to be directly linked with the index acute event.
Using appropriate controlled vocabulary and free search terms, we will systematically search Medline/PubMed, EMBASE and the Cochrane Library to identify controlled clinical trials and observational comparative effectiveness studies evaluating the safety, efficacy and/or clinical effectiveness of the selected medication (sample search strategies are available in the online appendix). We will also search the WHO International Clinical Trials Registry Platform search portal, the abstract proceedings of the European Respiratory Society, the American Thoracic Society, the Asian Pacific Society of Respirology, the European Academy of Allergy and Clinical Immunology, the American Academy of Allergy, Asthma and Immunology, and the World Allergy Organization, as well as the reference lists of all included studies. All sources will be searched from inception, without language limitations. We will follow standard methodology for screening titles, abstracts and the full text of all identified studies, as described previously.
The full study reference, study identifiers, details on the study design, eligibility criteria, predefined outcomes and potential confounding factors that were considered by the investigators, number and baseline characteristics of participants will be extracted by one investigator and will be cross-checked for validity by a second extractor. Details on the outcomes of interest from all included studies will be extracted by two investigators independently. Conflicts will be resolved through discussion and when needed adjudication by a third investigator.
Risk of bias of individual studies
We will use the second version of the Cochrane risk of bias (RoB2) tool for assessing risk of bias in the included RCTs 26 and the RELEVANT for assessing the risk of bias of observational studies. 27 Risk of bias of each included study will be evaluated by two investigators independently.
The RoB2 tool evaluates the following domains for potential risk of bias: (1) bias arising from the randomisation process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, (5) bias in selection of reported results and (6) any other potential source of bias. High risk of bias in any of these domains will result in an overall judgement of high risk of bias. In the absence of high-risk domains, unclear risk in any domain will lead to an overall judgement of unclear risk. All remaining trials will be considered to be of low risk of bias.
RELEVANT evaluates the quality of observational comparative effectiveness research studies across seven domains, which include background, design, measures, analysis, results, discussion/interpretation and conflicts of interest. Each domain includes primary and secondary items. It is suggested that studies not meeting the primary items of RELEVANT are of very low methodological quality (have ‘fatal flaws’) and should not be used to inform clinical recommendations. Therefore, we will exclude studies not meeting these criteria. We will consider of low risk of bias all studies meeting the secondary criteria of RELEVANT as well, and of high risk of bias studies that do not meet any of the secondary criteria.
For every comparison, we will use funnel plots, Egger’s regression and Begg’s rank tests to evaluate publication bias, if we are able to pool more than 10 studies.
Data synthesis
Data from controlled clinical trials or observational studies will be analysed separately. In addition, studies evaluating different comparators, will be analysed separately. If different doses of the index medication or comparator are evaluated across the included studies, we will consider grouping studies using similar doses, providing that their results are not significantly dissimilar.
For every analysis, I 2 statistic will be used to assess statistical heterogeneity. Substantial heterogeneity (I 2 >50%) will be explored using prespecified subgroup analyses (details in the next section). We will not perform meta-analyses in cases of considerable unresolved heterogeneity (I 2 >75%).
When it is considered meaningful, meta-analyses will be performed using the random-effects model, because we anticipate significant heterogeneity in our data. Results will be presented in the form of relative risk (95% CI) for dichotomous data, mean difference (95% CI) for continuous data and (HR, 95% CI) for time to event data. Meta-analyses will be performed using Review Manager V.5 (RevMan, http://community.cochrane.org/tools/review-production-tools/revman-5 ) and R statistics V.3.4.3 or newer (R Foundation for Statistical Computing, Vienna, Austria).
For dichotomous outcomes, the unit of analysis will preferably be participants, rather than events (ie, number of participants admitted to the intensive care unit, rather than number of admissions per participants).
Sensitivity and subgroup analyses
In sensitivity analyses for all comparisons, we will (1) use fixed effects models, (2) only include studies with low risk of bias, (3) exclude studies reporting limited adherence to the study drugs (<80%) and (4) evaluate separately studies assessing different doses of the index medication, which we may pool in the main analysis.
Subgroup analyses according to participants’ age, asthma phenotypes or, possibly, acute attack phenotypes will also by conducted, depending on data availability. In an additional subgroup analysis, we will evaluate separately trials utilising exploratory versus pragmatic study designs.
Certainty of the body of evidence
Certainty of the body of evidence, for every comparison will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. 28 GRADE assesses the certainty in a body of evidence as high, moderate, low or very low after considering the methodological quality of the included studies, imprecision, inconsistency, indirectness, publication bias, the magnitude of effect, dose response and confounders likely to minimise the effect. All decisions to upgrade or downgrade the quality of evidence will be transparent and justified in evidence profile and summary of evidence tables, in accordance with GRADE guidance. GRADEPro Software (2014; www.gradepro.org ) will be used for the development of these tables.
We will use GRADE methodology to assess the risk of bias associated with missing participant outcome data across the body of the available evidence. 29 GRADE suggests repeating the primary meta-analysis, imputing the most extreme assumptions about the values of the missing data, that the investigators consider plausible. Only if the analyses prove robust to this imputation, the risk of bias due to missing participant outcome data should be deemed low.
The impact that the risk of bias of individual studies and the confidence in the body of the evidence has on the results will be presented.
Ethics and dissemination
Ethical approval is not required for these SRs, since no primary data will be collected.
The findings of these evidence updates will be presented in national and international scientific conferences. They will also be submitted for publication in high-impact peer review journals. Plain English summaries of the final reports will be developed and shared with relevant patient organisations. Moreover, our results will be used to inform clinical recommendations that will be developed by the PeARL Think Tank. We anticipate that the overview of SRs will be completed by the end of 2021 and the remaining SRs by June 2022.
Patient and public involvement
The planned SRs were prioritised through a global, multi-stakeholder survey evaluating research priorities in childhood asthma, conducted by the PeARL Think Tank. 10 Among other stakeholders, this survey included responses from patients, patient caregivers and patient organisations. Moreover, two patient representatives (GDC and TAW) have joined the research group and provided input in this study protocol and they will also provide input throughout the study process.
We report on the methodology of a series of planned systematic evidence updates, aiming to evaluate maintenance management of childhood asthma, and the treatment of acute severe asthma attacks. Their design is informed by preliminary searches and the anticipated data availability. These SRs will be conducted by the PeARL group and will be used to inform clinical recommendations and future research needs. The need for high-quality evidence updates and clinical practice guidelines to improve the management of asthma in children is more urgent now, given the pressure that the unfolding COVID-19 pandemic pose on the healthcare systems, forcing us to reconsider our daily clinical practice. 30 31
Major strengths of our evidence update series are the inclusion of a wide evidence base, including data from RCTs and real-life comparative studies, the prospective design and strong methodology. The methodological quality of all available studies will be scrutinised and will aid the interpretation of our findings. Moreover, we will attempt to evaluate differential therapeutic response of different asthma phenotypes and age groups. We believe this analysis will be revealing, if adequate data is available, but may nevertheless reveal important gaps.
Guided by the available evidence, we will follow different strategies for the evidence updates on maintenance treatment of paediatric asthma and on management of acute severe asthma attacks. In view of the availability of ample published, up-to-date SRs on maintenance pharmacotherapy of childhood asthma, we chose to conduct an overview of SRs. We decided to focus on the most frequently used and thoroughly evaluated drug classes (ICS, LABA, LAMA, LTRA and biological therapies) and we expect to identify good quality data, which would inform clinical practice and research needs. Other, less frequently or experimentally used treatments will need to be evaluated in future studies. A potential limitation of this approach is that we might not be able to capture adequate data regarding the differential effectiveness of interventions across different severity groups, age groups or paediatric asthma phenotypes, if these have not been captured in existing SRs. Moreover, existing SRs may not capture some of the most recent studies, that may have been published after the SRs, although preliminary searches have revealed several very recently update meta-analyses.
The second SR, focusing on the management of acute attacks, will first evaluate a multitude of established and experimental treatments. With regard to the latter, this SR will reveal treatments that have been tested, appeared safe and efficacious and it may be worth to be further evaluated, but will also report on interventions that were tested, but did not appear efficacious, and therefore, further evaluation may not be beneficial. This wide approach would aid the prioritisation of interventions to be further validated in future clinical research studies.
