Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 01 October 2003

Working memory: looking back and looking forward
- Alan Baddeley 1
Nature Reviews Neuroscience volume 4 , pages 829–839 ( 2003 ) Cite this article
50k Accesses
3229 Citations
136 Altmetric
Metrics details
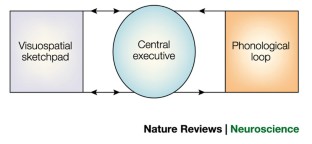
The concept of working memory assumes that a limited capacity system temporarily stores information and thereby supports human thought processes. One prevalent model of working memory comprises three components: a central executive, a verbal storage system called the phonological loop, and a visual storage system called the visuospatial sketchpad.
The phonological loop consists of a store that can hold memory traces for a few seconds, and an articulatory rehearsal process. Retrieval and re-articulation are used to refresh memory traces, and the span of working memory is limited by the amount of material that can be articulated before the first item fades from the store. Word length and similarity between items strongly influence performance on tests of verbal working memory.
Various models have been proposed to account for how serial order is remembered. In chaining models, each item is a cue for the next item, but these models run into problems with sequences in which an item recurs and with similarity effects. Contextual models assume that successive items are associated with a contextual cue or that recall of order is based on positional associations between items.
It has been proposed that the phonological loop evolved to facilitate the acquisition of language. In support of this, phonological loop capacity is a good predictor of second language learning. Future studies seem likely to link the phonological loop more closely to theories of language perception and production.
The visuospatial sketchpad is less well understood than the phonological loop. Spatial and visual working memory also have limited capacity and the two can be dissociated. Visuospatial working memory predicts success in fields such as architecture and engineering.
One model proposes that the sketchpad is divided into two components, a visual cache and a retrieval and rehearsal process called the 'inner scribe', analogous to the storage and articulatory components of the phonological loop.
The central executive is the least understood component of working memory. In one model, control is divided between 'automatic' habits or schemas, and an attentional system called the supervisory activating system that intervenes to overrule habitual control.
To move our concept of the supervisory activating system beyond a simple 'homunculus', we need to specify the processes attributed to it, and then to explain them. The processes needed are to focus, divide and switch attention, and to connect working memory with long-term memory. To account for the latter capacity, a fourth component of working memory has been proposed: the episodic buffer, a limited capacity store that binds information to form integrated episodes.
Neuroimaging and neuropsychology have provided evidence for localization of the components of working memory. The phonological loop is associated with the left temporoparietal region, and visuospatial working memory with analogous areas in the right hemisphere. The central executive is probably associated with the frontal lobes.
Work on the phonological loop, the visuospatial sketchpad and the central executive should be more closely linked to studies on language, visual processing and motor control, and executive control, respectively. In addition, more work is needed on what drives working memory.
The concept of working memory proposes that a dedicated system maintains and stores information in the short term, and that this system underlies human thought processes. Current views of working memory involve a central executive and two storage systems: the phonological loop and the visuospatial sketchpad. Although this basic model was first proposed 30 years ago, it has continued to develop and to stimulate research and debate. The model and the most recent results are reviewed in this article.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
The roles of attention, executive function and knowledge in cognitive ageing of working memory

Representation and computation in visual working memory

Long-term memory guides resource allocation in working memory
Andrade, J. Working Memory in Context (Psychology, Hove, Sussex, 2001).
Google Scholar
Miyake, A. & Shah, P. (eds) Models of Working Memory: Mechanisms of Active Maintenance and Executive Control (Cambridge Univ. Press, New York, 1999).
Book Google Scholar
Conway, A. R. A., Jarrold, C., Kane, M. J., Miyake, A. & Towse, J. N. Variation in Working Memory (Oxford Univ. Press, Oxford, in the press). References 1–3 provide excellent accounts of the current states of research on working memory, with reference 2 representing a range of theoretical approaches and highlighting their common features, reference 1 containing the view of a range of young British researchers on the strengths and weaknesses of the multi-component model, and reference 3 having more North American contributors and better reflecting approaches based on individual differences.
Miyake, A. & Shah, P. in Models of Working Memory: Mechanisms of Active Maintenance and Executive Control (eds Miyake, A. & Shah, P.) 28–61 (Cambridge Univ. Press, New York, 1999).
Cowan, N. in Models of Working Memory: Mechanisms of Active Maintenance and Executive Control (eds Miyake, A. & Shah, P.) 62–101 (Cambridge Univ. Press, New York, 1999). An alternative approach to working memory that focuses on attentional control. This leads to a different emphasis, but is not in any fundamental sense incompatible with a multi-component model.
Nairne, J. S. A feature model of immediate memory. Mem. Cogn. 18 , 251–269 (1990).
Article CAS Google Scholar
Neath, I. Modeling the effects of irrelevant speech on memory. Psychon. Bull. Rev. 7 , 403–423 (2000).
Article CAS PubMed Google Scholar
Nairne, J. S. Remembering over the short-term: the case against the standard model. Annu. Rev. Psychol. 53 , 53–81 (2002).
Article PubMed Google Scholar
Miller, G. A., Galanter, E. & Pribram, K. H. Plans and the Structure of Behavior (Holt, Rinehart & Winston, New York, 1960).
Baddeley, A. D. & Hitch, G. J. in Recent Advances in Learning and Motivation (ed. Bower, G. A.) 47–89 (Academic, New York, 1974).
Hebb, D. O. The Organization of Behavior (Wiley, New York, 1949).
Brown, J. Some tests of the decay theory of immediate memory. Q. J. Exp. Psychol. 10 , 12–21 (1958).
Article Google Scholar
Peterson, L. R. & Peterson, M. J. Short-term retention of individual verbal items. J. Exp. Psychol. 58 , 193–198 (1959).
Melton, A. W. Implications of short-term memory for a general theory of memory. J. Verbal Learn. Verbal Behav. 2 , 1–21 (1963).
Baddeley, A. D. Human Memory: Theory and Practice 2nd edn (Psychology, Hove, Sussex, 1997).
Atkinson, R. C. & Shiffrin, R. M. in The Psychology of Learning and Motivation: Advances in Research and Theory (ed. Spence, K. W.) 89–195 (Academic, New York, 1968).
Craik, F. I. M. & Lockhart, R. S. Levels of processing: a framework for memory research. J. Verbal Learn. Verbal Behav. 11 , 671–684 (1972).
Shallice, T. & Warrington, E. K. Independent functioning of verbal memory stores: a neuropsychological study. Q. J. Exp. Psychol. 22 , 261–273 (1970).
Vallar, G. & Papagno, C. in Handbook of Memory Disorders (eds Baddeley, A. D., Kopelman, M. D. & Wilson, B. A.) 249–270 (Wiley, Chichester, 2002). An excellent summary of the implications of the study of phonological working memory of neuropsychological patient studies.
Conrad, R. Acoustic confusion in immediate memory. B. J. Psychol. 55 , 75–84 (1964).
Conrad, R. & Hull, A. J. Information, acoustic confusion and memory span. B. J. Psychol. 55 , 429–432 (1964).
Baddeley, A. D. Short-term memory for word sequences as a function of acoustic, semantic and formal similarity. Q. J. Exp. Psychol. 18 , 362–365 (1966).
Baddeley, A. D. The influence of acoustic and semantic similarity on long-term memory for word sequences. Q. J. Exp. Psychol. 18 , 302–309 (1966).
Baddeley, A. D. Thomson, N. & Buchanan, M. Word length and the structure of short-term memory. J. Verbal Learn. Verbal Behav. 14 , 575–589 (1975).
Murray, D. J. Articulation and acoustic confusability in short-term memory. J. Exp. Psychol. 78 , 679–684 (1968).
Vallar, G. & Baddeley, A. D. Fractionation of working memory. Neuropsychological evidence for a phonological short-term store. J. Verbal Learn. Verbal Behav. 23 , 151–161 (1984).
Baddeley, A. D. & Wilson, B. Phonological coding and short-term memory in patients without speech. J. Mem. Lang. 24 , 490–502 (1985).
Caplan, D., Rochon, E. & Waters, G. S. Articulatory and phonological determinants of word-length effects in span tasks. Q. J. Exp. Psychol. 45 , 177–192 (1992).
Henson, R. N. A. in Working Memory in Perspective (ed. Andrade, J.) 151–174 (Psychology, Hove, Sussex, 2001). Offers a clear and useful summary of neuroimaging research viewed from a working memory perspective.
Henson, R. N. A., Norris, D. G., Page, M. P. A. & Baddeley, A. D. Unchained memory: error patterns rule out chaining models of immediate serial recall. Q. J. Exp. Psychol. 49 , 80–115 (1996).
Brown, G. D. A. Preece, T. & Hulme, C. Oscillator-based memory for serial order. Psychol. Rev. 107 , 127–181 (2000).
Murdock, B. B. TODAM2: A model for the storage and retrieval of item, associative, and serial order information. Psychol. Rev. 100 , 183–203 (1993).
Henson, R. N. A. Item repetition in short-term memory: Ranschburg repeated. J. Exp. Psychol. Learning Mem. Cogn. 24 , 1162–1181 (1998).
Baddeley, A. D. How does acoustic similarity influence short-term memory? Q. J. Psychol. 20 , 249–264 (1968).
CAS Google Scholar
Burgess, N. & Hitch, G. J. Towards a network model of the articulatory loop. J. Mem. Lang. 31 , 429–460 (1992).
Burgess, N. & Hitch, G. J. Memory for serial order: a network model of the phonological loop and its timing. Psychol. Rev. 106 , 551–581 (1999). A good example of one of a range of applications of computational modelling to the study of working memory.
Henson, R. N. A. Short-term memory for serial order. The start–end model. Cogn. Psychol. 36 , 73–137 (1998).
Page, M. A. & Norris, D. The primacy model: a new model of immediate serial recall. Psychol. Rev. 105 , 761–781 (1998).
Logie, R. H., Della Sala, S., Laiacona, M., Chalmers, P. & Wynn, V. Group aggregates and individual reliability: the case of verbal short-term memory. Mem. Cogn. 24 , 305–321 (1996). Probably the only study to attempt to quantify the magnitude and robustness of the phonological similarity and word-length effects.
Larsen, J. D. & Baddeley, A. D. Disruption of verbal STM by irrelevant speech, articulatory suppression and manual tapping: do they have a common source? Q. J. Exp. Psychol. (in the press).
Neath, I. Farley, L. A. & Surprenant, A. M. Directly assessing the relationship between speech and articulatory suppression. Q. J. Exp. Psychol. (in the press).
Hanley, J. R. & Bakopoulou, E. Irrelevant speech, articulatory suppression and phonological similarity: a test of the phonological loop model and the feature model. Psychon. Bull. Rev. 10 , 435–444 (2003). This study emphasizes the role of strategy in working memory, demonstrating powerful effects of strategic instructions to subjects.
Lovatt, P. & Avons, S. E. in Working Memory in Perspective (ed. Andrade, J.) 199–218 (Psychology, Hove, Sussex, 2001).
Brown, G. D. A. & Hulme, C. Modeling item length effects in memory span: no rehearsal needed? J. Mem. Lang. 34 , 594–621 (1995).
Neath, I. & Nairne, J. S. Word-length effects in immediate memory: overwriting trace-decay theory. Psychon. Bull. Rev. 2 , 429–441 (1995).
Cowan, N., Baddeley, A. D., Elliott, E. M. & Norris, J. List composition and the word length effect in immediate recall: a comparison of localist and globalist assumptions. Psychol. Bull. Rev. 10 , 74–79 (2003).
Baddeley, A. D., Chincotta, D., Stafford, L. & Turk, D. Is the word length effect in STM entirely attributable to output delay? Evidence from serial recognition. Q. J. Exp. Psychol. 55A , 353–369 (2002).
Dosher, B. A. & Ma, J. J. Output loss or rehearsal loop? Output-time versus pronunciation-time limits in immediate recall for forgetting-matched materials. J. Exp. Psychol. Learn. 24 , 316–335 (1998).
Lovatt, P., Avons, S. E. & Masterson, J. The word length effect and disyllabic words. Q. J. Exp. Psychol. 53 , 1–22 (2000).
Baddeley, A. D. & Andrade, J. Reversing the word-length effect: a comment on Caplan, Rochon and Waters. Q. J. Exp. Psychol. 47A , 1047–1054 (1994).
Caplan, D. & Waters, G. S. Articulatory length and phonological similarity in span tasks: a reply to Baddeley and Andrade. Q. J. Exp. Psychol. 47A , 1055–1062 (1994).
Service, E. Effect of word length on immediate serial recall depends on phonological complexity, not articulatory duration. Q. J. Exp. Psychol. 51A , 283–304 (1998).
Mueller, S. T., Seymour, T. L., Kieras, D. E. & Meyer, D. E. Theoretical implications of articulatory duration, phonological similarity, and phonological complexity in verbal working memory. J. Exp. Psychol. Learn. Mem. Cogn. (in the press). The most careful and sophisticated analysis of the roles of spoken duration and phonological similarity in verbal STM, leading to a clear and simple theoretical conclusion. A good account of potential pitfalls in this area of research is also provided.
Colle, H. A. Auditory encoding in visual short-term recall: effects of noise intensity and spatial location. J. Verbal Learn. Verbal Behav. 19 , 722–735 (1980).
Jones, D. M. in Attention: Selection, Awareness and Control (eds Baddeley, A. D. & Weiskrantz, L.) 87–104 (Clarendon, Oxford, 1993).
Jones, D. M. & Macken, W. J. Irrelevant tones produce an irrelevant speech effect: implications for phonological coding in working memory. J. Exp. Psychol. Learn. Mem. Cogn. 19 , 369–381 (1993).
Salame, P. & Baddeley, A. D. Disruption of short-term memory by unattended speech: implications for the structure of working memory. J. Verbal Learn. Verbal Behav. 21 , 150–164 (1982).
Colle, H. A. & Welsh, A. Acoustic masking in primary memory. J. Verbal Learn. Verbal Behav. 15 , 17–32 (1976).
Salame, P. & Baddeley, A. D. Phonological factors in STM: similarity and the unattended speech effect. Bull. Psychon. Soc. 24 , 263–265 (1986).
Jones, D. M. & Macken, W. J. Phonological similarity in the irrelevant speech effect. Within- or between-stream similarity? J. Exp. Psychol. Learn. Mem. Cogn. 21 , 103–115 (1995).
Larsen, J. D. Baddeley, A. D. & Andrade, J. Phonological similarity and the irrelevant speech effect: implications for models of short-term verbal memory. Memory 8 , 145–157 (2000).
Le Compte, D. C. & Shaibe, D. M. On the irrelevance of phonological similarity to the irrelevant speech effect. Q. J. Exp. Psychol. 50A , 100–118 (1997).
Jones, D., Beaman, M. & Macken, W. J. in Models of Short-Term Memory (ed. Gathercole, S.) 209–237 (Psychology, Hove, Sussex, 1996).
Page, M. A. & Norris, D. The irrelevant sound effect: what needs modeling and a tentative model. Q. J. Exp. Psychol. (in the press).
Macken, W. J. & Jones, D. M. Reification of phonological storage. Q. J. Exp. Psychol. (in the press).
Baddeley, A. D., Gathercole, S. E. & Papagno, C. The phonological loop as a language learning device. Psychol. Rev. 105 , 158–173 (1998). Summarizes the evidence supporting the view that the phonological loop serves as a device for facilitating language acquisition.