Next, meta-analyses of individual pharmacological interventions will be conducted to further assess the safety and clinical effectiveness of treatments for acute severe asthma attacks that will appear efficacious in our broad SR. In contrast to most previous meta-analyses, that may have been conducted, we will include both controlled clinical trials and observational comparative effectiveness studies. Due to limitations that have already been discussed, few controlled clinical trials are conducted in children. This leads several Cochrane SRs to report low or very low confidence in the body of evidence, due to the lack of data. 32–35 We believe that by incorporating data from observational studies we may be able to conclude more robust results. While observational studies are at a higher risk of bias, we will carefully evaluate this risk using the newly developed, thorough RELEVANT tool and we will discuss potential implications on our findings. The GRADE working groups provides transparent guidance for assessing the certainty in a body of evidence including data from different study designs (controlled clinical trials or observational studies); this guidance will be used for interpreting the findings of our meta-analyes.
Overall, we aim to develop evidence updates on the maintenance treatment of asthma and management of acute severe asthma attacks that will cover all available evidence, carefully considering methodological limitations. These will be used by the PeARL Think Tank for the development of clinical recommendations and to guide future clinical research.
Ethics statements
Patient consent for publication.
Not required.
Acknowledgments
AGM was supported by the National Institute of Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC). We thank Mrs Maria Kritikou for excellent administrative support of the study
- Stanojevic S ,
- Moores G , et al
- Global Initiative for Asthma
- British Thoracic Society
- Scottish Intercollegiate Guidelines Network
- Papadopoulos NG ,
- Čustović A ,
- Cabana MD , et al
- Arakawa H ,
- Carlsen K-H , et al
- Bacharier LB
- Krajinovic M ,
- Chauhan BF , et al
- Turner MA ,
- Catapano M ,
- Hirschfeld S
- Mathioudakis AG ,
- Custovic A ,
- Deschildre A , et al
- Miligkos M ,
- Papadopoulos NG
- Alimani GS ,
- Higgins JPT
- Liberati A ,
- Tetzlaff J , et al
- Thomson D ,
- Russell K ,
- Becker L , et al
- Dalziel SR ,
- Powell CV , et al
- Katsunuma T ,
- Fujisawa T ,
- Maekawa T , et al
- Alshehri M ,
- Almegamesi T ,
- Douglas LC ,
- Esteban-Cruciani N
- Mathioudakis A ,
- Chatzimavridou-Grigoriadou V ,
- Evangelopoulou E , et al
- Robroeks CMHHT ,
- van de Kant KDG ,
- van Vliet D , et al
- Tantichaiyakul P ,
- Preutthipan A
- Reeves BC ,
- Wells G , et al
- Pollock M ,
- Fernandes RM ,
- Sterne JAC ,
- Savović J ,
- Page MJ , et al
- Campbell JD ,
- Papadopoulos NG , et al
- Balshem H ,
- Helfand M ,
- Schünemann HJ , et al
- Guyatt GH ,
- Ebrahim S ,
- Alonso-Coello P , et al
- Custovic A , et al
- Chauhan BF ,
- Ducharme FM
- Normansell R ,
- Mathioudakis AG
- Stovold E , et al
- Knightly R ,
- Hughes R , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Twitter @mathioudakisag
AGM and MM contributed equally.
Contributors Study conception: AGM and NGP. Study design: AGM, MM and NGP. Preparation of the manuscript: AGM. Critical revision and final approval of the manuscript: AGM, MM, CB, GSA, AC, AD, FMD, OK, CM, ANG, WP, DP, AS, IOA, LB, AB, AB, MB, JAC-R, GDC, TC, ZD, WF, TF, JEG, JG, GH, EMH, DI, TJ, AK, RFL, PNLS, MJM, GAM, PM, MM, MM-A, KN, EP, HP, PMCP, PP, GR, IT, ST, VS, TAW, GWKW, PX, HJZ and NGP.
Funding This work was supported by the Respiratory Effectiveness Group (REG). REG has received support from AstraZeneca, Novartis and Sanofi for continued work on PeARL. (Award/Grant name: PeARL, Award/Grant Number: N/A). This is an investigator initiated study and the funders were not involved in the selection of the topic, or design of these systematic reviews. AGM was supported by the National Institute for Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC).
Competing interests AGM reports grants from Boehringer Ingelheim outside the submitted work. AC reports personal fees from Novartis, Regeneron / Sanofi, Thermo Fisher Scientific, Boehringer Ingelheim and Philips, outside the submitted work. LB reports personal fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura and Circassia outside the submitted work. TC reports grants and personal fees CSL Behring, Dyax, Takeda, BioCryst, Pharming, personal fees from Grifols, grants and non-financial support from GSK, Regeneron, Novartis/Genetech outside the submitted work. AD reports grants and personal fees from Stallergenes Greer, personal fees from Novartis, ALK, TEVA, GSK, MEDA-MYLAN, CHIESI, AImmune, DBV technologies and Astra Zeneca, outside the submitted work. ZD reports personal fees from academic affiliations, ZD acts as Executive and Scientific Medical Director at a phase I/II pharmacological unit (QPS-NL), which performs clinical studies for pharmaceutical companies. ZD reports personal fees from Astrazeneca, ALK, Aquilon, Boehringer Ingelheim, CSL, HAL Allergy, MSD, and Sanofi-Genzyme outside the submitted work. FMD reports grants from Thorasys; personal fees from Jean-Coutu Pharmaceuticals, unrestricted research funds from Novartis Canada, Teva and Trudell Medical, research grants from GlaxoSmithKline and MEDteq in partnership with Thorasys; honorarium for consultancy work from Covis Pharma and Teva; and honorarium as invited speaker from Covis Pharma, Pharmacy Brunet, outside the submitted work. JEG reports grants from NIH/NIAID, personal fees from Regeneron, Ena Theraputics and MedImmune outside the submitted work; personal fees and stock options from Meissa Vaccines Inc outside the submitted work. JG reports personal fees from GSK, Vifor Pharmaceuticals, Novartis, BV Pharma and AstraZeneca outside the submitted work. AK reports personal fees Astra Zeneca, Behring, Boehringer Ingelheim, Covis, GSK, NovoNordisk, Novartis, Griffols, Pfizer, Sanofi, Teva and Trudel, outside the submitted work. RFL reports grants from NIH, non-financial support from GlaxoSmithKline, Boehringer-Ingelheim, Merck, TEVA, American Academy of Allergy, Asthma and Immunology, grants from Clinical and Translational Science Award (NIH), Childhood Origins of ASThma (COAST) grant, AsthmaNet, personal fees from LSU, Elsevier, UpToDate, the University of Kentucky, ThermoFischer, and Food Allergy Research and Education (FARE) Network, outside the submitted work. CM reports personal fees from Novartis, GSK, Astra Zeneca, Thermo Fisher and Boehringer Ingelheim outside the submitted work. NGP reports personal fees from ALK, Novartis, Nutricia, HAL, Menarini/FAES Farma, Sanofi, Mylan/MEDA, Biomay, AstraZeneca, GSK, MSD, ASIT BIOTECH and Boehringer Ingelheim; grants from Gerolymatos International SA and Capricare outside the submitted work. WP reports grants from NIH; grants and personal fees from Genentech/Novartis, Sanofi/Rgeneron; personal fees GSK; non-financial support from Thermo Fisher, Lincoln Diagnostics, Alk Abello, and Monaghen, outside the submitted work. PP reports grants from Astra Zeneca, Chiesi and TEVA; personal fees from Astra Zeneca, TEVA, Novartis, Mundipharma, S&D Pharma, and GlaxoSmithKline outside the submitted work. DP reports grants from AKL Research and Development, British Lung Foundation, Respiratory Effectiveness Group and the UK National Health Service; grants and personal fees from Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, TEVA, Theravance and Zentiva (Sanofi Generics); personal fees from Cipla, GlaxoSmithKline, Kyorin and Merck; non-financial support from Efficacy and Mechanism Evaluation programme, Health Technology Assessment, outside the submitted work; DP also reports stock/stock options from AKL Research and Development which produces phytopharmaceuticals; and owns 74% of the social enterprise Optimum Patient Care (Australia and UK) and 74% of Observational and Pragmatic Research Institute (Singapore), outside the submitted work. GR reports personal fees from ALK, Allergen Therapeutics, Meda Plus, Merck; and a patent for the use of sublingual immunotherapy to prevent the development of allergy in at-risk infants, outside the submitted work. IT reports personal fees from Novartis, GSK, Boehringer Ingelheim and Astra Zeneca; grants from GSK Hellas, outside the submitted work. PX reports personal fees from Nutricia, Nestle, Friesland, Uriach, Novartis Pharma AG, and GlaxoSmithkline outside the submitted work.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Pathophysiology Description
Normal Physiology
- Air enters the body through the nose and mouth and travels done the trachea, into the smaller airways (bronchi and bronchioles), reaching the alveoli, balloon-like sacs at the end of the bronchioles.