Papagno, C., Valentine, T. & Baddeley, A. D. Phonological short-term memory and foreign language vocabulary learning. J. Mem. Lang. 30 , 331–347 (1991).
Papagno, C. & Vallar, G. Phonological short-term memory and the learning of novel words: the effect of phonological similarity and item length. Q. J. Exp. Psychol. 44A , 47–67 (1992).
Service, E. Phonology, working memory and foreign-language learning. Q. J. Exp. Psychol. 45A , 21–50 (1992).
Atkins, W. B. & Baddeley, A. D. Working memory and distributed vocabulary learning. App. Psycholinguistics 19 , 537–552 (1998).
Gathercole, S. E. & Adams, A. M. Children's phonological working memory: contributions of long-term knowledge and rehearsal. J. Mem. Lang. 33 , 672–788 (1994).
Gathercole, S. E. & Baddeley, A. D. Evaluation of the role of phonological STM in the development of vocabulary in children: a longitudinal study. J. Mem. Lang. 28 , 200–213 (1989).
Gathercole, S. E. & Baddeley, A. D. Phonological memory deficits in language-disordered children: is there a causal connection? J. Mem. Lang. 29 , 336–360 (1990).
Gathercole, S. E., Pickering, S., Hall, M. & Peaker, S. Dissociable lexical and phonological influences on serial recognition and serial recall. Q. J. Exp. Psychol. 54A , 1–30 (2001).
Houghton, G. Hartley, T. & Glasspool, D. W. in Models of Short-Term Memory (ed. Gathercole, S. E.) 101–128 (Psychology, Hove, Sussex, 1996).
Gathercole, S. E. Is nonword repetition a test of phonological memory or long-term knowledge? It all depends on the nonwords. Mem. Cogn. 23 , 83–94 (1995).
Thorn, A. S. C., Gathercole, S. E. & Frankish, C. R. Language familiarity effects in short-term memory: the role of output delay and long-term knowledge. Q. J. Exp. Psychol. 55A , 1363–1383 (2002).
O'Regan, J. K., Rensink, R. A. & Clark, J. J. Change-blindness as a result of 'mudsplashes'. Nature 398 , 34–34 (1999).
Simons, D. J. & Levin, D. T. Change blindness. Trends Cogn. Sci. 1 , 261–267 (1997).
O'Regan, J. K. Solving the real mysteries of visual-perception — the world as an outside memory. Can. J. Psychol. 46 , 461–488 (1992).
Luck, S. J. & Vogel, E. K. The capacity of visual working memory for features and conjunctions. Nature 390 , 279–281 (1997).
Vogel, E. K., Woodman, G. F. & Luck, S. J. Storage of features, conjunctions and objects in visual working memory. J. Exp. Psychol. Hum. Percept. Perf. 27 , 92–114 (2001).
Wheeler, M. E. & Treisman, A. M. Binding in short-term visual memory. J. Exp. Psychol. Gen. 131 , 48–64 (2002). A good example of the recent tendency for prominent researchers in visual attention to analyse related processes of visuospatial working memory.
Milner, B. Inter-hemispheric differences in the locatisation of psychological processes in man. B. Med. Bull. 27 , 272–277 (1971).
Della Sala, S., Gray, C., Baddeley, A. D., Allamano, N. & Wilson, L. Pattern span: a tool for unwelding visuo-spatial memory. Neuropsychologia 37 , 1189–1199 (1999).
Della Sala, S. & Logie, R. H. in Handbook of Memory Disorders (eds Baddeley, A. D., Kopelman, M. D. & Wilson, B. A.) 271–292 (Wiley, Chichester, 2002)
Smith, E. E. et al. Spatial versus object working memory: PET investigations. J. Cogn. Neurosci. 7 , 337–358 (1995).
Mishkin, M., Ungerleider, L. G. & Macko, K. O. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6 , 414 (1983).
Pickering, S. J. Cognitive approaches to the fractionation of visuo-spatial working memory. Cortex 37 , 470–473 (2001).
Smyth, M. M. & Pendleton, L. R. Space and movement in working memory. Q. J. Exp. Psychol. 42A , 291–304 (1990).
Logie, R. H. Visuo-spatial processing in working memory. Q. J. Exp. Psychol. 38A , 229–247 (1986).
Quinn, G. & McConnell, J. Irrelevant pictures in visual working memory. Q. J. Exp. Psychol. 49A , 200–215 (1996).
Purcell, A. T. & Gero, J. S. Drawings and the design process. Design Studies 19 , 389–430 (1998).
Verstijnen, I. M., van Leeuwen, C., Goldschimdt, G., Haeml, R. & Hennessey, J. M. Creative discovery in imagery and perception: combining is relatively easy, restructuring takes a sketch. Acta Psychol. 99 , 177–200 (1998).
Ghiselin, B. The Creative Process (Mentor, New York, 1952).
Finke, R. & Slayton, K. Explorations of creative visual synthesis in mental imagery. Mem. Cogn. 16 , 252–257 (1988).
Finke, R., Ward, T. B. & Smith, S. M. Creative Cognition: Theory, Research and Applications (MIT Press, Cambridge, Massachusetts, 1992)
Pearson, D. G., Logie, R. H. & Gilhooly, K. Verbal representations and spatial manipulation during mental synthesis. Eur. J. Cogn. Psychol. 11 , 295–314 (1999).
Pearson, D. G. in Working Memory in Perspective (ed. Andrade, J.) 33–59 (Psychology, Hove, Sussex, 2001).
Brandimonte, M. & Gerbino, W. Mental image reversal and verbal recoding: when ducks become rabbits. Mem. Cogn. 21 , 23–33 (1993).
Brandimonte, M., Hitch, G. J. & Bishop, D. Verbal recoding of visual stimuli in pairs mental image transformations. Mem. Cogn. 20 , 449–455 (1992).
Logie, R. H. Visuo-spatial Working Memory (Lawrence Erlbaum, Hove, Sussex, 1995).
Goldman-Rakic, P. W. Topography of cognition: parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 11 , 137–156 (1988). A review of studies in which single-unit recording in awake monkeys is used to analyse the nature of visuospatial working memory.
Goldman-Rakic, S. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil. Trans. R. Soc. Lond. B 351 , 1445–1453 (1996).
Baddeley, A. D. Working Memory (Oxford Univ. Press, Oxford, 1986).
Norman, D. A. & Shallice, T. in Consciousness and Self-regulation. Advances in Research and Theory (eds Davidson, R. J., Schwarts, G. E. & Shapiro, D.) 1–18 (Plenum, New York, 1986).
Shallice, T. From Neuropsychology to Mental Structure (Cambridge Univ. Press, Cambridge, 1988).
Shallice, T. in Principles of Frontal Lobe Function (eds Stuss, D. T. & Knight, R. T.) 261–277 (Oxford Univ. Press, New York, 2002).
Shallice, T. & Burgess, P. W. Deficits in strategy application following frontal lobe damage in men. Brain 114 , 727–741 (1991).
Bargh, J. A. & Ferguson, M. J. Beyond behaviorism: on the automaticity of higher mental processes. Psychol. Bull. 126 , 925–945 (2000). This article makes a strong case for the importance of implicit factors in determining social behaviour.
Bargh, J. A., Chen, M. & Burrows, L. Automaticity of social behavior: direct effects of trait construct and stereotype activation on action. J. Personality Soc. Psychol. 71 , 230–244 (1996).
Chartrand, T. L. & Bargh, J. A. The chameleon effect: the perception-behavior link and social interaction. J. Personality Soc. Psychol. 76 , 893–910 (1999).
Chartrand, T. L. & Bargh, J. A. in Self and Motivation: Emerging Psychological Perspectives (eds Tesser, A., Stapel, D. A. & Wood, J. V.) 13–41 (American Psychological Association, Washington DC, 2002).
Baumeister, R. F. & Exline, J. J. Self control, morality and human strength. J. Soc. Clin. Psychol. 19 , 29–42 (2000).
Baumeister, R. F., Bratslavsky, E., Muraven, M. & Tice, D. M. Ego-depletion: is the active self a limited resource? J. Personality Soc. Psychol. 74 , 1252–1265 (1998).
Muraven, M. & Baumeister, R. F. Self regulation and depletion of limited resources: does self control resemble a muscle? Psychol. Bull. 126 , 247–259 (2000).
McDougall, W. Outline of Psychology (Scribner, New York, 1923)
Attneave, F. in Sensory Communication (ed. Rosenblith, W.) 777–782 (MIT Press, Cambridge, Massachussetts, 1960).
Baddeley, A. D. Exploring the central executive. Q. J. Exp. Psychol. 49A , 5–28 (1996).
Baddeley, A. D., Bressi, S., Della Sala, S., Logie, R. & Spinnler, H. The decline of working memory in Alzheimer's disease: a longitudinal study. Brain 114 , 2521–2542 (1991).
Baddeley, A. D., Baddeley, H. A., Bucks, R. S. & Wilcock, G. K. Attentional control in Alzheimer's disease. Brain 124 , 1492–1508 (2001).
Baddeley, A. D. & Wilson, B. A. Prose recall and amnesia: implications for the structure of working memory. Neuropsychologia 40 , 1737–1743 (2002).
Logie, R. H., Della Sala, S., Wynn, V. & Baddeley, A. D. Visual similarity effects in immediate serial recall. Q. J. Exp. Psychol. 53A , 626–646 (2000).
Baddeley, A. D. & Andrade, J. Working memory and the vividness of imagery. J. Exp. Psychol. Gen. 129 , 126–145 (2000).
Baddeley, A. D. The episodic buffer: a new component of working memory? Trends Cogn. Sci. 4 , 417–423 (2000). This gives a brief account of the argument for assuming a fourth component to the working memory model.
Baars, B. J. A Cognitive Theory of Consciousness (Cambridge Univ. Press, Cambridge, 1988).
Baars, B. J. The conscious access hypothesis: origins and recent evidence. Trends Cogn. Sci. 6 , 47–52 (2002).
Dehaene, S. & Naccache, L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79 , 1–37 (2001). An excellent review of the neuropsychological and neuroimaging evidence for a limited capacity work-space system that is associated with conscious awareness.
Ericsson, K. A. & Kintsch, W. Long-term working memory. Psychol. Rev. 102 , 211–245 (1995).
Warrington, E. J., Logue, V. & Pratt, R. T. C. The anatomical localisation of selective impairment of auditory verbal short-term memory. Neuropsychologia 9 , 377–387 (1971).
Vallar, G., DiBetta, A. M. & Silveri, M. C. The phonological short-term store-rehearsal system: patterns of impairment and neural correlates. Neuropsychologia 35 , 795–812 (1997).
Paulesu, E., Frith, C. D. & Frackowiak, R. S. J. The neural correlates of the verbal component of working memory. Nature 362 , 342–345 (1993).
Jonides, J. et al. in The Psychology of Learning and Motivation (ed. Medin, D.) 43–88 (Academic, London, 1996). A good account of this group's work on applying neuroimaging to the study of both the phonological and visuospatial subsystems of working memory.
Jonides, J. & Smith, E. E. in Cognitive Neuroscience (ed. Rugg, M. D.) 243–276 (Psychology, Hove, Sussex, 1997).
Awh, E. et al. Dissociation of storage and retrieval in verbal working memory: evidence from positron emission tomography. Psychol. Sci. 7 , 25–31 (1996).
Smith, E. E. & Jonides, J. Working memory: a view from neuroimaging. Cogn. Psychol. 33 , 5–42 (1997).
Smith, E., Jonides, J. & Koeppe, R. A. Dissociating verbal and spatial working memory using PET. Cereb. Cortex 6 , 11–20 (1996).
DeRenzi, E. & Nichelli, P. Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex 11 , 341–353 (1975).
Hanley, J. R., Young, A. W. & Pearson, N. A. Impairment of the visuospatial sketch pad. Q. J. Exp. Psychol. 43A , 101–125 (1991).
Kosslyn, S. M. et al. Visual mental imagery activates topographically organised cortex: PET investigations. J. Cogn. Neurosci. 5 , 263–287 (1993).
Jonides, J. et al. Spatial working memory in humans as revealed by PET. Nature 363 , 623–625 (1993).
Awh, E., Jonides, J. & Reuter-Lorenz, P. A. Rehearsal in spatial working memory. J. Exp. Psychol. Hum. Percept. Perf. 24 , 780–790 (1998).
Wilson, F. A. W. Scalaidhe, S. & Goldman-Rakic, S. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260 , 1955–1958 (1993).
Levin, D. N. Warach, J. & Farah M. J. Two visual systems in mental imagery: dissociation of 'what' and 'where' in imagery disorders due to bilateral posterior cerebral lesions. Neurology 35 , 1010–1018 (1985).
Owen, A. M. The functional organisation of working memory processes within the human lateral frontal cortex: the contribution of functional neuroimaging. Eur. J. Neurosci. 9 , 1329–1339 (1997).
Stuss, D. T. & Knight, R. T. Principles of Frontal Lobe Function (Oxford Univ. Press, New York, 2002).
Braver, T. S. et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5 , 49–62 (1997).
Cohen, J. D. et al. Temporal dynamics of brain activation during a working memory task. Nature 386 , 604–608 (1997).
Frith, C. D., Friston, K. J., Liddle, P. F. & Frackowiak, R. S. J. Willed action in the prefrontal cortex in man: a study with PET. Proc. R. Soc. Lond. B 244 , 241–246 (1991).
Jahanshahi, M., Dirnberger, G., Fuller, R. & Frith, C. D. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage 12 , 713–725 (2000).
Baddeley, A. D. Emslie, H., Kolodny, J. & Duncan, J. Random generation and the executive control of working memory. Q. J. Exp. Psychol. 51A , 818–852 (1998).
Duncan, J. & Owen, A. M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23 , 475–483 (2000). A meta-analysis of neuroimaging studies of attentional control that argues for the dependence of diverse executive processes on a limited anatomical region of the right frontal lobe.
D'Esposito, M. et al. The neural basis of the central executive system of working memory. Nature 378 , 279–281 (1995).
Klingberg, T. Concurrent performance of two working memory tasks: potential mechanisms of interference. Cereb. Cortex 8 , 593–601 (1998).
Fletcher, T. C. et al. Brain systems for encoding and retrieval of auditory-verbal memory: an in vivo study in humans. Brain 118 , 401–416 (1995).
Goldberg, T. E. et al. Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage 7 , 296–303 (1998).
Damasio, A. R. Descarte's Error: Emotion, Reason, and the Human Brain (Putnam, New York, 1994).
Hume, D. An Enquiry Concerning Human Understanding (Hackett, Indianapolis, 1772/1993).
Lewin, K. Field Theory in Social Science (Harper, New York, 1951).
Kennard, C. & Swash, M. (Eds) Hierarchies in Neurology: reappraisal of Jacksonian Concept (Springer, Berlin, 1989).
Craik, K. J. W. The Nature of Explanation (Cambridge Univ. Press, London, 1943).
Broadbent, D. E. Levels, hierarchies and the locus of control. Q. J. Exp. Psychol. 29 , 181–201 (1977).
Frith, C. D., Blakemore, S. J. & Wolpert, D. M. Abnormalities in the awareness and control of action. Phil. Trans. R. Soc. Lond. B 355 , 1771–1788 (2000). A stimulating attempt to provide an account of the control of action based on a range of experimental phenomena and neuropsychological deficits. It makes a potentially important case for the possible extension of a model of motor control to the more general control of action and behaviour.