- The alveoli are surrounded by thin capillaries that allow for the exchange of gases between the pulmonary system and the blood.
- Upon inhalation, the lungs fill with oxygen-enriched air providing oxygenation for the blood. Upon exhalation, carbon dioxide is removed from the lungs which has been removed from the blood through alveolar-capillary exchange.
- 4 liters of air enter the respiratory tract while 5 liters of blood diffuse through the capillaries every minute (McCance & Huether, 2019)

Childhood Asthma is a disorder caused by chronic inflammation of the bronchial mucosa. It is the most prevalent chronic pediatric disease , and ½ of the total asthma cases are developed and diagnosed during childhood. Asthma is caused by a complex interaction between environment and genetic susceptibility and presents more severely in younger children due to smaller airway diameters (McCance & Huether, 2019).
Pathophysiology
- Episodic attacks caused by an IgE mediated Type I hypersensitivity immune over-response that results in reversible bronchial hyperresponsiveness, bronchoconstriction, mucosal edema, increased mucous production and airway obstruction that leads to ventilation-perfusion (V/Q) mismatch, hypoxemia and expiratory airway obstruction.
- IL-4 activates eosinophils and B lymphocytes
- IL-5 causes activation and proliferation of eosinophils what can result in direct tissue injury, bronchial hyperresponsiveness, epithelial damage and formation of scarring of the airway
- IL-8 activates neutrophils that invoke a hyper reactive immune response
- IL-9 promotes mast cell proliferation
- IL-13 results in decreased mucociliary function and causes bronchoconstriction and airway remodeling
- IL-25 Increases occurrence of airway remodeling
- Inflammatory mediators activate inflammatory cells and cause vasodilation, edema, bronchospasms, mucous secretion and increased capillary permeability (McCance & Huether, 2019)
- Recruitment of lymphocytes, eosinophils, basophils and neutrophils through chemotaxis causes a release of inflammatory mediators resulting in bronchospasms, excess mucous secretion and edema
- Prolonged smooth muscle contraction leads to scar tissue and collagen matrix formation which can lead to airway scarring
- Accumulation of mucous and debris caused by impaired mucociliary function form plugs in the airways that result in airway remodeling
- Mucous plugs cause air trapping and hyperinflation distal to the obstruction that can result in hypoxemia and increased work of breathing (McCance & Huether, 2019)
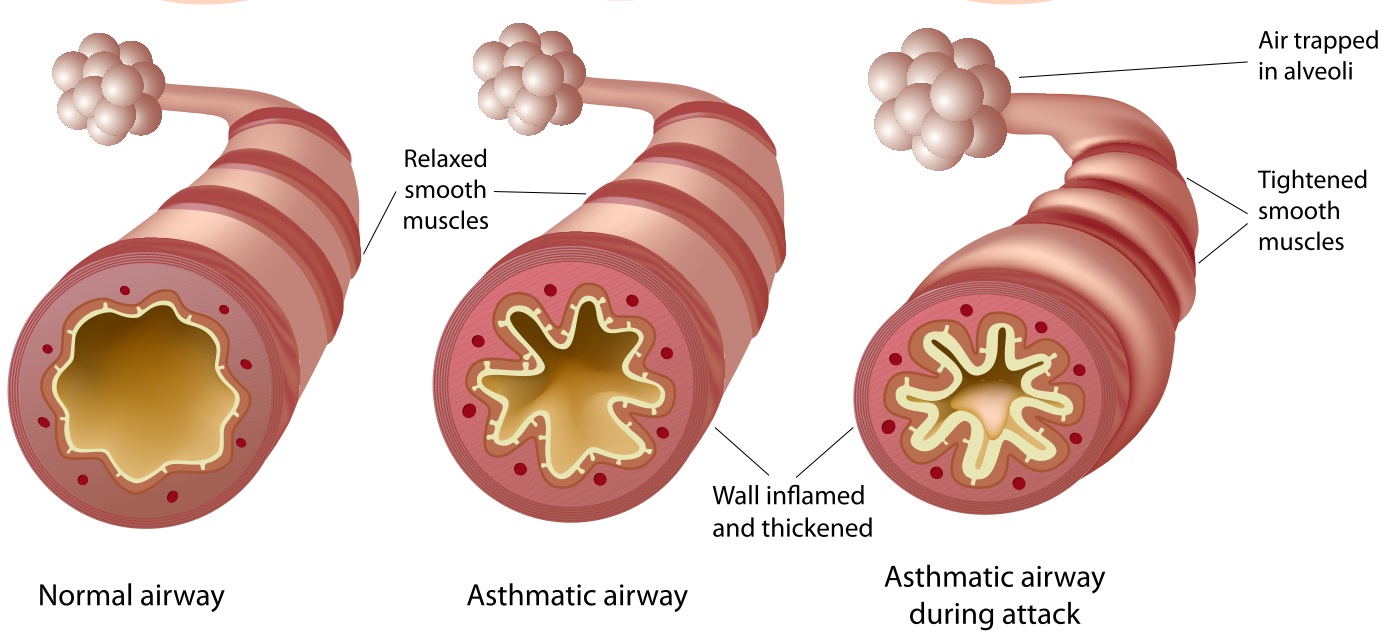
[ Simple Bio, 2019]
- Asymptomatic between episodes
- Nonproductive cough
- Tachycardia
- Prolonged expiration
- Wheezing upon expiration
Diagnostic Tools / Tests
- Rule out other disorders / diseases that present similarly to asthma
- Lung function test (spirometry)
- Exhaled nitric oxide test
- Thorough medical history including family member history
- Allergy testing (Asthma Diagnosis, 2019)
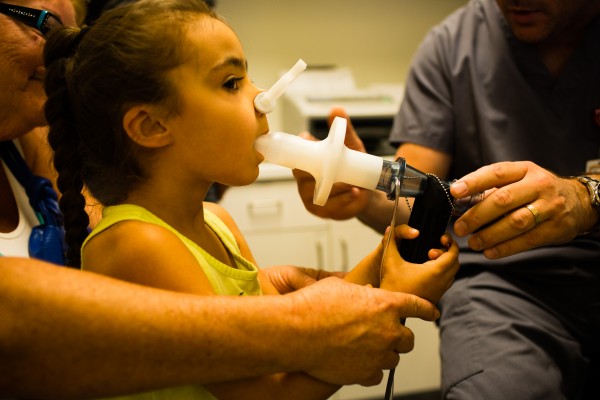
[Pulmonary Function Testing, 2019]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Effectiveness of pediatric asthma education program in the context of a general hospital in France: A retrospective real-life study
Affiliations.
- 1 Service de pédiatrie, Groupe hospitalier Sud Ile de France, 77000 Melun, France.
- 2 Univ Paris Est Creteil, INSERM, IMRB, F-94010 Creteil, France.
- 3 AP-HP, hôpital Henri-Mondor, Service de Santé Publique, F-94010, Créteil, France.
- 4 Service de pédiatrie, Centre intercommunal de Créteil, 94010 Créteil Cedex, France.