Daneman, M. & Carpenter, A. Individual differences in working memory and reading. J. Verbal Learn. Verbal Behav. 19 , 450–466 (1980).
Daneman, M. & Merikle, M. Working memory and language comprehension: A meta-analysis. Psychon. Bull. Rev. 3 , 422–433 (1996).
Turner, M. L. & Engle, R. W. Is working memory capacity task-dependent? J. Mem. Lang. 28 , 127–154 (1989).
Bayliss, D. M., Jarrold, C., Gunn, D. M. & Baddeley, A. D. The complexities of complex span: Explaining individual differences in working memory in children and adults. J. Exp. Psychol. Gen. 132 , 71–92 (2003).
Ormrod, J. E. & Cochran, K. F. Relationship of verbal ability and working memory to spelling achievement and learning to spell. Reading Res. Instruction 28 , 33–43 (1988).
Kyllonen, C. & Stephens, D. L. Cognitive abilities as the determinants of success in acquiring logic skills. Learn. Individual Diff. 2 , 129–160 (1990).
Kiewra, K. A. & Benton, S. L. The relationship between information processing ability and note-taking. Contemp. Edu. Psychol. 13 , 3–44 (1988).
Engle, R. W. Carullo, J. J. & Collins, K. W. Individual differences in working memory for comprehension and following directions. J. Edu. Res. 84 , 253–262 (1991).
Kyllonen, C. & Christal, R. E. Reasoning ability is (little more than) working memory capacity. Intelligence 14 , 389–433 (1990).
Engle, R. W. Kane, M. J. & Tuholski, S. W. in Models of Working Memory: Mechanisms of Active Maintenance and Executive Control (eds Miyake, A. & Shah, P.) 102–134 (Cambridge Univ. Press, Cambridge, 1999).
Miyake, A., Friedman, N. P., Rettinger, D. A., Shah, P. & Heggarty, M. How are visuo-spatial working memory, executive functioning, and spatial abilities related: latent-variable analysis. J. Exp. Psychol. Gen. 130 , 621–640 (2001).
Miyake, A. et al. The unity and diversity of executive functions and their contributions to complex 'frontal lobe' tasks: a latent variable analysis. Cogn. Psychol. 41 , 49–100 (2000). A clear account of the application of the statistical technique of latent variable analysis to the analysis of executive processes in working memory.
Kane, M. J. & Engle, R. W. The role of prefrontal cortex in working-memory capacity, executive attention and general fluid intelligence: an individual differences perspective. Psychon. Bull. Rev. 4 , 637–671 (2002).
Spearman, C. The Abilities of Man (Macmillan, London, 1927).
Thurstone, L. L. Primary Mental Abilities (Univ. Chicago Press, Chicago, 1938).
Carroll, J. B. Human Cognitive Abilities (Cambridge Univ. Press, Cambridge, 1993).
Herrnstein, R. J. & Murray, C. The Bell Curve: Intelligence and Class Structure in American Life (Free Press, New York, 1994).
Kamin, L. J. Intelligence: The Battle for the Mind (H. J. Eysenck versus Leon Kamin) (Macmillan, London, 1981).
Brooks, L. R. Spatial and verbal components in the act of recall. Can. J. Psychol. 22 , 349–368 (1968).
Martin, J. H. Neuroanatomy: Text and Atlas 2nd edn (Appleton & Lange, Stamford, Connecticut, 1996).
Download references
Author information
Authors and affiliations.
Department of Psychology, University of York, York, YO10 5DD, UK
Alan Baddeley
You can also search for this author in PubMed Google Scholar
Related links
Further information, mit encyclopedia of cognitive sciences.
neural basis of working memory
working memory
(BA). Korbinian Brodmann (1868–1918) was an anatomist who divided the cerebral cortex into numbered subdivisions on the basis of cell arrangements, types and staining properties (for example, the dorsolateral prefrontal cortex contains subdivisions, including BA 46, BA 9 and others). Modern derivatives of his maps are commonly used as the reference system for discussion of brain-imaging findings.
Unable to speak because of defective articulation.
Having an impairment of the ability to perform certain voluntary movements, often including speech.
McDougall proposed the term 'conative' to denote the activity of mental striving or the will, as opposed to cognitive and affective or emotional processes.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Baddeley, A. Working memory: looking back and looking forward. Nat Rev Neurosci 4 , 829–839 (2003). https://doi.org/10.1038/nrn1201
Download citation
Issue Date : 01 October 2003
DOI : https://doi.org/10.1038/nrn1201
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Association of auditory processing abilities and employment in young women.
- Yoshita Sharma
The Egyptian Journal of Otolaryngology (2024)
Pan-cortical electrophysiologic changes underlying attention
- Ronald P. Lesser
- W. R. S. Webber
- Diana L. Miglioretti
Scientific Reports (2024)
Exploring the impact of intensified multiple session tDCS over the left DLPFC on brain function in MCI: a randomized control trial
- M. Pupíková
- I. Rektorová
The role of teaching solfeggio considering memory mechanisms in developing musical memory and hearing of music school students
Current Psychology (2024)
Far Transfer Effects of Trainings on Executive Functions in Neurodevelopmental Disorders: A Systematic Review and Metanalysis
- Clara Bombonato
- Benedetta Del Lucchese
- Chiara Pecini
Neuropsychology Review (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- A-Z Publications
Annual Review of Psychology
Volume 66, 2015, review article, the cognitive neuroscience of working memory.
- Mark D'Esposito 1 , and Bradley R. Postle 2
- View Affiliations Hide Affiliations Affiliations: 1 Helen Wills Neuroscience Institute and Department of Psychology, University of California, Berkeley, California 94720; email: [email protected] 2 Departments of Psychology and Psychiatry, University of Wisconsin, Madison, Madison, Wisconsin 53719; email: [email protected]
- Vol. 66:115-142 (Volume publication date January 2015) https://doi.org/10.1146/annurev-psych-010814-015031
- First published as a Review in Advance on September 19, 2014
- © Annual Reviews
For more than 50 years, psychologists and neuroscientists have recognized the importance of a working memory to coordinate processing when multiple goals are active and to guide behavior with information that is not present in the immediate environment. In recent years, psychological theory and cognitive neuroscience data have converged on the idea that information is encoded into working memory by allocating attention to internal representations, whether semantic long-term memory (e.g., letters, digits, words), sensory, or motoric. Thus, information-based multivariate analyses of human functional MRI data typically find evidence for the temporary representation of stimuli in regions that also process this information in nonworking memory contexts. The prefrontal cortex (PFC), on the other hand, exerts control over behavior by biasing the salience of mnemonic representations and adjudicating among competing, context-dependent rules. The “control of the controller” emerges from a complex interplay between PFC and striatal circuits and ascending dopaminergic neuromodulatory signals.
Article metrics loading...
Full text loading...
Literature Cited
- Akil M , Kolachana BS , Rothmond DA , Hyde TM , Weinberger DR , Kleinman JE . 2003 . Catechol- O -methyltransferase genotype and dopamine regulation in the human brain. J. Neurosci. 23 : 2008– 13 [Google Scholar]
- Alvarez GA , Cavanagh P . 2004 . The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychol. Sci. 15 : 106– 11 [Google Scholar]
- Anderson DE , Serences JT , Vogel EK , Awh E . 2014 . Induced alpha rhythms track the content and quality of visual working memory representations with high temporal precision. J. Neurosci. 34 : 7587– 99 [Google Scholar]
- Arnsten A . 1997 . Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 11 : 151– 62 [Google Scholar]
- Arnsten KT , Cai JX , Murphy BL , Goldman-Rakic PS . 1994 . Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116 : 143– 51 [Google Scholar]
- Awh E , Jonides J . 2001 . Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci. 5 : 119– 26 [Google Scholar]
- Awh E , Jonides J , Reuter-Lorenz PA . 1998 . Rehearsal in spatial working memory. J. Exp. Psychol.: Hum. Percept. Perform. 24 : 780– 90 [Google Scholar]
- Azuar C , Reyes P , Slachevsky A , Volle E , Kinkingnehun S . et al. 2014 . Testing the model of caudo-rostral organization of cognitive control in the human with frontal lesions. NeuroImage 84 : 1053– 60 [Google Scholar]
- Baddeley A . 1986 . Working Memory New York: Oxford Univ. Press [Google Scholar]
- Baddeley A , Hitch GJ . 1974 . Working memory. Psychology of Learning and Motivation G Bower 8 47– 89 New York: Academic Press The paper that introduces the highly influential multiple-component model of working memory. [Google Scholar]
- Badre D . 2008 . Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn. Sci. 12 : 193– 200 [Google Scholar]
- Badre D . 2012 . Opening the gate to working memory. Proc. Natl. Acad. Sci. USA 109 : 19878– 79 [Google Scholar]
- Badre D , D'Esposito M . 2007 . Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J. Cogn. Neurosci. 19 : 2082– 99 [Google Scholar]
- Badre D , D'Esposito M . 2009 . Is the rostro-caudal axis of the frontal lobe hierarchical?. Nat. Rev. Neurosci. 10 : 659– 69 A synthesis of the evidence that the rostral-caudal functional gradient observed along the frontal cortex is hierarchical. [Google Scholar]
- Badre D , Frank MJ . 2012 . Mechanisms of hierarchical reinforcement learning in cortico-striatal circuits 2: evidence from fMRI. Cereb. Cortex 22 : 527– 36 [Google Scholar]
- Badre D , Hoffman J , Cooney JW , D'Esposito M . 2009 . Hierarchical cognitive control deficits following damage to the human frontal lobe. Nat. Neurosci. 12 : 515– 22 [Google Scholar]
- Bannon MJ , Roth RH . 1983 . Pharmacology of mesocortical dopamine neurons. Pharmacol. Rev. 35 : 53– 68 [Google Scholar]
- Barbas H , Pandya DN . 1991 . Patterns of connections of the prefrontal cortex in the rhesus monkey associated with cortical architecture. Frontal Lobe Function and Dysfunction HS Levin, H Eisenberg, AL Benton 35– 58 Oxford, UK: Oxford Univ. Press [Google Scholar]
- Bays PM , Husain M . 2008 . Dynamic shifts of limited working memory resources in human vision. Science 321 : 851– 54 [Google Scholar]
- Bentin S , Allison T , Puce A , Perez E , McCarthy G . 1996 . Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 8 : 551– 65 [Google Scholar]
- Bilder RM , Volavka J , Lachman HM , Grace AA . 2004 . The catechol- O -methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29 : 1943– 61 [Google Scholar]
- Blumenfeld RS , Nomura EM , Gratton C , D'Esposito M . 2013 . Lateral prefrontal cortex is organized into parallel dorsal and ventral streams along the rostro-caudal axis. Cereb. Cortex 23 : 2457– 66 [Google Scholar]
- Braver TS , Cohen JD . 1999 . Dopamine, cognitive control, and schizophrenia: the gating model. Prog. Brain Res. 121 : 327– 49 [Google Scholar]
- Braver TS , Gray JR , Burgess GC . 2008 . Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. Variation in Working Memory ARA Conway, C Jarrold, MJ Kane, A Miyake, JN Towse 76– 106 Oxford, UK: Oxford Univ. Press [Google Scholar]
- Brown RM , Crane AM , Goldman PS . 1979 . Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 168 : 133– 50 [Google Scholar]
- Brozoski TJ , Brown RM , Rosvold HE , Goldman PS . 1979 . Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205 : 929– 32 [Google Scholar]
- Burgess PW , Dumontheil I , Gilbert SJ . 2007 . The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 11 : 290– 98 [Google Scholar]
- Buzsáki G , Draguhn A . 2004 . Neuronal oscillations in cortical networks. Science 304 : 1926– 29 [Google Scholar]
- Camps M , Cortés R , Gueye B , Probst A , Palacios JM . 1989 . Dopamine receptors in human brain: autoradiographic distribution of D 2 sites. Neuroscience 28 : 275– 90 [Google Scholar]
- Chao LL , Knight RT . 1998 . Contribution of human prefrontal cortex to delay performance. J. Cogn. Neurosci. 10 : 167– 77 [Google Scholar]
- Chatham CH , Badre D . 2013 . Working memory management and predicted utility. Front. Behav. Neurosci. 7 : 83 [Google Scholar]
- Chen AJ , Britton M , Turner GR , Vytlacil J , Thompson TW , D'Esposito M . 2012 . Goal-directed attention alters the tuning of object-based representations in extrastriate cortex. Front. Hum. Neurosci. 6 : 187 [Google Scholar]
- Christoff K , Ream JM , Geddes LP , Gabrieli JD . 2003 . Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav. Neurosci. 117 : 1161– 68 [Google Scholar]
- Christophel TB , Hebart MN , Haynes JD . 2012 . Decoding the contents of visual short-term memory from human visual and parietal cortex. J. Neurosci. 32 : 12983– 89 [Google Scholar]
- Cohen JD , Braver TS , Brown JW . 2002 . Computational perspectives on dopamine function in prefrontal cortex. Curr. Opin. Neurobiol. 12 : 223– 29 [Google Scholar]
- Constantinidis C , Franowicz MN , Goldman-Rakic PS . 2001 . The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat. Neurosci. 4 : 311– 16 [Google Scholar]
- Cools R , D'Esposito M . 2009 . Dopaminergic modulation of flexible control in humans. Dopamine Handbook A Bjorklund, SB Dunnett, LL Iversen, SD Iversen 249– 60 Oxford, UK: Oxford Univ. Press [Google Scholar]
- Cools R , D'Esposito M . 2011 . Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69 : e113– 25 A review of evidence from studies with experimental animals, healthy humans, and patients with Parkinson's disease, which demonstrate that optimum levels of dopamine are necessary for successful cognitive control. [Google Scholar]
- Cools R , Robbins TW . 2004 . Chemistry of the adaptive mind. Philos. Transact. A Math. Phys. Eng. Sci. 362 : 2871– 88 [Google Scholar]
- Cools R , Sheridan M , Jacobs E , D'Esposito M . 2007 . Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J. Neurosci. 27 : 5506– 14 [Google Scholar]
- Corbetta M , Miezin FM , Dobmeyer S , Shulman GL , Petersen SE . 1990 . Attentional modulation of neural processing of shape, color, and velocity in humans. Science 248 : 1556– 59 [Google Scholar]
- Courtney SM , Ungerleider LG , Keil K , Haxby JV . 1997 . Transient and sustained activity in a distributed neural system for human working memory. Nature 386 : 608– 11 [Google Scholar]
- Cowan N . 1995 . Attention and Memory: An Integrated Framework New York: Oxford Univ. Press [Google Scholar]
- Crespo-Garcia M , Pinal D , Cantero JL , Díaz F , Zurrón M , Atienza M . 2013 . Working memory processes are mediated by local and long-range synchronization of alpha oscillations. J. Cogn. Neurosci. 25 : 1343– 57 [Google Scholar]
- Cummings JL . 1993 . Frontal-subcortical circuits and human behavior. Arch. Neurol. 50 : 873– 80 [Google Scholar]
- Curtis CE , Rao VY , D'Esposito M . 2004 . Maintenance of spatial and motor codes during oculomotor delayed response tasks. J. Neurosci. 24 : 3944– 52 [Google Scholar]
- D'Ardenne K , McClure SM , Nystrom LE , Cohen JD . 2008 . BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319 : 1264– 67 [Google Scholar]
- D'Esposito M . 2007 . From cognitive to neural models of working memory. Philos. Trans. R. Soc. B 362 : 761– 72 [Google Scholar]
- D'Esposito M , Postle B , Rypma B . 2000 . Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp. Brain Res. 133 : 3– 11 [Google Scholar]
- Devinsky O , D'Esposito M . 2003 . Neurology of Cognitive and Behavioral Disorders New York: Oxford Univ. Press [Google Scholar]
- Duncan J . 2001 . An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2 : 820– 29 [Google Scholar]
- Durstewitz D , Seamans JK . 2008 . The dual-state theory of prefrontal cortex dopamine function with relevance to catechol- O -methyltransferase genotypes and schizophrenia. Biol. Psychiatry 64 : 739– 49 [Google Scholar]
- Durstewitz D , Seamans JK , Sejnowski TJ . 2000a . Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J. Neurophysiol. 83 : 1733– 50 [Google Scholar]
- Durstewitz D , Seamans JK , Sejnowski TJ . 2000b . Neurocomputational models of working memory. Nat. Neurosci. 3 : Suppl. 1184– 91 [Google Scholar]
- Eichenbaum H . 2013 . Memory on time. Trends Cogn. Sci. 17 : 81– 88 [Google Scholar]
- Emrich SM , Riggall AC , Larocque JJ , Postle BR . 2013 . Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short-term memory. J. Neurosci. 33 : 6516– 23 [Google Scholar]
- Epstein R , Kanwisher N . 1998 . A cortical representation of the local visual environment. Nature 392 : 598– 601 [Google Scholar]
- Erickson MA , Maramara LA , Lisman J . 2010 . A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J. Cogn. Neurosci. 22 : 2530– 40 [Google Scholar]
- Ester EF , Anderson DE , Serences JT , Awh E . 2013 . A neural measure of precision in visual working memory. J. Cogn. Neurosci. 25 : 754– 61 A powerful demonstration, with MVPA encoding models, that the precision of neural representations in sensory cortex determines the precision of STM. [Google Scholar]
- Fell J , Axmacher N . 2011 . The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12 : 105– 18 [Google Scholar]
- Feredoes E , Heinen K , Weiskopf N , Ruff C , Driver J . 2011 . Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci. USA 108 : 17510– 15 [Google Scholar]
- Fiebach CJ , Rissman J , D'Esposito M . 2006 . Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron 51 : 251– 61 [Google Scholar]
- Frank MJ , Badre D . 2012 . Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 1: computational analysis. Cereb. Cortex 22 : 509– 26 [Google Scholar]
- Frank MJ , Loughry B , O'Reilly RC . 2001 . Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn. Affect. Behav. Neurosci. 1 : 137– 60 [Google Scholar]
- Frank MJ , O'Reilly RC . 2006 . A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav. Neurosci. 120 : 497– 517 [Google Scholar]
- Freedman DJ , Riesenhuber M , Poggio T , Miller EK . 2001 . Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291 : 312– 16 [Google Scholar]
- Freedman DJ , Riesenhuber M , Poggio T , Miller EK . 2003 . A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J. Neurosci. 23 : 5235– 46 [Google Scholar]
- Fries P . 2005 . A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9 : 474– 80 [Google Scholar]
- Funahashi S , Bruce CJ , Goldman-Rakic PS . 1989 . Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 61 : 331– 49 [Google Scholar]
- Fuster JM . 1990 . Prefrontal cortex and the bridging of temporal gaps in the perception-action cycle. Ann. N. Y. Acad. Sci. 608 : 318– 29 discussion 330– 36 [Google Scholar]
- Fuster JM . 2004 . Upper processing stages of the perception-action cycle. Trends Cogn. Sci. 8 : 143– 45 [Google Scholar]
- Fuster JM . 2008 . The Prefrontal Cortex Oxford, UK: Elsevier [Google Scholar]
- Fuster JM , Alexander GE . 1971 . Neuron activity related to short-term memory. Science 173 : 652– 54 [Google Scholar]
- Fuster JM , Bauer RH , Jervey JP . 1985 . Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 330 : 299– 307 [Google Scholar]
- Gazzaley A , Cooney JW , McEvoy K , Knight RT , D'Esposito M . 2005 . Top-down enhancement and suppression of the magnitude and speed of neural activity. J. Cogn. Neurosci. 17 : 507– 17 A study using fMRI and ERP that provides converging evidence that both the magnitude of neural activity and the speed of neural processing are modulated by top-down influences. [Google Scholar]
- Gazzaley A , Rissman J , D'Esposito M . 2004 . Functional connectivity during working memory maintenance. Cogn. Affect. Behav. Neurosci. 4 : 580– 99 [Google Scholar]
- Goldman-Rakic PS . 1987 . Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. Handbook of Physiology: The Nervous System F Plum, VB Mountcastle 373– 417 Bethesda, MD: Am. Physiol. Soc. [Google Scholar]
- Goldman-Rakic PS . 1995 . Cellular basis of working memory. Neuron 14 : 477– 85 [Google Scholar]
- Goldman-Rakic PS , Lidow MS , Gallager DW . 1990 . Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J. Neurosci. 10 : 2125– 38 [Google Scholar]
- Grace AA . 2000 . The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95 : Suppl. 2 S119– 28 [Google Scholar]
- Hamidi M , Tononi G , Postle BR . 2008 . Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Res. 1230 : 202– 10 [Google Scholar]
- Hamidi M , Tononi G , Postle BR . 2009 . Evaluating the role of prefrontal and parietal cortices in memory-guided response with repetitive transcranial magnetic stimulation. Neuropsychologia 47 : 295– 302 [Google Scholar]
- Han X , Berg AC , Oh H , Samaras D , Leung HC . 2013 . Multi-voxel pattern analysis of selective representation of visual working memory in ventral temporal and occipital regions. NeuroImage 73 : 8– 15 [Google Scholar]
- Harrison SA , Tong F . 2009 . Decoding reveals the contents of visual working memory in early visual areas. Nature 458 : 632– 35 [Google Scholar]
- Haxby JV , Gobbini MI , Furey ML , Ishai A , Schouten JL , Pietrini P . 2001 . Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293 : 2425– 30 [Google Scholar]
- Higo T , Mars RB , Boorman ED , Buch ER , Rushworth MFS . 2011 . Distributed and causal influence of frontal operculum in task control. Proc. Natl. Acad. Sci. USA 108 : 4230– 35 [Google Scholar]
- Hillyard SA , Hink RF , Schwent VL , Picton TW . 1973 . Electrical signs of selective attention in the human brain. Science 182 : 177– 80 [Google Scholar]
- Itskov V , Hansel D , Tsodyks M . 2011 . Short-term facilitation may stabilize parametric working memory trace. Front. Comput. Neurosci. 5 : 40 [Google Scholar]
- Jerde TA , Merriam EP , Riggall AC , Hedges JH , Curtis CE . 2012 . Prioritized maps of space in human frontoparietal cortex. J. Neurosci. 32 : 17382– 90 [Google Scholar]
- Kanwisher N , McDermott J , Chun MM . 1997 . The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17 : 4302– 11 [Google Scholar]
- Kimberg DY , D'Esposito M . 2003 . Cognitive effects of the dopamine receptor agonist pergolide. Neuropsychologia 41 : 1020– 27 [Google Scholar]
- Kimberg DY , D'Esposito M , Farah MJ . 1997 . Effects of bromocriptine on human subjects depend on working memory capacity. NeuroReport 8 : 3581– 85 [Google Scholar]
- Koechlin E , Ody C , Kouneiher F . 2003 . The architecture of cognitive control in the human prefrontal cortex. Science 302 : 1181– 85 [Google Scholar]
- Koechlin E , Summerfield C . 2007 . An information theoretical approach to prefrontal executive function. Trends Cogn. Sci. 11 : 229– 35 [Google Scholar]
- Kouneiher F , Charron S , Koechlin E . 2009 . Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 12 : 939– 45 [Google Scholar]
- Kubota K , Niki H . 1971 . Prefrontal cortical unit activity and delayed alternation performance in monkeys. J. Neurophysiol. 34 : 337– 47 [Google Scholar]
- LaRocque JJ , Lewis-Peacock JA , Drysdale AT , Oberauer K , Postle BR . 2013 . Decoding attended information in short-term memory: an EEG study. J. Cogn. Neurosci. 25 : 127– 42 [Google Scholar]
- Lee SH , Kravitz DJ , Baker CI . 2013 . Goal-dependent dissociation of visual and prefrontal cortices during working memory. Nat. Neurosci. 16 : 997– 99 [Google Scholar]
- Lee TG , D'Esposito M . 2012 . The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J. Neurosci. 32 : 15458– 66 [Google Scholar]
- Lewis-Peacock JA , Drysdale AT , Oberauer K , Postle BR . 2012 . Neural evidence for a distinction between short-term memory and the focus of attention. J. Cogn. Neurosci. 24 : 61– 79 [Google Scholar]
- Lewis-Peacock JA , Postle BR . 2008 . Temporary activation of long-term memory supports working memory. J. Neurosci. 28 : 8765– 71 [Google Scholar]
- Lewis-Peacock JA , Postle BR . 2012 . Decoding the internal focus of attention. Neuropsychologia 50 : 470– 78 [Google Scholar]
- Liebe S , Hoerzer GM , Logothetis NK , Rainer G . 2012 . Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat. Neurosci. 15 : 456– 62 S1– 2 [Google Scholar]
- Luciana M , Collins PF . 1997 . Dopaminergic modulation of working memory for spatial but not object cues in normal humans. J. Cogn. Neurosci. 9 : 330– 47 [Google Scholar]
- Luck SJ , Vogel EK . 1997 . The capacity of visual working memory for features and conjunctions. Nature 390 : 279– 81 [Google Scholar]
- Luck SJ , Vogel EK . 2013 . Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 17 : 391– 400 An authoritative summary of evidence supporting slots models of STM capacity limits. [Google Scholar]
- Ma WJ , Husain M , Bays PM . 2014 . Changing concepts of working memory. Nat. Neurosci. 17 : 347– 56 A comprehensive review of psychophysical and neural evidence for single-resource models of STM capacity limits. [Google Scholar]
- Magnussen S . 2000 . Low-level memory processes in vision. Trends Neurosci. 23 : 247– 51 [Google Scholar]
- Magnussen S , Greenlee MW . 1999 . The psychophysics of perceptual memory. Psychol. Res. 62 : 81– 92 [Google Scholar]
- McElree B . 1998 . Attended and non-attended states in working memory: accessing categorized structures. J. Mem. Lang. 38 : 225– 52 [Google Scholar]
- McElree B . 2006 . Accessing recent events. Psychol. Learn. Motiv. 46 : 155– 200 [Google Scholar]
- Meyer-Lindenberg A , Kohn PD , Kolachana B , Kippenhan S , McInerney-Leo A . et al. 2005 . Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat. Neurosci. 8 : 594– 96 [Google Scholar]
- Meyers EM , Freedman DJ , Kreiman G , Miller EK , Poggio T . 2008 . Dynamic population coding of category information in inferior temporal and prefrontal cortex. J. Neurophysiol. 100 : 1407– 19 [Google Scholar]
- Miller BT , D'Esposito M . 2005 . Searching for “the top” in top-down control. Neuron 48 : 535– 38 [Google Scholar]
- Miller BT , Vytlacil J , Fegen D , Pradhan S , D'Esposito M . 2011 . The prefrontal cortex modulates category selectivity in human extrastriate cortex. J. Cogn. Neurosci. 23 : 1– 10 [Google Scholar]
- Miller EK , Cohen JD . 2001 . An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24 : 167– 202 An influential model of how the PFC implements top-down control for the flexible control of behavior. [Google Scholar]
- Miller EK , Erickson CA , Desimone R . 1996 . Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J. Neurosci. 16 : 5154– 67 [Google Scholar]
- Miller GA , Galanter E , Pribham KH . 1960 . Plans and the Structure of Behavior New York: Holt, Rinehart and Winston [Google Scholar]
- Mongillo G , Barak O , Tsodyks M . 2008 . Synaptic theory of working memory. Science 319 : 1543– 46 [Google Scholar]
- Moran J , Desimone R . 1985 . Selective attention gates visual processing in the extrastriate cortex. Science 229 : 782– 84 [Google Scholar]
- Muller U , Von Cramon DY , Pollmann S . 1998 . D1- versus D2-receptor modulation of visuospatial working memory in humans. J. Neurosci. 18 : 2720– 28 [Google Scholar]
- Nelissen N , Stokes M , Nobre AC , Rushworth MF . 2013 . Frontal and parietal cortical interactions with distributed visual representations during selective attention and action selection. J. Neurosci. 33 : 16443– 58 [Google Scholar]
- Oberauer K . 2001 . Removing irrelevant information from working memory: a cognitive aging study with the modified Sternberg task. J. Exp. Psychol.: Learn. Mem. Cogn. 27 : 948– 57 [Google Scholar]
- Oberauer K . 2002 . Access to information in working memory: exploring the focus of attention. J. Exp. Psychol.: Learn. Mem. Cogn. 28 : 411– 21 [Google Scholar]
- Oberauer K . 2005 . Control of the contents of working memory—a comparison of two paradigms and two age groups. J. Exp. Psychol.: Learn. Mem. Cogn. 31 : 714– 28 [Google Scholar]
- Oberauer K . 2009 . Design for a working memory. Psychol. Learn. Motiv. 51 : 45– 100 [Google Scholar]
- Oberauer K . 2013 . The focus of attention in working memory—from metaphors to mechanisms. Front. Hum. Neurosci. 7 : 673 [Google Scholar]
- Palva JM , Monto S , Kulashekhar S , Palva S . 2010 . Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. USA 107 : 7580– 85 [Google Scholar]
- Pesaran B , Pezaris JS , Sahani M , Mitra PP , Andersen RA . 2002 . Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 5 : 805– 11 [Google Scholar]
- Petrides M . 2000 . Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. J. Neurosci. 20 : 7496– 503 [Google Scholar]
- Postle BR . 2006 . Working memory as an emergent property of the mind and brain. Neuroscience 139 : 23– 38 [Google Scholar]
- Postle BR . 2011 . What underlies the ability to guide action with spatial information that is no longer present in the environment?. Spatial Working Memory A Vandierendonck, A Szmalec 87– 101 Hove, UK: Psychology [Google Scholar]
- Postle BR . 2015 . The cognitive neuroscience of visual short-term memory. Curr. Opin. Behav. Sci. 1 40– 46 A summary of the novel insights provided by MVPA, including the possibility that elevated activity reflects the focus of attention rather than working memory storage per se. [Google Scholar]
- Postle BR , Idzikowski C , Sala SD , Logie RH , Baddeley AD . 2006 . The selective disruption of spatial working memory by eye movements. Q. J. Exp. Psychol. ( Hove ) 59 : 100– 20 [Google Scholar]
- Pribram KH , Ahumada A , Hartog J , Roos L . 1964 . A progress report on the neurological processes disturbed by frontal lesions in primates. The Frontal Cortex and Behavior JM Warren, K Akert 28– 55 New York: McGraw-Hill [Google Scholar]
- Pycock CJ , Kerwin RW , Carter CJ . 1980 . Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature 286 : 74– 76 [Google Scholar]
- Ramnani N , Owen AM . 2004 . Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5 : 184– 94 [Google Scholar]
- Riggall AC , Postle BR . 2012 . The relationship between working memory storage and elevated activity as measured with functional magnetic resonance imaging. J. Neurosci. 32 : 12990– 98 [Google Scholar]
- Rigotti M , Barak O , Warden MR , Wang X-J , Daw ND . et al. 2013 . The importance of mixed selectivity in complex cognitive tasks. Nature 497 : 585– 90 [Google Scholar]
- Rissman J , Gazzaley A , D'Esposito M . 2004 . Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage 23 : 752– 63 [Google Scholar]
- Romo R , Brody CD , Hernández A , Lemus L . 1999 . Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399 : 470– 73 [Google Scholar]
- Roux F , Uhlhaas PJ . 2014 . Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information?. Trends Cogn. Sci. 18 : 16– 25 [Google Scholar]
- Ruff CC . 2013 . Sensory processing: Who's in (top-down) control?. Ann. N. Y. Acad. Sci. 1296 88– 107 [Google Scholar]
- Saalmann YB , Pinsk MA , Wang L , Li X , Kastner S . 2012 . The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337 : 753– 56 [Google Scholar]
- Samson Y , Wu JJ , Friedman AH , Davis JN . 1990 . Catecholaminergic innervation of the hippocampus in the cynomolgus monkey. J. Comp. Neurol. 298 : 250– 63 [Google Scholar]
- Sauseng P , Klimesch W , Schabus M , Doppelmayr M . 2005 . Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 57 : 97– 103 [Google Scholar]
- Sawaguchi T . 2001 . The effects of dopamine and its antagonists on directional delay-period activity of prefrontal neurons in monkeys during an oculomotor delayed-response task. Neurosci. Res. 41 : 115– 28 [Google Scholar]
- Sawaguchi T , Goldman-Rakic PS . 1991 . D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251 : 947– 50 [Google Scholar]
- Serences JT , Ester EF , Vogel EK , Awh E . 2009 . Stimulus-specific delay activity in human primary visual cortex. Psychol. Sci. 20 : 207– 14 [Google Scholar]
- Shallice T . 1982 . Specific impairments of planning. Philos. Trans. R. Soc. B 298 : 199– 209 [Google Scholar]
- Shohamy D , Adcock RA . 2010 . Dopamine and adaptive memory. Trends Cogn. Sci. 14 : 464– 72 [Google Scholar]
- Silvanto J , Cattaneo Z . 2010 . Transcranial magnetic stimulation reveals the content of visual short-term memory in the visual cortex. NeuroImage 50 : 1683– 89 [Google Scholar]