- 5 Unité transversale d'Education thérapeutique du Patient, Groupe hospitalier Sud Ile de France, 77000 Melun, France.
- PMID: 39170434
- PMCID: PMC11337717
- DOI: 10.1016/j.heliyon.2024.e35356
Objective: To assess the feasibility and effectiveness of a pediatric asthma education program delivered in the context of a French suburban general hospital.
Design: Monocentric retrospective study including children with asthma in Melun, Île-de-France, from January to December 2019. Data collected concerned asthma management, symptoms, education, and knowledge.
Results: We included 262 patients with a median age of 4.5 years. Asthma education (AE) was taught to 226 (86 %) children, 36 with minimal education (ME), 155 (69 %) with an unstructured asthma education program (USEP) and 71 (31 %) a structured asthma education program (SEP). Patients with an SEP had better knowledge of the disease and its treatment as compared with those with a USEP or ME (p < 0.05). Lung function was evaluated for 70 % of children with ME, 90 % with a USEP (p = 0.144) and 77 % an SEP (p = 0.455). Allergy testing was assessed for 42 % of children with ME, 69 % a USEP (p = 0.020) and 57 % an SEP (p = 0.185). Almost all children with USEP (93 %) and SEP (94 %) also had a written asthma action plan as compared with 49 % of the children with ME (p < 0.001). Also, 76 % of children with ME did not have an asthma follow-up as compared with 37 % with a USEP and 52 % an SEP. Overall, 69 % of children with ME had at least one hospitalization within the year as compared with 32 % with a USEP (p = 0.001) and 59 % an SEP (p = 0.506).
Conclusions: An asthma education program delivered in a general hospital resulted in increased disease knowledge for children and their caregivers, together with reduced acute interventions.
Keywords: Asthma; Care pathway; Educational program; Follow-up.
© 2024 The Authors.
PubMed Disclaimer
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Epaud reports a relationship with 10.13039/501100014088AstraZeneca Pharmaceuticals LP that includes: board membership, speaking and lecture fees, and travel reimbursement. Epaud reports a relationship with 10.13039/100031283Sanofi that includes: board membership and speaking and lecture fees. Epaud reports a relationship with GSK that includes: board membership, speaking and lecture fees, and travel reimbursement. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Serebrisky D., Wiznia A. Pediatric asthma: a global epidemic. Ann Glob Health. 2019;85 doi: 10.5334/aogh.2416. [published Online First: 2019/02/12] - DOI - PMC - PubMed
- Stern J., Pier J., Litonjua A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020;42:5–15. doi: 10.1007/s00281-020-00785-1. [published Online First: 2020/02/06] - DOI - PubMed
- Szefler S.J., Fitzgerald D.A., Adachi Y., et al. A worldwide charter for all children with asthma. Pediatr. Pulmonol. 2020;55:1282–1292. doi: 10.1002/ppul.24713. [published Online First: 2020/03/07] - DOI - PMC - PubMed
- Delmas M.C., Guignon N., Leynaert B., Moisy M., Marguet C., Fuhrman C. Augmentation de la prevalence de l'asthme chez le jeune enfant en France. Rev. Mal. Respir. 2017;34:525–534. doi: 10.1016/j.rmr.2016.09.002. [published Online First: 2016/12/07] - DOI - PubMed
- Expert Panel Working Group of the National Heart L, Blood Institute a, coordinated National Asthma E Focused updates to the asthma management guidelines: a report from the national asthma education and prevention program coordinating committee expert panel working group. J. Allergy Clin. Immunol. 2020;146:1217–1270. et al. 2020. doi: 10.1016/j.jaci.2020.10.003 [published Online First: 2020/12/08] - PMC - PubMed
Related information
Linkout - more resources, full text sources.
- Elsevier Science
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Pediatr
Asthma Action Plans: An International Review Focused on the Pediatric Population
Francesco pegoraro.
1 Department of Health Sciences, University of Florence, Florence, Italy
Marzio Masini
2 Allergy Unit, Department of Pediatrics, Meyer Children’s University Hospital, Florence, Italy
Mattia Giovannini
3 Pediatric Allergy Group, Department of Women and Children’s Health, School of Life Course Sciences, King’s College London, London, United Kingdom
Simona Barni
Francesca mori, george du toit.
4 Children’s Allergy Service, Evelina London Children’s Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
5 Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King’s College London, London, United Kingdom
Irene Bartha
Enrico lombardi.
6 Pulmonary Unit, Department of Pediatrics, Meyer Children’s University Hospital, Florence, Italy
Associated Data
Introduction.
Asthma is a chronic respiratory disease that affects more than 339 million people worldwide ( 1 ). It is the most common chronic respiratory disease in children, and its prevalence varies widely between nations ( 2 , 3 ), ranging from 2.6% in Albanian children to 26.5% in Singaporean teenagers ( 4 ). Management of asthma and especially of its exacerbations is a main concern in children since asthma activity may contribute to causing bronchial remodeling in the future ( 5 – 7 ). Several longitudinal studies have shown that uncontrolled asthma in childhood is associated with a permanent decrease in lung function well into adulthood. Furthermore, lower values of Forced Expiratory Volume in one second (FEV1) in childhood asthma are directly associated with an increased risk of developing Chronic Obstructive Pulmonary Disease (COPD) in adulthood ( 5 , 8 , 9 ). Thus, optimal asthma control in children is of paramount importance for preventing life-long debilitating respiratory conditions.
Asthma action plans (AAPs) are a set of written instructions for the patients to follow in their day-to-day management, as their asthma worsens or in case of exacerbation. These plans are usually personalized, according to patients’ clinical characteristics, controller treatments, common triggers, and other factors. AAPs are supposed to facilitate the recognition of exacerbations or declining lung function, and also contain instructions on how to manage or modify, to an extent, the long-term controller therapy in case of worsening clinical manifestations.
Asthma action plans should be designed to be clear and practical sets of algorithmic instructions, with eye-catching and distinct features to facilitate patient understanding and compliance: they usually employ a three-zone, “traffic light” scheme, ranging from a green zone (which corresponds to an absence of signs and symptoms, with no need to modify therapy) to a red zone (in which patients are instructed on how to recognize clinical manifestations of generally acute and severe loss of control and to seek immediate medical attention together with the administration of rescue therapy). A yellow zone is usually present as well, corresponding to a progressively declining control and worsening of clinical manifestations, with instructions on how to escalate controller therapy accordingly. This last point seems the more complex and delicate one, and to date, guidelines struggle to give clear-cut and practical indications on how to structure it ( 10 – 12 ). A recent work from Kouri et al. ( 13 ) gave a valuable tool on how to implement action plans, especially regarding the yellow zone in the adult asthmatic population. Unfortunately, the same attention has not been paid to pediatric AAPs, even though a suboptimal control of asthma in childhood puts patients at risk of long-term complications, as mentioned earlier ( 5 , 8 , 9 ).
Pediatric AAPs pose peculiar problems in drafting, given the characteristics of the population to whom they are directed: e.g., different response to therapy, suboptimal compliance especially in adolescents, and the mandatory involvement and education of parents or legal guardians, as well as other figures as teachers, are all variables that should be taken into account in a pediatric AAPs but are not constantly considered, and when they are considered, they are implemented in vastly different ways.
Several studies have shown the benefits of providing action plans to asthma patients, both in the adult ( 14 , 15 ) and pediatric population ( 16 ), resulting in reduced frequency of exacerbations, fewer visits to the Emergency Department, and an overall improvement of the quality of life. Following this, international asthma recommendations suggest that all asthmatic patients receive a written action plan ( 10 – 12 ). The importance of these plans got even greater during the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, considering the possibility of an exacerbation during a national lockdown and the potential difficulties in contacting doctors. However, an overwhelming number of patients are not provided with an action plan ( 17 – 21 ).
This study aims to review and compare the characteristics and differences between action plans currently proposed by English-speaking societies and, most importantly, their applicability and limitations in the pediatric population (<18-year-old).
Materials and Methods
Asthma action plan collection.