- Singer W . 2009 . Distributed processing and temporal codes in neuronal networks. Cogn. Neurodyn. 3 : 189– 96 [Google Scholar]
- Smith EE , Jonides J . 1999 . Storage and executive processes of the frontal lobes. Science 283 : 1657– 61 [Google Scholar]
- Sreenivasan KK , Curtis CE , D'Esposito M . 2014a . Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 18 : 82– 89 A review of recent neural evidence for sensorimotor-recruitment models and for non-activity-based mechanisms for working memory storage. [Google Scholar]
- Sreenivasan KK , Vytlacil J , D'Esposito M . 2014b . Distributed and dynamic storage of working memory stimulus information in extrastriate cortex. J. Cogn. Neurosci. 26 : 1141– 53 [Google Scholar]
- Stokes MG , Kusunoki M , Sigala N , Nili H , Gaffan D , Duncan J . 2013 . Dynamic coding for cognitive control in prefrontal cortex. Neuron 78 : 364– 75 [Google Scholar]
- Sugase-Miyamoto Y , Liu Z , Wiener MC , Optican LM , Richmond BJ . 2008 . Short-term memory trace in rapidly adapting synapses of inferior temporal cortex. PLOS Comput. Biol. 4 : e1000073 [Google Scholar]
- Tallon-Baudry C , Kreiter A , Bertrand O . 1999 . Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis. Neurosci. 16 : 449– 59 [Google Scholar]
- Theeuwes J , Olivers CN , Chizk CL . 2005 . Remembering a location makes the eyes curve away. Psychol. Sci. 16 : 196– 99 [Google Scholar]
- Venkatraman V , Rosati AG , Taren AA , Huettel SA . 2009 . Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J. Neurosci. 29 : 13158– 64 [Google Scholar]
- Verstynen TD , Badre D , Jarbo K , Schneider W . 2012 . Microstructural organizational patterns in the human corticostriatal system. J. Neurophysiol. 107 : 2984– 95 [Google Scholar]
- Vogel EK , Woodman GF , Luck SJ . 2001 . Storage of features, conjunctions and objects in visual working memory. J. Exp. Psychol.: Hum. Percept. Perform. 27 : 92– 114 [Google Scholar]
- Wallis JD , Anderson KC , Miller EK . 2001 . Single neurons in prefrontal cortex encode abstract rules. Nature 411 : 953– 56 [Google Scholar]
- Wang M , Vijayraghavan S , Goldman-Rakic PS . 2004 . Selective D2 receptor actions on the functional circuitry of working memory. Science 303 : 853– 56 [Google Scholar]
- Wang XJ . 1999 . Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19 : 9587– 603 [Google Scholar]
- Wang XJ . 2001 . Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24 : 455– 63 [Google Scholar]
- Warden MR , Miller EK . 2010 . Task-dependent changes in short-term memory in the prefrontal cortex. J. Neurosci. 30 : 15801– 10 [Google Scholar]
- Williams SM , Goldman-Rakic PS . 1993 . Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cereb. Cortex 3 : 199– 222 [Google Scholar]
- Yeterian EH , Pandya DN , Tomaiuolo F , Petrides M . 2012 . The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48 : 58– 81 [Google Scholar]
- Zaksas D , Bisley JW , Pasternak T . 2001 . Motion information is spatially localized in a visual working-memory task. J. Neurophysiol. 86 : 912– 21 [Google Scholar]
- Zanto TP , Rubens MT , Thangavel A , Gazzaley A . 2011 . Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 14 : 656– 61 [Google Scholar]
- Article Type: Review Article
Most Read This Month
Most cited most cited rss feed, job burnout, executive functions, social cognitive theory: an agentic perspective, on happiness and human potentials: a review of research on hedonic and eudaimonic well-being, sources of method bias in social science research and recommendations on how to control it, mediation analysis, missing data analysis: making it work in the real world, grounded cognition, personality structure: emergence of the five-factor model, motivational beliefs, values, and goals.
SYSTEMATIC REVIEW article
A systematic review on predictors of working memory training responsiveness in healthy older adults: methodological challenges and future directions.

- 1 Department of Medical Psychology | Neuropsychology & Gender Studies, Center for Neuropsychological Diagnostics and Intervention (CeNDI), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- 2 Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Faculty of Medicine and University Hospital of Cologne, University of Cologne, Cologne, Germany
Background: Research on predictors of working memory training responsiveness, which could help tailor cognitive interventions individually, is a timely topic in healthy aging. However, the findings are highly heterogeneous, reporting partly conflicting results following a broad spectrum of methodological approaches to answer the question “who benefits most” from working memory training.
Objective: The present systematic review aimed to systematically investigate prognostic factors and models for working memory training responsiveness in healthy older adults.
Method: Four online databases were searched up to October 2019 (MEDLINE Ovid, Web of Science, CENTRAL, and PsycINFO). The inclusion criteria for full texts were publication in a peer-reviewed journal in English/German, inclusion of healthy older individuals aged ≥55 years without any neurological and/or psychiatric diseases including cognitive impairment, and the investigation of prognostic factors and/or models for training responsiveness after targeted working memory training in terms of direct training effects, near-transfer effects to verbal and visuospatial working memory as well as far-transfer effects to other cognitive domains and behavioral variables. The study design was not limited to randomized controlled trials.
Results: A total of 16 studies including n = 675 healthy older individuals with a mean age of 63.0–86.8 years were included in this review. Within these studies, five prognostic model approaches and 18 factor finding approaches were reported. Risk of bias was assessed using the Quality in Prognosis Studies checklist, indicating that important information, especially regarding the domains study attrition, study confounding, and statistical analysis and reporting, was lacking throughout many of the investigated studies. Age, education, intelligence, and baseline performance in working memory or other cognitive domains were frequently investigated predictors across studies.
Conclusions: Given the methodological shortcomings of the included studies, no clear conclusions can be drawn, and emerging patterns of prognostic effects will have to survive sound methodological replication in future attempts to promote precision medicine approaches in the context of working memory training. Methodological considerations are discussed, and our findings are embedded to the cognitive aging literature, considering, for example, the cognitive reserve framework and the compensation vs. magnification account. The need for personalized cognitive prevention and intervention methods to counteract cognitive decline in the aging population is high and the potential enormous.
Registration: PROSPERO, ID CRD42019142750.
Introduction
The promotion of healthy aging constitutes a major goal given the demographic change that the world's population is facing ( Parish et al., 2019 ). One key aspect of healthy aging is the maintenance of cognitive functions by preventing or delaying the onset of clinically relevant cognitive dysfunction or even reversing age-related cognitive decline ( Lustig et al., 2009 ). Cognitive decline is one of the most feared aspects in aging ( Deary et al., 2009 ), as it reduces the quality of life of both the aging individual and his/her relatives and increases the burden on care providers and the public healthcare system. Decline of executive functions, working memory, processing speed, and memory—cognitive functions that are essential for everyday functioning—is the most prominent cognitive alteration in healthy aging ( Paraskevoudi et al., 2018 ). Especially working memory, a capacity-limited system for short-term storage and manipulation of information, is of fundamental importance for general cognitive functioning and is seen as a key function and processing resource for other cognitive abilities ( Salthouse, 1990 ; Chai et al., 2018 ).
Cognitive training interventions, as a non-pharmacological intervention and prevention method, have gained increased scientific interest ( Lustig et al., 2009 ). A recent meta-analysis of Chiu et al. (2017) on broad cognitive interventions in healthy older adults clearly indicated the potential of cognitive interventions to counteract cognitive decline. However, some issues such as the degree of transfer to untrained tasks and long-term effects remain a matter of debate. In this context, working memory has become a main target for cognitive training interventions. The role of working memory as a processing resource for other cognitive abilities ( Salthouse, 1990 ; Chai et al., 2018 ) implies that working memory improvements after targeted working memory training (WMT) might naturally lead to positive transfer effects to other cognitive functions and even fluid intelligence ( Au et al., 2015 ). Despite a general consensus regarding the effectiveness of targeted WMT regarding direct training effects (i.e., effects in trained working memory tasks over the course of training) and near-transfer effects (i.e., effects in untrained working memory tasks), evidence on far-transfer effects (i.e., effects in untrained domains) for different populations including healthy older adults has not convincingly been shown (for recent meta-analyses see e.g., Melby-Lervåg et al., 2016 ; Weicker et al., 2016 ; Soveri et al., 2017 ; Sala et al., 2019 ; Teixeira-Santos et al., 2019 ). Given those heterogeneous results concerning effects after WMT, identifying modifying, so-called prognostic or moderating, factors (including both individual- and training-related characteristics) of WMT responsiveness seems highly relevant.
In general, a prognostic factor is defined as any measure that, among people with a given condition (e.g., the process of aging), is associated with a subsequent outcome (e.g., changes in cognition after certain interventions) ( Riley et al., 2013 ). In prognostic research, prognostic factor finding studies and prognostic model studies are distinguished: prognostic factor finding studies aim at establishing one or several variables as independent prognostic factors associated with an outcome. In contrast, prognostic model studies identify more than one prognostic factor, assign relative weight to each prognostic factor, and estimate the model's predictive performance through calibration and discrimination ( Moons et al., 2009 ). Identifying prognostic factors for individual treatment response to WMT would take into account individual differences in cognitive plasticity and following responsiveness to cognitive training interventions ( Baltes and Lindenberger, 1988 ; Noack et al., 2009 ; Bürki et al., 2014 ). It would further contribute to the development of an encompassing approach in terms of a “personalized” or “precision medicine” ( Hingorani et al., 2013 ) in healthy aging and the prevention of cognitive decline, for example, in the context of Alzheimer's disease ( Reitz, 2016 ; Berkowitz et al., 2018 ).
The latest meta-analysis on WMT for healthy older adults ( Teixeira-Santos et al., 2019 ) included a broad moderator analysis for WMT responsiveness. Despite training-related variables (e.g., training dose and length, number of sessions, training type), study population characteristics (e.g., age, education, general cognitive ability, baseline performance) were considered as moderating variables ( Teixeira-Santos et al., 2019 ). The meta-analysis mainly identified training-related characteristics as moderating variables for WMT response: for example, longer training durations in hours were associated with smaller effect sizes across studies ( Teixeira-Santos et al., 2019 ). Note, however, that whereas prognostic factors are, per definition, measured and investigated on an individual-person level, the moderator analysis approach within the standard meta-analytical approach investigates modifying factors on an aggregated, study-wide level, i.e., across many individuals (e.g., mean age of participants, mean years of education). Therefore, interindividual variance of those parameters and corresponding differential training outcomes within the single-study populations are neglected in the meta-analysis of Teixeira-Santos et al. (2019) . A focus on research using prognostic approaches on a single-study level would therefore substantially expand upon already existing data.
Prognostic research on treatment responsiveness after WMT has received increasing interest on a single-study level as well. However, data are inconclusive yet, as findings are highly heterogeneous and inconsistent, and prognostic approaches are often considered as an add-on analysis beyond standard effectiveness evaluations only. It seems that, especially if an intervention did not yield an overall positive effect, researchers tend to exploratively analyze prognostic factors of training responsiveness. One could argue that conducting prognostic analyses on null effects might be dealing with pure noise. However, prognostic research is obliged to detangle predictors of systematic retest effects, such as practice effects or regression to the mean, from predictors of treatment response ( Hingorani et al., 2013 ). Therefore, it is tremendously important to compare the prognostic factors between a control group and the group receiving the treatment of interest ( Hingorani et al., 2013 ). To anticipate one weakness of prognostic research in the context of cognitive interventions including WMT so far, prognostic effects are often investigated with data of the experimental group only.
Two of the most frequently investigated prognostic factors for WMT responsiveness are baseline performance in working memory or the respective cognitive outcome and general cognitive ability (e.g., Zinke et al., 2014 ; Borella et al., 2017b ; Matysiak et al., 2019 ). For both, inconsistent findings exist, which can be discussed within the compensation vs. magnification framework ( Lövdén et al., 2012 ). Following the compensation account, individuals with lower baseline performance would show higher training benefits because they have more room for improvement. On the contrary, the magnification hypothesis constitutes that individuals with higher abilities would benefit most, as they have more resources “to acquire, implement, and sharpen effortful cognitive strategies” ( Lövdén et al., 2012 ). Similar inconsistent evidence exists, e.g., for age (e.g., Borella et al., 2013 , 2014 , 2017b ; Zinke et al., 2014 ; Simon et al., 2018 ) and other demographic factors such as education ( Clark et al., 2016 ; Mondini et al., 2016 ; Borella et al., 2017b ; Matysiak et al., 2019 ) and sex ( Rahe et al., 2015 ; Matysiak et al., 2019 ; Roheger et al., 2019 ). Furthermore, motivational processes ( West et al., 2008 ; Kalbe et al., 2018 ) and personality traits ( Studer-Luethi et al., 2012 ; Double and Birney, 2016 ) might constitute important individual characteristics predicting training responsiveness as well. Finally, genetic variation ( Brehmer et al., 2009 ; Bellander et al., 2011 ; Bäckman and Nyberg, 2013 ) and brain imaging parameters ( Stern, 2009 ; Heinzel et al., 2014a ) might reflect meaningful proxies for the potential to engage in cognitive plasticity following cognitive training interventions. To summarize, a broad spectrum of potential prognostic factors to predict individual training responsiveness is discussed; however, data are inconclusive yet. Therefore, systematic reviews and meta-analyses to summarize existing evidence about prognostic factors and models of individual treatment response in the context of cognitive interventions in general and WMT in particular are urgently needed but missing so far.
On the basis of the aforementioned considerations, the present systematic review aimed to systematically investigate prognostic factors and models for WMT responsiveness in healthy older adults. We further aimed to meta-analyze groups of “similar” prognostic effect measures to quantitatively investigate the predictive performance of the different prognostic factors. However, to anticipate one limitation of this work, data on prognostic factors after WMT were too heterogeneous and too poorly reported to conduct this meta-analysis after all.
Our systematic review question was defined using the PICOTS system as proposed by the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies (CHARMS) ( Moons et al., 2014 ; Debray et al., 2017 ; Riley et al., 2019 ). Our target population (P) consisted of healthy (i.e., absence of any neurological or psychiatric disease) older (aged ≥ 55 years) individuals. The target intervention (I) was single-domain WMT. No comparator factor (C) is being considered. The outcome variables (O) for this review are training and near-transfer effects to the domains of verbal and visuospatial working memory as well as far-transfer effects to other cognitive domains and behavioral variables, if applicable, operationalized with objective and standardized instruments, after targeted WMT. The timing (T) of recording the relevant variables is the baseline assessment for prognostic factors and all time points of measurement for outcome variables, including follow-ups. The setting (S) was supposed to be a non-clinical one to gain prognostic information on possibilities of enhancing cognitive functioning and the prevention of cognitive decline in cognitively healthy individuals.
The preregistered review protocol of the present systematic review can be accessed through https://www.crd.york.ac.uk/PROSPERO/ (ID: CRD42019142750). The reporting follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for systematic reviews and meta-analyses ( Moher et al., 2009 ). The PRISMA checklists for abstracts and systematic reviews are displayed in Supplementary Material 1 .
Search Strategy
As prognostic studies are often not indexed, a broad and rather unspecific search filter was used ( Riley et al., 2019 ). We conducted a systematic search throughout four online databases up to October 2019: MEDLINE Ovid, Web of Science Core Collection, CENTRAL, and PsycINFO. A series of keywords which were expected to appear in the title or the abstract of any study containing analyses on prognostic factors or models for WMT success was created. The keywords used can be grouped into three main categories. The first category aimed to identify studies including healthy older adults as participants (e.g., “healthy elderly,” “healthy aging,” “older adults”). The second category was used to detect a broad spectrum of interventional studies not only covering “working memory training” but also a broader spectrum of cognitive interventions (e.g., “cognitive training,” “reasoning training”) and even interventional studies per se (e.g., “training,” “intervention”). This broad intervention category was built to ensure the search strategy to cover all kinds of WMT that are differentially labeled in literature. The third category was included to ensure (working) memory to be a central construct of the included studies (“memory”). In addition to the systematic database search, the reference lists of all relevant full texts, review articles, and current treatment guidelines were hand-searched for further suitable articles. Further information and full search strings for each database can be obtained from Supplementary Material 2 .
Study Selection and Data Extraction
Title and abstract screening with predefined eligibility criteria was conducted by two reviewers (AKF and MR or AO and MR) in Covidence Systematic Review Software (Veritas Health Innovation, available at www.covidence.org ). Then, the full-text articles were screened for final inclusion in the systematic review by two reviewers (AO and MR). If a full text was not available online, we contacted the corresponding authors and asked them to provide the full-text publication within 2 weeks of time. If no consensus was reached between the two reviewers (AO and MR), the plan was to discuss the case with a third author (NS) until a final consensus was reached; however, this option was not needed. Relevant data considering general study characteristics (e.g., participants' demographics, WMT features) and prognostic factor and/or model analyses were independently extracted by two reviewers (AO and MR) according to the CHARMS checklist ( Moons et al., 2014 ).
Eligibility Criteria
The inclusion criteria for our systematic review were (i) full-text research article publication until October 2019 in a peer-reviewed journal in English or German, (ii) inclusion of healthy older individuals aged ≥55 years without any neurological and/or psychiatric diseases including cognitive impairment (mild cognitive impairment or dementia) as well as uncorrected seeing or hearing impairments assessed via self-report, and (iii) investigation of prognostic factors and/or models for training responsiveness in terms of direct training and near-transfer effects to verbal and visuospatial working memory as well as far-transfer effects to other cognitive domains and behavioral variables, operationalized with objective and standardized instruments, after targeted WMT.
Age of ≥55 years was chosen as a cutoff, as we, on the one hand, wanted to provide an objective age cutoff for individuals within the included studies and, on the other hand, did not want to exclude studies including healthy older individuals just below the frequently used cutoff of ≥60 years (e.g., Soveri et al., 2017 ; Sala et al., 2019 ). Targeted WMT was defined as a cognitive training either computerized, with paper–pencil tasks, or mixed, which is administered either on personal devices or in individual or group settings, with a minimum of two training sessions. When multi-domain trainings were examined, working memory had to be the main component of the program (defined as being the main target in at least 80% of the exercises). Verbal and visuospatial working memory, i.e., direct training and near-transfer effects, were defined as primary outcomes, with direct training effects constituting effects in trained working memory tasks over the course of training and with near-transfer effects constituting effects in untrained working memory tasks. Other cognitive far-transfer outcomes (i.e., effects in untrained cognitive domains, e.g., global cognition, memory, fluid intelligence, executive functions, attention) and clinical and patient-centered outcomes (e.g., depressive symptoms, quality of life) were considered as secondary outcomes. Both primary and secondary outcomes needed to be assessed with established and objective psychometric instruments.
For the systematic review, we considered all prognostic factors (e.g., sociodemographic factors, cognitive abilities at the entry of training, brain imaging parameters, genetic parameters, personality traits, training-related characteristics), which investigate critical aspects of WMT responsiveness. As outlined in the introduction, a prognostic factor is defined as any measure that, among people with a given condition (e.g., the process of aging), is associated with a subsequent outcome (e.g., changes in cognition after certain interventions) ( Riley et al., 2013 ). Prognostic factor finding studies aim at establishing one or several variables as independent prognostic factors associated with an outcome. In contrast, prognostic model studies identify more than one prognostic factor, assign relative weights to each prognostic factor, and estimate the model's predictive performance through calibration and discrimination ( Moons et al., 2009 ). We included all studies investigating prognostic factors and/or prognostic models regardless of whether or not significant general training effects and/or significant relationships between prognostic factors and training responsiveness were found.
Quality Assessment
Using the Quality in Prognosis Studies (QUIPS) checklist ( Hayden et al., 2013 ), risk of bias of the included studies was examined independently by two reviewers (AO and MR) across six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, adjustment for other prognostic factors, statistical analyses, and reporting. Each domain was overall rated with high, moderate, or low risk, depending on the rating in the corresponding items. A detailed description of the QUIPS checklist, including each item and the overall judgment rules implemented by the two reviewers, is presented in Supplementary Material 3 . Instead of using two different risk of bias assessment tools [QUIPS ( Hayden et al., 2013 ) for prognostic factor finding studies and Prediction Model Risk of Bias Assessment Tool ( Wolff et al., 2019 ) for prognostic model studies], risk of bias of both prognostic factor finding and prognostic model studies was assessed with the QUIPS tool to get a comparable risk of bias rating.
Data Analysis
Initially and as stated in the pre-registration of the study, we aimed to meta-analyze groups of “similar” prognostic effect measures with a random effects approach to investigate the predictive performance of the different prognostic factors. However, after data extraction, we had to ascertain that data on prognostic factors after WMT were too heterogeneous and too poorly reported to conduct this meta-analysis. The main reason was that we were not able to compute comparable effect size measures (e.g., odds ratios, hazard ratios) to meta-analyze the prognostic effects reported in the studies due to the fact that either data were not reported and could not be assessed within studies or data were not consistent enough across studies to pool the results. Therefore, the systematic review focused on the qualitative directionality of the prognostic effects reported in the included studies rather than their magnitude.
A total of 12,966 records were identified through our database search. After removing duplicates, titles and abstracts of 9,583 records were screened for eligibility. As prognostic analyses are often not indexed, title and abstract screening focused on the content-related criteria “healthy older individuals” and “working memory training.” Thus, 138 full texts were screened for eligibility. Finally, n = 16 studies were included in the present systematic review [for details on study flow and reasons for exclusion, see Figure 1 (PRISMA flow diagram)].
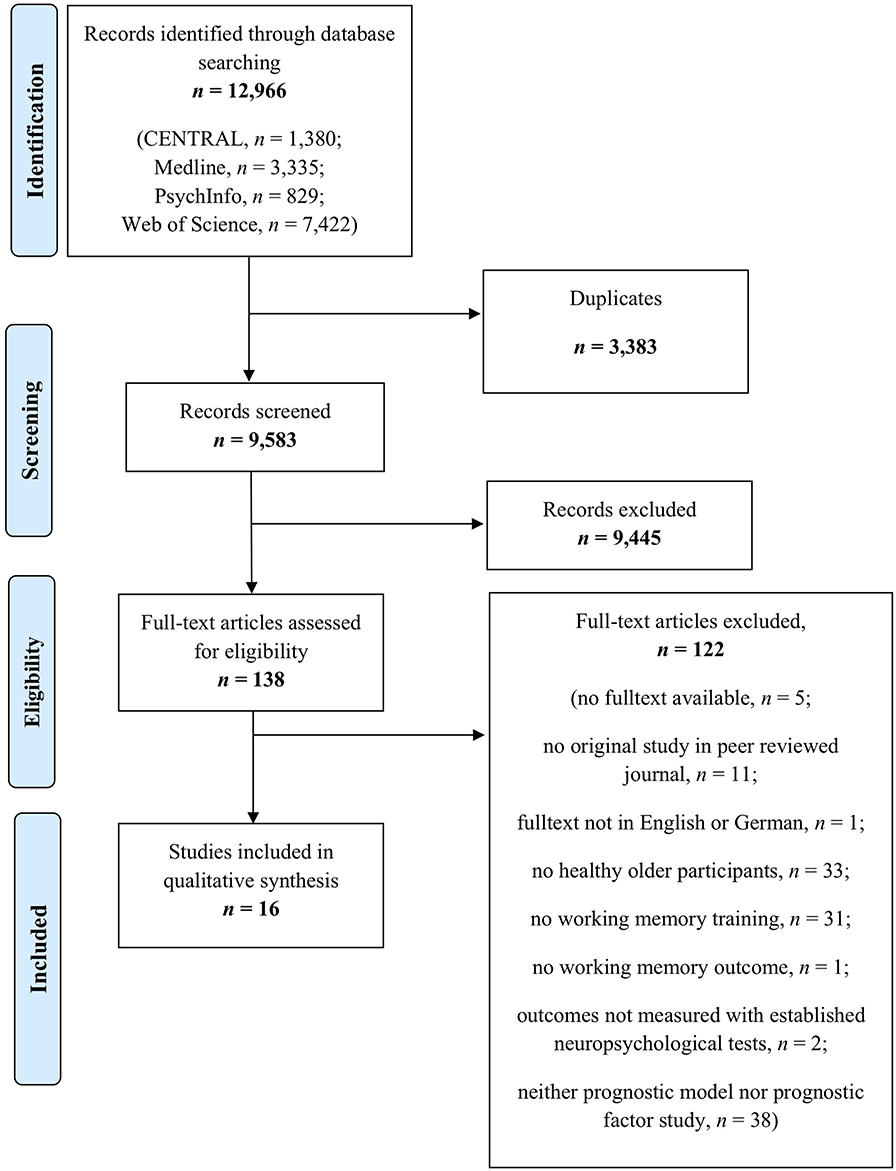
Figure 1 . Preferred reporting items for systematic reviews and meta-analyses flow diagram.
Descriptive Characteristics of the Included Studies
Within the 16 studies, n = 675 healthy older individuals, with age ranging from a mean of 63.0 years ( Brehmer et al., 2011 ) to 86.8 years ( Zinke et al., 2012 ), were investigated, of which 63% were women. Years of formal education ranged from a mean of 5.72 years ( Borella et al., 2013 ) to 18.65 years ( Tusch et al., 2016 ). Throughout the studies, different training regimes that varied in terms of setting, number of sessions, total time of training, and training tasks were applied. The number of training sessions ranged from three ( Borella et al., 2013 , 2014 , 2017a , b , c ; Brum et al., 2018 ) to 25 ( Brehmer et al., 2011 ; McAvinue et al., 2013 ; Tusch et al., 2016 ; Simon et al., 2018 ; Matysiak et al., 2019 ), with the total time of training ranging from 105 min ( Brum et al., 2018 ) to 1,000 min ( Tusch et al., 2016 ); 44% of trainings addressed verbal working memory only and 50% followed a mixed approach, addressing both verbal and visuospatial working memory. Only one study conducted a multi-domain WMT, as next to working memory tasks one executive control task was included within the training regime ( Zinke et al., 2014 ). All training regimes were conceptualized as adaptive, except for those studies in which adaptivity was investigated as a prognostic factor for WMT responsiveness ( Brehmer et al., 2011 ; Tusch et al., 2016 ; Simon et al., 2018 ; Weicker et al., 2018 ).
In total, nine studies applied digital WMT: four studies used commercially available digital WMT programs (Cogmed and WOME/ RehaCom ® ) ( Brehmer et al., 2011 ; Tusch et al., 2016 ; Simon et al., 2018 ; Weicker et al., 2018 ), three studies used a digital n-back training ( Heinzel et al., 2014a , b ; Matysiak et al., 2019 ), and two used a study–individual composition of digital WMT tasks ( McAvinue et al., 2013 ; Borella et al., 2014 ). Five studies used a WMT with the Categorization Working Memory Span (CWMS) Task based on audio recordings ( Borella et al., 2013 , 2017a , b , c ; Brum et al., 2018 ); however, all of these studies were conducted by the same group of researchers. Only two studies used paper–pencil WMT ( Zinke et al., 2012 , 2014 ) (for details on the study, participants, and training characteristics, see Table 1 ).