We collected AAPs recommended by the found English-speaking organizations, i.e., from the United Kingdom (Asthma United Kingdom), Ireland (Asthma Society of Ireland), the United States (American Academy of Allergy Asthma & Immunology, Asthma and Allergy Foundation of America, American Lung Association, National Institutes of Health), Canada (Asthma Canada), New Zealand (Asthma Respiratory Foundation New Zealand), Australia (National Asthma Council Australia), and South Africa (Allergy Foundation South Africa) and they were evaluated. We initially screened for pediatric AAPs and then included adult AAPs when a society did not provide a plan specifically addressed to children. When both adult and pediatric plans were available from the same society, we included both to allow a comparison between them. AAPs designed by single centers (hospitals and clinics) or subnational organizations were not included.
Asthma Action Plan Analysis
A literature search on the main interfaces (PubMed, Embase, Cochrane) and the websites of English-speaking organizations was performed from January 2010 to October 2021, to identify updated recommendations on how to design asthma action plans ( 11 – 13 , 15 , 22 – 24 ) and to collect all the essential features/items for effective AAPs. Each AAP was then evaluated to define whether it conformed to such features. During the examination, we identified items that we had not considered; when such items were deemed potentially helpful, they were included in the analysis. Finally, 27 items were deemed worthy inclusion: their detailed description is reported in Supplementary Table 1 .
A total of 16 plans provided by 10 organizations were included in the analysis ( Supplementary Table 2 ). Five organizations (50%) provided specific plans for adult and pediatric patients, one (10%) additional plan included information that could fit both adults and children, whereas four plans (40%) lacked any reference to children’s care.
The detailed summary of our analysis is reported in Supplementary Table 3 . Among plans, written information for the parent or guardian was included in four (40%). All but one plan (90%) pointed out an emergency contact, while a phone or web address for asthma education was available in four (40%). A space for a photograph was added to one plan only (10%). All plans included information on the date of the last review, the referring clinician who revised it, and the patient’s personal best peak flow. Potential factors exacerbating the patient’s asthma, such as environmental triggers or physical exercise, were detailed in six (60%) and five (50%) plans, respectively.
A section to report additional, personalized clinical characteristics was included in five plans (50%). All plans followed a severity-based three-step approach to asthma exacerbation management—only the Asthma and Respiratory Foundation of New Zealand designed an additional four-step adult plan.
A detailed description of the sequential management steps was provided in all cases, including a list of asthma medications for every day and exacerbation use. Four plans (40%) included an additional description of asthma medication colors for better recognition in emergency settings, whereas two (20%) included an explanation of asthma medication functioning. The need for a spacer for therapy administration and information on oral glucocorticoids use were both detailed in six plans (60%). Additional detailed information on how to administer therapy was reported in two plans (20%). Only one plan (10%) included an explicit authorization to administer medications in case of emergency. Three plans (30%) included extra information on asthma first-aid procedures, whereas two plans (20%) focused on recovery and follow-up after an exacerbation. Additional information on comorbidities and asthma-independent therapies were included in three plans (30%). No plan mentioned anaphylaxis potentially associated with asthma exacerbation, nor its management.
It has been documented that providing asthmatic patients with a written action plan results in a better asthma control and reduced visits to the Emergency Department, both in adults and children ( 16 , 24 ). However, out of all the action plans we examined, only five provided a specific action plan targeted toward children. This finding raises concerns since optimal pediatric asthma management is crucial for preventing a long-term permanent decline in lung function resulting from poor asthma control ( 8 , 9 , 25 ). Moreover, asthma in children and adults are considered two different entities worthy of different therapeutic approaches, as stated in the latest Global Initiative for Asthma (GINA) report ( 12 ), in which different indications are given even between different age ranges among pediatric patients.
We found substantial heterogeneity even among action plans that included a pediatric version, especially when considering aspects that might appear trivial in the adult setting but gain great importance when dealing with children. Specific instructions provided toward the caregiver on how to behave in the event of an exacerbation were provided in a varied way. The identification of medications by their color, instructions on how to administer therapy (including the use of a spacer), and the presence of comorbidities are of fundamental importance since signs and symptoms of asthma might have to be managed by people other than the patient, and especially by people who might be inexperienced in the treatment of asthma, like teachers and trainers. Unexpectedly, an important element such as the authorization to administer therapy in emergencies was mentioned in only one plan.
In addition to differences when dealing with the specific pediatric issues of asthma management, we noticed differences in the inclusion of steps that could be deemed crucial both in a pediatric (and in an adult setting), as the lack of explicit recommendation of the use of systemic corticosteroids in half of the plans, even though their use is recommended in both the GINA report and the CTS guidelines ( 12 , 26 ). When looking specifically at “red zones”, only one out of all the plans mentioned the use of systemic corticosteroids.
It must be acknowledged that including corticosteroids in an action plan is not easy, considering the different ways of using it in terms of ways of administration (oral versus inhaled) and indications (rescue versus maintenance treatment). Likewise, other therapeutic approaches, such as ipratropium bromide and the maintenance and reliever therapy (MART), might find their place in AAPs in the future.
We focused on the different recommended sequences of actions to enact in the event of a severe exacerbation, the escalation of therapy, and the need for assistance ( Figure 1 ). As expected, the two most important actions to perform in case of severe exacerbation (i.e., “administer reliever” and “call an ambulance”) are always present—despite not always reported in the same order. However, other significant steps and recommendations are often not explicitly stated.

Instructions provided by the action plans in case of acute asthma exacerbation. get help : the plan recommends seeking assistance (either medical or not) during an exacerbation; reliever : the plan recommends administering the reliever drug; call doctor : the plan recommends contacting the family/emergency physician; stay calm : the plan recommends not panicking and following the instructions included in the plan; ER : the plan recommends reaching the nearest emergency room; OCS : the plan recommends taking oral corticosteroids; Epi : the plan recommend administering epinephrine if indicated. AAAI, American Academy of Allergy Asthma & Immunology; AAFA, Asthma and Allergy Foundation of America; AC, Asthma Canada; AFSA, Allergy Foundation South Africa; ALA, American Lung Association; ARFNZ, Asthma Respiratory Foundation New Zealand; ASI, Asthma Society of Ireland; AUK, Asthma United Kingdom; NAC, National Asthma Council Australia; NIH, National Institutes of Health.
Only two of them explicitly advised the caregiver to get help, an important step to describe when instructing someone who might be inexperienced in administering medication in a potentially critical setting and whose attention might be monopolized by the distressed child and might not be able to call for an ambulance, another crucial step, indeed mentioned in all the action plans we examined.
We believe that instructing the child and the caregiver holds significant weight in both the medications to take and the behavior to adopt. Directions like “stay calm,” “sit straight,” and “take slow, steady breaths” can help tremendously in an asthma exacerbation. However, most of the examined documents lacked practical instructions on the actions to take in an acute loss of signs and symptoms control, only referencing the medications to take/administer.
Despite being a valuable tool in managing asthma in adults and children, we found that AAPs show significant variability and different degrees of comprehensiveness in their approach to the asthmatic patient. AAPs are not frequently adapted to the peculiarities of the pediatric patient, and those flaws are especially apparent when considering an emergency setting and the caregiver’s involvement. Providing caregivers with clear and easy-to-follow instructions is essential when dealing with emergency situations. Moreover, it is mandatory to ensure the completeness of AAPs, including other situations that might associate with asthma.
Overall, AAPs are effective tools for the management of patients with asthma. However, their heterogeneity will have to be addressed to improve AAP quality and effectiveness. Indeed, even if a certain grade of variability could be normal due to differences in several populations, the effectiveness of the management of asthma patients could improve with the standardization of the items included in AAPs. Moreover, it would be of the utmost importance for pediatric AAPs to be conceived with a 360-degrees view of the child in mind as a different and unique entity, worthy of a dedicated approach.