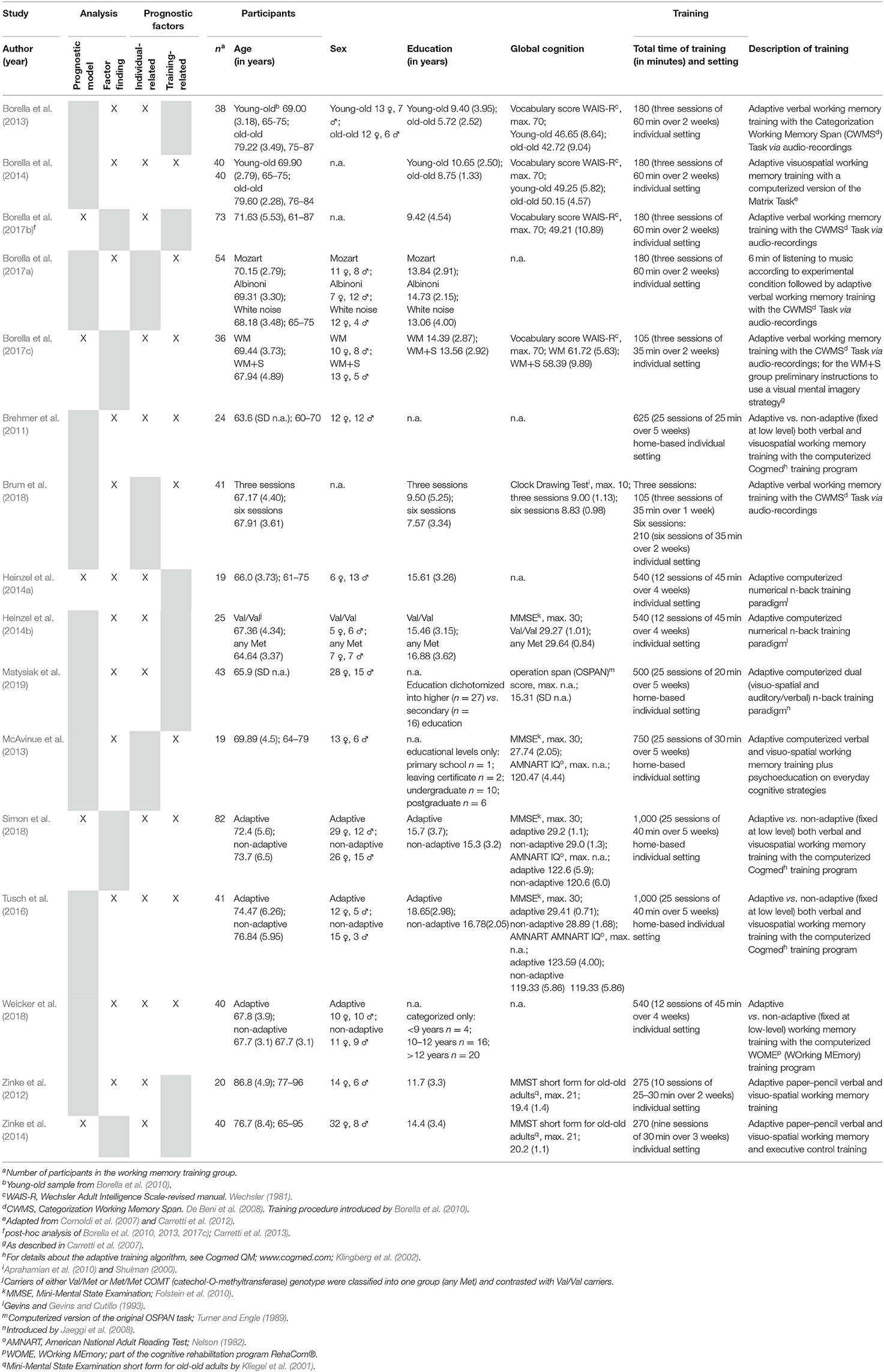
Table 1 . Study objectives, participants' demographics, and working memory training characteristics.
Reporting Quality and Risk of Bias
Table 2 reports the risk of bias per study across six domains evaluated with the QUIPS checklist ( Hayden et al., 2013 ). A detailed risk of bias assessment on a single item level rather than QUIPS domain ratings can be obtained from the corresponding author. Important information is lacking throughout many of investigated studies, especially regarding the domains study attrition, study confounding, and statistical analysis and reporting. Most notably, the appropriate selection of the analysis plan and reporting of both the statistical analyses and results are often fragmentary. Only for the domains of prognostic factor measurement and outcome measurement were the majority of studies rated with low risk. In summary, the reporting quality was partly insufficient, and the results should be interpreted cautiously.
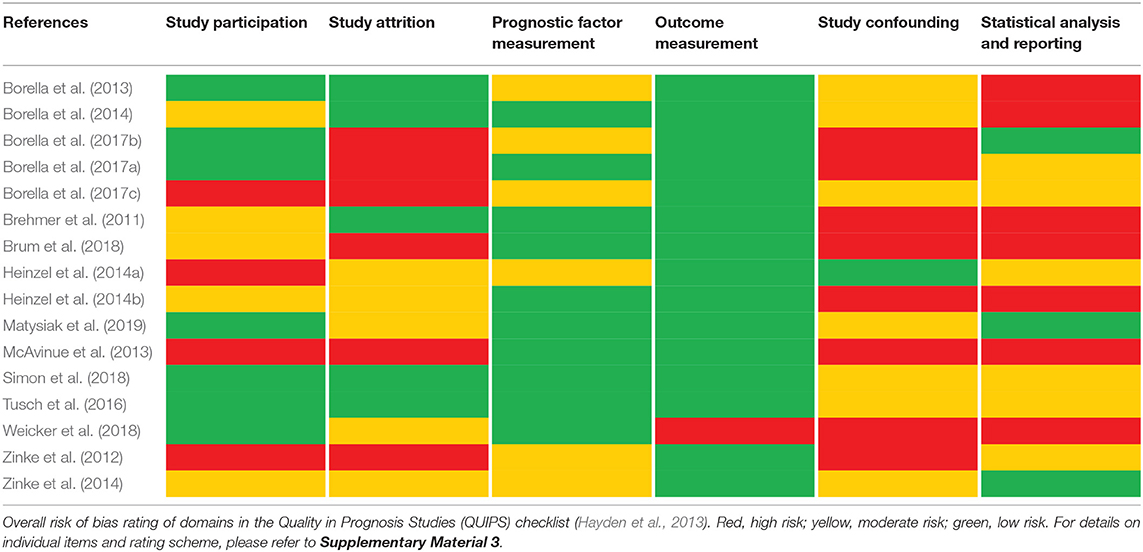
Table 2 . Risk of bias assessment using the QUIPS checklist.
Unfortunately, the initially planned meta-analysis could not be performed as the applied analytical approaches, as described below, were too heterogeneous and the reported results did not allow to compute comparable effect size measures (e.g., odds ratios, hazard ratios) across studies to meta-analyze the prognostic effects. Therefore, only a systematic review focusing on the directionality of prognostic effects rather than their magnitude was performed.
Prediction Analyses and Outcome Measures
Seven of the 16 prognostic studies used more than one prediction analysis account to predict WMT responsiveness (one study included both a prediction model and a factor finding approach; six studies included more than one factor finding approach, i.e., investigated the prognostic value of one or several variables with at least two different approaches). Five studies investigated prediction models, three of which used hierarchical regression analyses ( Heinzel et al., 2014a ; Zinke et al., 2014 ; Borella et al., 2017c ) with change scores or relative change scores as dependent variables. One study used Bayesian modeling approach ( Borella et al., 2017b ) and one used linear mixed effect modeling ( Simon et al., 2018 ), both with time as one predictor, therefore abandoning the use of change scores as dependent variable. Ten studies were factor finding studies, including a total of 18 factor finding analysis approaches: seven used a generalized linear model approach (e.g., ANOVA) ( Brehmer et al., 2011 ; Zinke et al., 2012 ; Borella et al., 2014 ; Heinzel et al., 2014b ; Tusch et al., 2016 ; Brum et al., 2018 ; Weicker et al., 2018 ), one used ANCOVA ( Borella et al., 2017a ), five used Pearson correlations ( Brehmer et al., 2011 ; Zinke et al., 2012 ; McAvinue et al., 2013 ; Heinzel et al., 2014a ; Tusch et al., 2016 ), and one used linear regressions ( Weicker et al., 2018 ) and one Linear Mixed Models ( Matysiak et al., 2019 ). Three studies used a (descriptive) comparison of effect sizes ( Borella et al., 2013 , 2014 ; Brum et al., 2018 ). For the generalized mixed model approach, 71% used time as a predictor and only 29% used raw or standardized change scores as a dependent variable. For ANCOVA, the post-test score was used as a dependent variable. Pearson correlations and linear regressions used (standardized) change scores as dependent variables; for the linear mixed model, time was used as a predictor. None of the studies compared prognostic factors or models between the trained group and a passive control group, i.e., they analyzed the data of trained groups only. To summarize, even though prediction approaches were highly heterogeneous, analyses were comparable within the different approaches.
We defined verbal and visuospatial working memory, i.e., direct training and near-transfer effects, as primary outcomes. Most of the included studies distinguished between these two domains; however, four studies did not ( Brehmer et al., 2011 ; Zinke et al., 2012 ; Simon et al., 2018 ; Weicker et al., 2018 ), and four studies addressed verbal working memory only ( Heinzel et al., 2014a , b ; Tusch et al., 2016 ; Matysiak et al., 2019 ). Three of the 16 included studies (18.8%) investigated direct training effects (i.e., effects in trained tasks) only ( Heinzel et al., 2014a , b ; Matysiak et al., 2019 ). The majority of studies (62.5%) investigated a combination of direct training, near-transfer (i.e., untrained working memory tasks), and several far-transfer measures, defined as secondary outcomes in our systematic review. Frequently investigated far-transfer cognitive domains were executive functions (including verbal fluency, reasoning, inhibition, set shifting, and executive control), processing speed (short-term), memory, and fluid intelligence. Only one study investigated non-cognitive outcomes (anxiety and depression) ( McAvinue et al., 2013 ). Only three of the included studies (18.8%) did not apply a prognostic approach for at least one direct training outcome and instead focused on near- and far-transfer effects only ( McAvinue et al., 2013 ; Tusch et al., 2016 ; Simon et al., 2018 ). Most studies used objective and standardized neuropsychological assessment tools. Others, for example, studies assessing (verbal) working memory by n-back tasks (25%), compared n-back task levels within different points of time or used indexes from signal detection theory ( Heinzel et al., 2014a , b ; Tusch et al., 2016 ; Matysiak et al., 2019 ) (for details on prediction analyses and outcomes, see Table 3 and Supplementary Material 4 ).
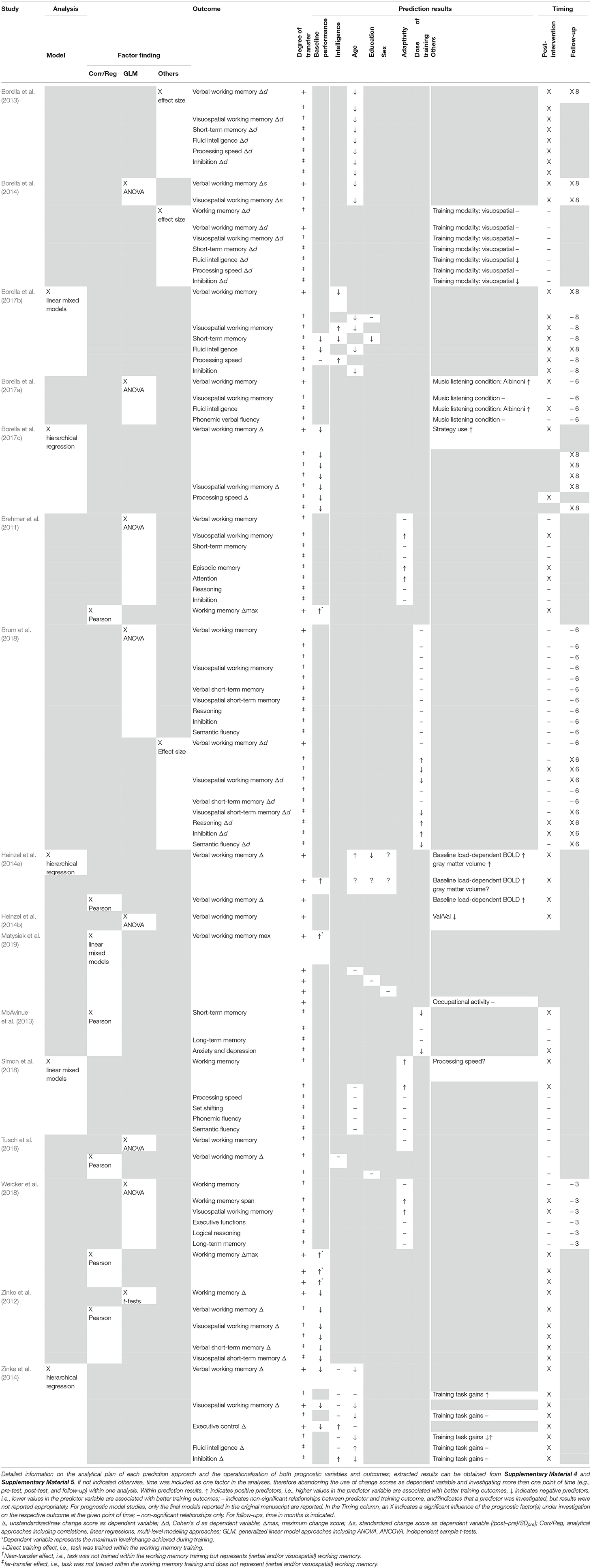
Table 3 . Prognostic analyses, outcomes, results, and timing.
Predictor Variables and Prediction Results
Several different predictors for WMT responsiveness were investigated, including individual-related sociodemographic factors (e.g., age, sex, education), cognitive variables (baseline performance, intelligence, processing speed), and biological factors (genes, brain metabolism) as well as training-related factors (e.g., adaptivity, dose of training). There were 13 analysis approaches that investigated individual-related prognostic factors only, two analysis approaches investigated a combination of individual- and training-related characteristics, and eight analysis approaches investigated training-related characteristics only as predictors for WMT responsiveness. The results of the prognostic analyses are reported in Table 3 . As in most cases, the direction of predictor effects did not vary systematically between single-outcome variables, and within prognostic factor finding vs. prognostic model studies, we decided to not further distinguish between different outcome variables and prognostic factor finding vs. prognostic model studies but indicate if prognostic effects were found for direct training and/or near- and/or far-transfer effects only. The described patterns of prognostic effects only reflect frequencies of observed prognostic relationships and do not take into account risk of bias and further methodological shortcomings of the underlying studies.
Age was investigated in four of five prognostic model studies and three of 18 factor finding approaches. With only few exceptions for single-outcome measures reporting positive or non-significant relationships, age was consistently found to be a negative predictor for WMT responsiveness across direct training as well as both near- and far-transfer effects, i.e., younger participants benefited more from the training than older participants independent of outcome measures. Note, however, that age as a continuous variable was dichotomized into young-olds vs . old-olds for three analytical approaches investigating age as a predictor for WMT responsiveness ( Borella et al., 2013 , 2014 ; Simon et al., 2018 ).
Education was investigated within two prognostic model and two factor finding approaches. Education most frequently constituted a negative predictor for direct training as well as near- and far-transfer effects ( Heinzel et al., 2014a ; Borella et al., 2017b ); however, some analyses do not yield a significant relationship at all ( Tusch et al., 2016 ; Matysiak et al., 2019 ). Whereas education was treated as a continuous variable in most studies, Matysiak et al. (2019) dichotomized the variable for their analysis. Sex was investigated in one prognostic model and one factor finding approach but was not found to be a significant predictor of WMT responsiveness in direct training effects ( Heinzel et al., 2014a ; Matysiak et al., 2019 ) and was not investigated in any prognostic approach on near- and/or far-transfer measures.