Author Contributions
MG and EL conceptualized the work. FP, MM, and IB collected the data and drafted the manuscript. FP, MM, MG, SB, FM, GdT, IB, and EL analyzed the data. MG, SB, FM, GdT, and EL critically revised the manuscript. All authors approved the final version of the manuscript as submitted.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.874935/full#supplementary-material
- Introduction
- Conclusions
- Article Information
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Freed GL , Bogan B , Nicholson A , Niedbala D , Woolford S. Error Rates in Race and Ethnicity Designation Across Large Pediatric Health Systems. JAMA Netw Open. 2024;7(9):e2431073. doi:10.1001/jamanetworkopen.2024.31073
Manage citations:
© 2024
- Permissions
Error Rates in Race and Ethnicity Designation Across Large Pediatric Health Systems
- 1 Michigan Child Health Equity Collaborative, Ann Arbor
- 2 Child Health Evaluation and Research Center, University of Michigan, Ann Arbor
- 3 C. S. Mott Children’s Hospital and Von Voigtlander Women’s Hospital, Ann Arbor, Michigan
- 4 Corewell Health Helen DeVos Children’s Hospital, Grand Rapids, Michigan
- 5 Children’s Hospital of Michigan, Detroit
Question How accurate are racial and ethnic designations for children in electronic medical records (EMRs)?
Findings In this cross-sectional study of race and ethnicity data accuracy that included 4333 participants, rates of exact match of parental report of race with racial designation in the EMR ranged from 41% to 78% across 3 health systems. Rates of ethnicity matching between parental report and the EMR ranged from 65% to 95% across the health systems.
Meaning Error rates of these magnitudes raise doubts regarding data, suggesting either the presence or absence of inequities and disparities in specific areas of clinical care, and may undermine strategies to improve care.
Importance Without knowledge of the degree of misattribution in racial and ethnic designations in data, studies run the risk of missing existing inequities and disparities and identifying others that do not exist. Further, accuracy of racial and ethnic designations is important to clinical care improvement efforts and health outcomes.
Objective To determine the error rate of racial and ethnic attribution in the electronic medical records (EMRs) across the 3 largest pediatric health systems in Michigan.
Design, Setting, and Participants This cross-sectional study collected race and ethnicity data from parents in outpatient clinics, emergency departments, and inpatient units at the 3 largest pediatric health systems in Michigan. A total of 1594 parents or guardians participated at health system A, 1537 at health system B, and 1202 at health system C from September 1, 2023, to January 31, 2024. Parent or guardian report of race and ethnicity for a child was used as the gold standard for comparison with the designation in the EMR.
Exposure Race and ethnicity designations in the EMR. Options for race designation across the health systems ranged from 6 to 49; options for ethnicity, from 2 to 10.
Main Outcomes and Measures Matching occurred in 3 stages. First, the exact racial and ethnic designations made by parents for their child were compared with what was found in the EMR. Second, for any child whose parent selected more than 1 racial category or for whom more than 1 appeared in the EMR, the designation of a minoritized racial group was used for matching purposes. Third, starting with the product of stage 2, racial designations were combined or collapsed into 6 (health systems A and C) or 5 (health system B) designations.
Results A total of 4333 survey responses were included in the analysis. The greatest error rate across the health systems occurred with the exact match of parental report of racial designation with the EMR, which ranged from 41% to 78% across the health systems. Improvement in the matching rate for each health system occurred with consolidation of race options provided. Differences between the health systems narrowed at the final consolidation to varying from 79% to 88% matching. Ethnicity matching between the EMR and the parental report ranged from 65% to 95% across the health systems. Missing race or ethnicity data in the EMR was counted as a nonmatch. Rates of missing racial data varied across the health systems from 2% to 10%. The health system with the greatest number of options for race and ethnicity had the highest error rates.
Conclusions and Relevance Although there will always be some misattribution of race and ethnicity in the EMR, the results of this cross-sectional study suggest that significant error in these data may undermine strategies to improve care. It is unclear whether those in an organization who determine the number of potential categories are the same persons who use those data to investigate potential disparities and inequities.
Numerous studies have demonstrated health inequities or disparities in the outcomes, processes, or patient experiences in the care of adults and children. 1 , 2 Many of these studies 3 - 7 have focused on variation in care relative to the racial and/or ethnic characteristics of a sample or a population of patients. Inherent to the precision of the findings of these studies is a clear understanding of the mechanisms by which the racial and ethnic characteristics were determined and the degree of accuracy of those designations. 8 , 9 This is true both for studies of large public or private datasets as well as studies using the electronic medical record (EMR) within a given health system, practice, or hospital. 10 Without knowledge of the degree of misattribution in racial and ethnic designations, studies run the risk of missing existing inequities and disparities and identifying some that do not exist. Further, accuracy of racial and ethnic designations is important to clinical care improvement efforts and health outcomes.
Further complicating this issue is the range in the number of potential categories used by health systems for racial and ethnic attribution. Some health systems have chosen consistency with the US Census Bureau, while others have expanded the choices available for patients as part of inclusivity programs.
Another vexing issue has been the proportion of missing data for race and ethnicity in some data sources. Investigators have either removed individuals with missing data or used a variety of strategies to account for them; the degree of veracity and the impact of those efforts is highly variable. 11 - 13 Further, and potentially more importantly, verification of the degree of accuracy of racial and ethnic attribution in secondary data or EMRs is rarely undertaken, 14 especially for children, 2 raising potential validity issues regarding the results of many published studies using these variables. 15 - 18 This study sought to determine the error rate of racial and ethnic attribution in the EMRs across the 3 largest pediatric health systems in the state of Michigan.
This study was determined to be exempt from review by the University of Michigan Medical School Institutional Review Board owing to its classification as a quality improvement project. Informed consent was not required. We followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Each health system had a process in place for entry of race and ethnicity information at the time of initial registration as a patient and for verification at the time of check in for outpatient and/or inpatient care. However, none of the health systems had verified the regularity with which those processes were followed. We used parent or guardian report of race and ethnicity for the child as the gold standard for comparison with the designation in the EMR.
In the present study, for each health system (A, B, and C), the specific options for the classification of race and ethnicity available in the EMR were identified ( Table 1 ). Health system A used 17 racial category options and 2 ethnicity options; health system B, 6 racial category options and 2 ethnicity options; and health system C, 49 racial category options and 10 ethnicity options. These options were then placed verbatim on printed data collection forms developed separately for each health system, resulting in 3 distinct data collection forms. Each health system used separate choices for race and ethnicity. The remainder of the forms provided identical wording describing the purpose of the study and requesting participation, as well a request to record their child’s name and birthdate. The forms were available in English, Spanish, and Arabic and were written at a sixth grade level.
At each health system, parents were approached in a variety of outpatient clinics or the emergency department, either by a member of the research team or a staff member of the health system. They were requested to participate in a study to better understand the accuracy of racial and ethnic attribution in the EMR. Those who agreed to participate were provided with the form and an envelope in which to place the completed form and asked to select as many categories as they desired to categorize the race and ethnicity of their child. They were instructed to place the envelope in a designated box in the clinic or to return it to the person who provided it to them. Fewer than 5% of those approached at any site declined to participate. The data collection goal of the study was to achieve a convenience sample of 1200 to 1500 children at each health system.
Members of the research team then identified the demographic information for each child as recorded in the demographic section of the EMR of each health system and recorded it on the same data collection form completed by the parent or guardian. Data for both the parent report and the medical record were entered into a REDCap (research electronic data capture) database. Verification of accuracy of data entry was conducted for 5% of the sample, and no errors were found.
Data analysis for matching of race category occurred in 3 stages. Stages were developed to determine the effect on the match rate by combining different racial categories in the parental report and the EMR.
In stage 1, the exact racial and ethnic designations made by parents or guardians for their child were compared with what was found in the EMR. A match required all designations to be the same in both the parental designation and the medical record. For example, if a parent selected both White and Black for racial designation, then both White and Black needed to be present in the medical record for a correct match to be designated.
In stage 2, for any child for whom the parent or guardian selected more than 1 racial category or for whom more than 1 appeared in the EMR, the designation of a minoritized racial group was used for matching purposes. When there was more than 1 minoritized racial group category selected, we prioritized designation for matching in the following order: Black or African American; American Indian, Alaska Native, or Pacific Islander; any Asian category used by the health system; Middle Eastern or North African; and other (eg, if the parent selected both White and Black, then in stage 2 the child was coded as of Black race). Other was a specific category for each health system that parents could choose and that appeared in the medical record.
In stage 3, starting with the product of stage 2, we then combined and collapsed racial designations into 6 (health systems A and C) or 5 (health system B) designations. The difference was due to health systems A and C having a separate Middle Eastern or North African designation that was not used by health system B. The 5 categories in common were American Indian or Alaska Native, Asian, Black or African American, White, and other. Matching was assessed to determine overall results from each of these 3 stages and, additionally for stage 3, the proportion of racial designations in the EMR that matched those provided by the parent or guardian in the survey and the proportion of racial designations provided by the parent or guardian in the survey that matched those found in the EMR.
For health systems A and B, the only ethnicity options in the medical record were Hispanic or non-Hispanic. Health system C had 10 ethnic response options ( Table 1 ).
For health systems A and B, an absolute match rate was determined between parental report and the medical record. For health system C, an initial absolute match rate was conducted using all their ethnic designations. Subsequently, all ethnicity designations were collapsed to create a dichotomous selection between Hispanic and non-Hispanic, consistent with health systems A and B.
A total of 4333 parents or guardians completed the surveys. There were 1594 parents or guardians who completed the survey at health system A, 1537 at Health system B, and 1202 at health system C. The greatest error rate across the health systems occurred with stage 1 matching, which ranged from 41% to 78% across the health systems. Improvement in the matching rate for each health system occurred with each subsequent matching stage. Differences between the health systems narrowed with stage 3 matching rates varying from 79% to 88% ( Figure 1 ). Thus, in one health system, as many as 1 in 5 children were misidentified for race in the EMR and as many as 1 in 9 in the other two ( Figure 2 ). Rates of missing racial data varied across the health systems from 2% to 10%.
Results of stage 3 racial matching for each health system are shown in Table 2 . For each health system, there was error in the match rates across the racial designations. For example, regarding the accuracy of the designations in the EMR, for all health systems, at least 97% of those who were identified as Black in the EMR were also identified as Black on the survey. In contrast, for those who were identified as Black in the survey, the EMR contained a designation of Black ranging from 71% to 91% across the health systems. This indicated that the EMR undercounted children of Black race in all health systems but by varying degrees.
For those identified as White in the EMR, surveys confirmed that designation between 80% and 96% across the health systems. For those who were identified as White in the survey, the EMR contained the same designation for those same children from 77% to 96% across the health systems.
Ethnic designation was completed by parents or guardians on the same survey form as for racial designation. Missing ethnicity data in the EMR were counted as a nonmatch. Rates of missing ethnicity data in the EMR varied from missing race data in the EMR across the health systems and ranged from 1% to 11% ( Figure 2 ).
Ethnicity matching between the EMR and the survey ranged from 65% to 95% across the health systems. For those designated as Hispanic in the EMR, 68% to 94% of parents reported their children to be Hispanic, depending on the health systems. For those who were identified as Hispanic in the survey, the EMR contained a designation of Hispanic ranging from 33% to 81% of the time across the health systems ( Table 3 ).
Among the most important findings from this study is the range of misattribution for race and ethnicity across the 3 health systems as well as the variability in the proportion of children with missing data. As similar comparative studies, to our knowledge, have not been conducted previously, it is unclear if these results represent a best- or a worse-case scenario. Regardless, they indicate a clear need for those who conduct studies to examine the presence of racial and ethnic disparities and inequities to assess the accuracy of the data used in those assessments.
As race and ethnicity are social constructs, there will never be an absolute gold standard for their designation in the EMR. However, if there is a desire or a societal imperative to assess the potential for disparities and inequities in care, some type of gold standard must be used to assess for accuracy in the EMR. The designation of a gold standard is inherently more complex for children than for adults. While adults can most often self-declare, children have the added complexity of the source of race and ethnicity designation used; race and ethnicity could be the mother, father, other caregiver, or, depending on age, the individual themselves (eg, if an adolescent). In this study, parental report was chosen as the gold standard.
Most previous studies of disparities and inequities using medical records, claims, or national datasets have not reported any assessments of either the accuracy of racial and ethnic designations or the rates of missing data within those data sources. 19 , 20 Thus, the validity of such studies may be called into question. Depending on the degree of error in these variables, some of these studies may have missed disparities and inequities that exist and/or found some that do not. Either way, such occurrences would dilute true efforts to improve the care provided to children. 21
Over the past several years, some health systems have expanded the number of categories available to patients for race and ethnicity to promote a sense of greater inclusivity. Although laudable, our data indicate that the greater the number of categories, the greater the potential for error in the designation of race and ethnicity in the EMR. 22 It is unclear whether those in an organization who determine the number of potential categories are the same persons who will use those data to investigate potential disparities and inequities. If not, we suggest greater coordination within an organization may help to balance the goal of inclusivity with the reality of the use of data collected. At the least, transparent and deliberate communication regarding the need to consolidate or group race and ethnicity data for accurate analyses should occur. These efforts should also account for the White House Office of Management and Budget’s statistical policy directive Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity. 23 This directive, announced in March 2024, provides up to 18 months for federal agencies to develop action plans regarding the incorporation of several changes to the way race and ethnicity data are collected. These changes include using one combined question for race and ethnicity and allowing respondents to select as many options as ally with how they identify, and the inclusion of a category of Middle Eastern or North African. Also of note, state and local jurisdictions may provide different guidance than federal authorities.
There are also complexities involved for health systems when attempting to improve the accuracy of race and ethnicity in the EMR. Depending on the health system, the origin of the EMR designations can be from a variety of sources, including registration personnel, self-service patient portal use, or clinic or hospital check-in staff. 24 Often there are protocols in place for entering race and ethnicity into the medical record that may or may not be followed. For example, health system staff may be uncomfortable asking about race and ethnicity and simply enter their best assessment of the child. Increased training of health system personnel may be required to improve consistency and accuracy of such information placed into the EMR.
This study has some limitations. Parents were only offered the opportunity to complete the form in English, Spanish, or Arabic. Those who were only fluent in a different language would have been excluded. However, there are not large populations in the catchment area of these 3 hospitals that do not speak one of these 3 languages. Thus, we believe the impact on the study results is small.
Although the size of the sample provides face validity for verification of race and ethnicity data, the sample was not obtained in a randomized manner. Thus, results cannot be used to reflect the exact racial and ethnic distribution of the patient populations at each health system. Additionally, our use of parental or guardian report as the gold standard may have varied depending on the parent or guardian present with the child. If a different parent or guardian had accompanied the child, they may have completed the survey differently. Finally, we are unable to determine whether the error varies by the age of the child at the time of initial registration.
This cross-sectional study found a range of misattribution for race and ethnicity and variability in the proportion of children with missing data. Without assessment of racial and ethnic disparities and inequities, efforts to measure improvement in health care for marginalized populations cannot occur. Although there will always be some misattribution of race and ethnicity in the EMR, significant error in these data may undermine strategies to improve care and raise doubts regarding studies that previously identified inequities and disparities. Health care systems should make efforts to assess the fidelity of their data and then ensure the data available are as accurate as possible. Further, once an understanding of the causes of missing data and misattribution are determined, attempts should be made to develop statistical correction factors to evaluate previous studies of disparities and inequities using those data and to account for discrepancies while new measurement and documentation strategies are established.
Accepted for Publication: July 8, 2024.
Published: September 3, 2024. doi:10.1001/jamanetworkopen.2024.31073
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Freed GL et al. JAMA Network Open .
Corresponding Author: Gary L. Freed, MD, MPH, Child Health Evaluation and Research Center, University of Michigan, 2800 Plymouth Rd, Building 16, Room G034E, Ann Arbor, MI 48109 ( [email protected] ).
Author Contributions: Drs Freed and Woolford had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Freed, Niedbala, Woolford.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Freed, Nicholson, Woolford.
Critical review of the manuscript for important intellectual content: Bogan, Nicholson, Niedbala, Woolford.
Obtained funding: Freed.
Administrative, technical, or material support: Freed, Bogan, Nicholson.
Supervision: Bogan, Nicholson, Niedbala.
Conflict of Interest Disclosures: Dr Woolford reported receiving grant funding from the Michigan Health Endowment Fund outside the submitted work. No other disclosures were reported.
Funding/Support: This study was supported by the Michigan Department of Health and Human Services Medicaid Administrative Match Program (Drs Freed and Woolford).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 1 .
Additional Contributions: The Michigan Child Health Equity Collaborative team members Sheng Koivu, BA, Corinne Davis, Cheyney Dobson, PhD, Julie McCormick, MA, Jennifer Stout, RN, BSN, Michael Happy, BA, and Jessamyn Ressler-Maerlender, MPH, assisted in data collection; Niko Kaciroti, PhD, and Harlan McCaffery, MS, assisted with data analysis. They did not receive compensation greater than their salary.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

IMAGES
VIDEO
COMMENTS
In recent years, it has gained increasing notoriety in schools and in the media. Population-based studies have shown that 20% to 35% of children with allergies experience bullying. In many cases (31% in one recent study ), this bullying is related directly to the food allergy. From a medical perspective, there are little published data ...
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
Asthma Case Study. Asthma affects about 6.1 million children in the US under 18 years of age, making it one of the most common chronic childhood disorders (American Lung Association, 2021). Asthma occurs as a result of a stimulus which can range from allergens, cigarette smoke, changes in temperature, stress, or exercise.
Asthma is a chronic respiratory disease characterised by episodes of wheeze, cough, and shortness of breath. Around 14% of children worldwide have a diagnosis of asthma, making it the most common chronic respiratory disease of childhood. 1. Poor asthma control is associated with a number of negative effects on children and families.
Asthma is the the most common chronic respiratory condition of childhood worldwide, with around 14% of children and young people affected. Despite the high prevalence, paediatric asthma outcomes are inadequate, and there are several avoidable deaths each year. Characteristic asthma features include wheeze, shortness of breath and cough, which are typically triggered by a number of possible ...
One of the award-winning case studies focused on using embedded, evidence-based care guidelines to control pediatric asthma and an alert system that triggered when patients were about to be discharged before a home management plan was created. CHOC drove down the average length of stay for pediatric asthma patients from 2.14 days to 1.72 days.
Case 9-2013 — A 9-Year ... As a pediatric pulmonologist, I always consider cystic fibrosis in a child with lung disease. ... Naessens, T, Grooten, J. Where asthma and hypersensitivity ...
Pediatric asthma is characterized by variable expiratory airway limitation and persistent respiratory symptoms, including wheezing, coughing, shortness of breath, and chest tightness. Asthma often starts in childhood, with nearly half of all infants wheezing in their first year, and most developing persistent asthma by age 6. A complex interplay between genetic predisposition and environmental ...
Single dose Dexamethasone is the preferred oral corticosteroid for Pediatric Asthma. A study out of the Annals of EM entitled ... 3 Mixed Studies. A limited case series has reported the effectiveness of a bolus (2 mg/kg) followed by a continuous infusion (2 to 3 mg/kg/h) of ketamine in children with severe asthma who were approaching ...
Asthma is a complex disease that involves physiological, environmental, and psychosocial factors. This paper reviews childhood asthma case management by social service professionals, lay health workers, and nurses, and it presents a new randomized controlled study using nurse case management in a local community coalition. Evidence suggests the common factor for success involves case managers ...
Summary. This chapter presents a case study of a 10-year-old girl with moderate intermittent asthma diagnosed at age 4 and was admitted to the pediatric intensive care unit with status asthmaticus. The case study includes details about history of present illness, past medical history, past surgical history, family history, and current status.
Introduction Clinical recommendations for childhood asthma are often based on data extrapolated from studies conducted in adults, despite significant differences in mechanisms and response to treatments. The Paediatric Asthma in Real Life (PeARL) Think Tank aspires to develop recommendations based on the best available evidence from studies in children. An overview of systematic reviews (SRs ...
Patient Presentation. A 12 year old girl, Annie, enters the Pediatrician's office with complaints of dyspnea, wheezing, and chest tightness. The history of the illness from the family includes: A recent upper respiratory infection with cough, congestion and runny nose with a low grade fever of 100.8 degrees Fahrenheit.
Quick-Relief Medication for All Patients: SABA as needed for symptoms. Intensity of treatment depends on severity of symptoms: up to 3 treatments at 20-minute intervals as needed. Short course of systemic oral corticosteroids may be needed. Caution: Increasing of b-agonist or use >2x/week for symptoms control indicates.
•People with asthma are at risk for flu complications even if their asthma is well controlled. The flu causes inflamed airways and lungs, which can cause an acute asthma exacerbation. They are more likely to develop pneumonia or serious health problems from the flu. It is recommended that every patient with asthma and their families get ...
A recently published study examined the effect of long-term treatment with inhaled budesonide on adult height in children with asthma. 7 This study observed a cohort of 142 children with asthma who had received a mean dose of inhaled budesonide of 412 μg daily. The final adult height of children with asthma was compared with that of control ...
UNFOLDING Reasoning Case Study: STUDENT Pediatric Asthma History of Present Problem: Jared Johnson is a 10 year-old African-American boy with a history of moderate persistent asthma. He is being admitted to the pediatric unit of the hospital from the walk-in clinic with an acute asthma exacerbation. Jared started complaining of increased chest ...
What does your child's room once per week (Also wet mop the child's room and parent should also vacuum all carpets and furniture weekly using a HEPA filter. this will decrease the child's exposure to airborne irritants and allergens That can trigger the asthma attack) Study with Quizlet and memorize flashcards containing terms like A nurse is ...
Childhood Asthma is a disorder caused by chronic inflammation of the bronchial mucosa. It is the most prevalent chronic pediatric disease, and ½ of the total asthma cases are developed and diagnosed during childhood. Asthma is caused by a complex interaction between environment and genetic susceptibility and presents more severely in younger children due to smaller airway diameters (McCance ...
Moreover, in a real-life study of 104 children and adolescents with severe allergic refractory asthma followed over 1 year, treatment with omalizumab resulted in good asthma control in 67% of the cases (p < 0.001), while FEV 1 improved by 4.9% (p = 0.02) and exacerbation rates and healthcare utilisation decreased approximately by 30% (p < 0.001) .
Study with Quizlet and memorize flashcards containing terms like A nurse is caring for an adolescent who is experiencing status asthmaticus. Which of the following actions should the nurse take?, A nurse is assessing a school-age child who has asthma and is experiencing a severe exacerbation. Which of the following findings should the nurse expect?, A nurse is teaching the parents of a school ...
Asthma is a chronic disease that is characterized by narrowing and inflammation of the airways. The obstruction of the airways occurs due to three processes that occur with asthma: inflammatory cell infiltration, mucus hypersecretion with mucus plug formation, and smooth muscle contraction (Hashmi et al., 2022).
Objective: To assess the feasibility and effectiveness of a pediatric asthma education program delivered in the context of a French suburban general hospital. Design: Monocentric retrospective study including children with asthma in Melun, Île-de-France, from January to December 2019. Data collected concerned asthma management, symptoms, education, and knowledge.
AAPs are supposed to facilitate the recognition of exacerbations or declining lung function, and also contain instructions on how to manage or modify, to an extent, the long-term controller therapy in case of worsening clinical manifestations. Asthma action plans should be designed to be clear and practical sets of algorithmic instructions ...
Asthma, when untreated, may lead to serious implications, especially in the pediatric population. Understanding the perceptions and needs of children and their caregivers may optimize asthma management. This study was aimed to analyze the perceptions of children and their caregivers regarding asthma and its pharmacotherapy.
BACKGROUND AND OBJECTIVES. Victims of natural disasters are exposed to air pollution, changes in living conditions, and physical/emotional stress, which leads to exacerbation of asthma. The study aimed to examine the association between being victims of a natural disaster and asthma medication prescriptions among children and adolescents by comparing those affected and unaffected by the 2018 ...
Inherent to the precision of the findings of these studies is a clear understanding of the mechanisms by which the racial and ethnic characteristics were determined and the degree of accuracy of those designations. 8,9 This is true both for studies of large public or private datasets as well as studies using the electronic medical record (EMR ...