Baseline performance in working memory tasks and/or outcome measures was the most frequently investigated prognostic factor (four of five prognostic model studies and five of 18 factor finding approaches). For both near- and far-transfer outcomes, baseline working memory and/or baseline performance in outcome measure was consistently found to be a negative predictor for WMT responsiveness ( Zinke et al., 2012 ; Borella et al., 2017b , c ), i.e., individuals with lower performance at baseline improved more from WMT than individuals with higher baseline performance. However, for analyses on direct training effects, heterogeneous results appear, with some analyses indicating baseline working memory and/or baseline performance in outcome measure to be a positive predictor ( Brehmer et al., 2011 ; Heinzel et al., 2014a ; Weicker et al., 2018 ; Matysiak et al., 2019 ), i.e., individuals with higher baseline performance in training tasks achieving higher WMT task gains than individuals with lower baseline performance. Baseline performance, as a continuous variable, was dichotomized into high vs . low performers by median split in two of the analytical approaches ( Zinke et al., 2012 ; Matysiak et al., 2019 ).
Intelligence was investigated within two of five prognostic model studies and one of 18 factor finding approaches. For direct transfer effects, the prognostic value remains unclear ( Zinke et al., 2014 ; Borella et al., 2017b ). Furthermore, whereas there does not seem to be a significant predictive value when intelligence is investigated as a prognostic factor for near-transfer effects ( Zinke et al., 2014 ; Tusch et al., 2016 ) or when evidence points to different prognostic directions ( Borella et al., 2017b ), a different pattern emerges for far-transfer effects: if significant, for the majority of far-transfer effect outcomes, the analyses indicate intelligence to be a positive predictor of gains after WMT ( Zinke et al., 2014 ; Borella et al., 2017b ), i.e., individuals with higher intelligence show larger far-transfer effects after targeted WMT than individuals with lower intelligence.
In the only study (prognostic model and prognostic factor) investigating a functional imaging parameter as predictor for WMT gains, individuals with a blood oxygen level-dependent (BOLD) response pattern more similar to younger adults (i.e., higher load-dependent network Delta scores) showed higher direct WMT gains ( Heinzel et al., 2014a ). Only one study investigated a genetic factor, yielding carriers of the Val/Val catechol-O-methyltransferase (COMT) genotype to show less direct training effects after WMT than the carriers of any Met COMT genotype ( Heinzel et al., 2014b ).
With regard to training-related prognostic factors, the prognostic effects of dose of training (investigated within two studies) were mixed for both near- and far-transfer effects ( McAvinue et al., 2013 ; Brum et al., 2018 ), only marginally comparable between studies because of different prognostic factor operationalizations and not investigated for direct training effects. Adaptivity was investigated within four studies and, if significant, showed to be a positive predictor for WMT responsiveness ( Brehmer et al., 2011 ; Simon et al., 2018 ; Weicker et al., 2018 ), with adaptive training regimes yielding better results than non-adaptive training regimes, especially for near-transfer effects.
This systematic review is the first one to evaluate prognostic factors and models for WMT responsiveness in healthy older adults. Within the 16 studies meeting our inclusion criteria, five prognostic model approaches and 18 factor finding approaches were included. One of the main findings is that the methodological and reporting quality of prognostic research within the evaluation of WMT regimes in healthy older adults is often insufficient; therefore, no meta-analysis could be conducted and no clear conclusions can be drawn from the systematic review. Age, education, intelligence, and baseline performance in working memory or other cognitive domains were frequently investigated predictors across studies. However, given the methodological shortcomings of the included studies, emerging patterns of prognostic effects across direct training as well as near- and far-transfer effects will have to survive sound methodological replication in future attempts to promote precision medicine approaches in the context of WMT.
First, our findings will be discussed within the methodological framework of prognostic research; secondly, they will be related to the theoretical framework of cognitive aging and embedded into other prognostic research literature in the field of cognitive interventions, and thirdly, they will be linked to findings from a prognostic review on memory trainings in healthy older adults ( Roheger et al., 2020 ).
Methodological Considerations
Several methodological considerations and implications can be derived from the present systematic review. First of all, it has confirmed that prognostic research in the area of WMT in healthy older adults is not yet fully established and is rather premature. The prognostic framework is usually not indexed, and the specific mention of the prognostic approach in titles or abstracts is limited as well ( Riley et al., 2019 ). For example, within our included studies, only five studies used a prediction-related terminology in their titles ( Heinzel et al., 2014a , b ; Zinke et al., 2014 ; Borella et al., 2017b ; Matysiak et al., 2019 ).
Furthermore, large heterogeneity appears throughout the included studies with regard to study design (e.g., randomized controlled trials vs. cohort studies vs. post hoc analyses) and the applied analytical approaches. The applied analytical approaches did not only differ widely per se but have differing suitability to answer the question “who benefits most” from WMT regimes in healthy older adults. In general, a prognostic factor is defined as any measure that, among people with a given condition, is associated with a subsequent outcome ( Riley et al., 2013 ), therefore implying at least some kind of a causal relationship. The majority of studies in our systematic review, however, used group comparisons (e.g., by ANOVA, t -test, comparison of effect sizes) to investigate the influence of a group characteristic on a given outcome. Despite the fact that these approaches can only state whether the compared groups differ from one another and not whether the investigated group characteristic linearly correlate with or even causally predict the investigated outcome, another important point needs to be highlighted: Whereas some investigated prognostic factors are innately categorical (e.g., sex, training modality, adaptivity), originally continuous predictors (e.g., age, baseline performance) were frequently dichotomized into artificial groups, for example, young-olds vs. old-olds ( Borella et al., 2013 , 2014 ; Simon et al., 2018 ) and high vs. low performers ( Zinke et al., 2012 ; Matysiak et al., 2019 ). Dichotomization of both dependent and independent variables is strongly discouraged as it results in loss of information, possible misunderstandings of actual continuous relationships, and severe loss of power ( Dawson and Weiss, 2012 ; Moreau et al., 2016 ; Fernandes et al., 2019 ).
Another frequently used analytical approach was the computation of correlation coefficients between predictor variables and change scores in outcome measures after WMT. However, no causal interferences can be derived from correlation analyses ( Bewick et al., 2003 ). Furthermore, correlations, for example, between baseline performance and change scores (which is obtained by subtracting baseline performance from post-training performance), are less more than pure statistical artifacts ( Smoleń et al., 2018 ). Smoleń et al. (2018 ) discuss that, unfortunately, even more advanced methods such as multiple regressions and linear mixed models do not guarantee the correct assessment of relationships between predictor variables and respective outcomes. According to the authors, the only correct method would be to use direct modeling of correlations between latent true measures and gain by structural equation modeling ( Smoleń et al., 2018 ). Future research on prognostic factors regarding (working memory) training responsiveness should apply advanced statistical methods such as latent difference score models or growth curve analyses as highly flexible statistical approaches from the structural equation modeling background. On the one hand, this would allow to circumvent several statistical fallacies clinical trial data often include, such as violations of multivariate normality assumptions, non-linear change trajectories, and missing data patterns ( Newsom, 2015 ). On the other hand, it would allow to explore the (statistical) properties of change through training without actually calculating change scores and with highly flexible options to model interdependencies between several variables ( Smoleń et al., 2018 ).
In this context, one immense problem arises within prognostic research on cognitive intervention programs per se and WMT in particular: the lack of statistical power due to small sample sizes. Prognostic research requires large sample sizes, with a representative distribution of individuals' characteristics and values across the prognostic factors of interest. Especially for (cognitive) training studies, researchers are confronted with the challenge to overcome a self-selection bias to not only engage highly educated, active, and motivated individuals within their trials ( Oswald et al., 2006 ; Schubert et al., 2014 ). As prognostic research in this field often arises as an (explorative) add-on or post hoc analysis of former data from randomized controlled trials, sample size calculations at the stage of study design (if present at all) do only take into account the sample size needed to evaluate the effectiveness of a training regime (by comparing the experimental group against at least one control group). For future research in the field of personalized prevention and treatment approaches for healthy aging, we encourage to emphasize the outstanding importance of prognostic research by focusing on the prognostic aim already during study design.
Importantly, as already discussed in the introduction, prognostic analyses should always include data of at least one control group as well to detangle predictors of specific treatment response from general prognostic factors of retest effects such as practice effects and regression to the mean ( Hingorani et al., 2013 ). None of the studies included in this systematic review followed this recommendation. Therefore, the identified prognostic relationships might represent systematic relationships; however, they might exist in both treated and untreated individuals and, therefore, not represent true predictors of treatment response.
Beyond that, however, the large body of data on WMT effectiveness for healthy older adults bears the enormous potential of post hoc prognostic analyses, for example, as executed by Borella et al. (2017b) . Within the tradition of evaluating similar WMT regimes, over the years several randomized controlled trials to investigate the efficacy of similar training regimes were carried out in this study group. As Borella et al. (2017b) recognized large variability in the effectiveness of WMT across individuals on the one hand and large heterogeneity across results on earlier investigations on the influence of individual characteristics on training outcomes on the other hand, they merged the data of four earlier training studies ( Borella et al., 2010 , 2013 , 2017c ; Carretti et al., 2013 ) to investigate an individual's characteristics related to WMT gains in a larger sample. In other words, they conducted a tiny-scale individual participant data (IPD) meta-analysis, the gold standard for meta-analytical approaches. At this point, it should be noted that Borella et al. (2017b) included data of participants from the training groups of Borella et al. (2013) and Borella et al. (2017c) , two studies included in our systematic review as well. Therefore, the prognostic results of these three studies are not fully independent. However, we did not exclude the two earlier works, as the exclusion would not have changed the results on the (qualitative) directional prognostic effects. For future IPD meta-analysis, the IPD data of either the four mentioned studies or Borella et al. (2017b) should be included only.
Regarding the analytical approaches used and the results of this review, it should further be mentioned that the recommendation to focus on adjusted results to reveal whether a certain index factor contributes independently and above other prognostic factors ( Riley et al., 2019 ) could not be met entirely: most of the included studies in this review investigated only one prognostic factor per analysis. However, as established prognostic factors did not (yet) exist in the context of WMT responsiveness, analytical approaches excluding possibly important confounding variables are (at least in parts) comprehensible as well. For future prognostic research in this field, however, we recommend to include baseline performance and age as a minimum set of control variables when investigating further prognostic factors.
Prognostic Factors for Working Memory Training Responsiveness
Several different predictors for WMT responsiveness were investigated, including individual-related sociodemographic factors (e.g., age, sex, education), cognitive variables (baseline performance, intelligence), biological factors (brain metabolism, genes) as well as training-related factors (e.g., adaptivity, dose of training). Given the methodological shortcomings of the included studies discussed above, no clear conclusions regarding prognostic effects can be drawn. Emerging patterns based on frequently observed prognostic effects will have to survive sound methodological replication in future attempts to promote precision medicine approaches in the context of WMT. Some inconsistent findings might be due to statistical and psychometric artifacts, uncontrolled extraneous influences, or the absence of convincing robust prognostic relationships at all. Nevertheless, we would like to provide a contextual framework for the discussion of possible predictors for WMT responsiveness beyond pure methodological issues.
The most frequently investigated predictor was baseline performance. Despite the many different statistical approaches and poor reporting quality in most studies, baseline performance was, with exceptions for direct training effects only ( Brehmer et al., 2011 ; Heinzel et al., 2014a ; Weicker et al., 2018 ; Matysiak et al., 2019 ), identified as a negative predictor, i.e., individuals with lower baseline performance are the ones that benefit most from WMT in terms of performance on neuropsychological tests in the domains of working memory and other cognitive functions (e.g., executive functions, short-term memory). Therefore, most inconsistencies regarding the directionality of the prognostic effect of baseline performance could be elucidated when taking a look at the operationalization of the dependent variables. The finding of baseline performance being a negative predictor for cognitive intervention responsiveness is also common for targeted memory trainings ( Roheger et al., 2020 ) as well as other cognitive intervention approaches such as multidomain cognitive trainings ( Whitlock et al., 2012 ; López-Higes et al., 2018 ; Roheger et al., 2019 ). However, opposing findings exist as well, indicating that higher baseline performance might be indicative for cognitive intervention success ( Fairchild et al., 2013 ; Willis and Caskie, 2013 ). However, given the lack of comparisons of prognostic factors between WMT and control groups within the included studies, the frequently observed negative associations between baseline performance and change through training might simply represent effects of regression to the mean ( Smoleń et al., 2018 ). This statistical artifact causes negative correlations between baseline performance and gain by noisy repeated measurements, where extreme values at the first point of time tend to be closer to the mean at the second point of time, without reflecting a real change ( Smoleń et al., 2018 ).
Nevertheless, baseline performance as a predictor for training responsiveness can be discussed within the compensation vs. magnification framework ( Lövdén et al., 2010 , 2012 ). Following this account, individuals with lower baseline performance would show higher training benefits because they have more room for improvement, whereas individuals with higher baseline performance already perform at ceiling, leaving less room for improvement. Improvements across individuals performing less optimal at baseline might therefore represent some kind of flexibility rather than plasticity. According to Lövdén et al. (2010 ), flexibility represents “the capacity to optimize the brain's performance within current structural constraints, using the available range of existing representational states.” Beyond this flexibility, plasticity denotes the capacity for extending the range of representational states, where flexibility then operates. This understanding of plasticity, however, fits better with the magnification hypothesis, constituting that individuals with higher cognitive abilities would benefit most, as they have more resources “to acquire, implement, and sharpen effortful cognitive strategies” ( Lövdén et al., 2012 ).
Within our systematic review, we also found hints for this dualism between compensation vs. magnification or rather flexibility vs. plasticity. Whereas, our findings regarding baseline performance in neuropsychological test measures might rather reflect mechanisms following the compensation account, our findings regarding age as a possibly negative predictor and intelligence as a possibly positive predictor for WMT responsiveness are more interpretable in terms of the magnification account. Higher (crystallized) intelligence might constitute the required “hardware” to utilize the possibilities given by WMT to extend the cognitive repertoire and, in the broadest sense, reflecting cognitive plasticity. This perspective is strengthened considering our finding that intelligence seems to be a positive predictor for gains after WMT for far-transfer effects only. Whereas, lower baseline performance might be predictive for both near- and far-transfer effects (interpreted in terms of the compensation account and flexibility: if there is room for improvement, performance will be optimized by training), higher cognitive abilities might be especially beneficial for far-transfer effects, i.e., to transfer direct training effects to untrained cognitive domains. The magnification account might additionally be able to explain our finding that baseline performance in trained tasks sometimes emerged as a positive predictor for direct training effects. As most WMT regimes adapted their difficulty to user performance across the course of training and no ceiling effects could be expected, higher initial levels might represent general cognitive ability rather than task-specific baseline, and participants with higher initial levels in training tasks might be more able to utilize the whole potential of the training regime.
The second most frequently investigated predictor was age, indicating that older individuals might benefit less from WMT than younger individuals, even within the cohort of healthy older adults above the age of 55. Age might be a proxy for the course of the interplay between neural and cognitive plasticity, which yields a higher potential for plastic changes in younger age than in old-old age ( Burke and Barnes, 2006 ; Greenwood and Parasuraman, 2010 ; Li, 2013 ). Due to age-related reductions in processing resources ( Park and Bischof, 2013 ; Paraskevoudi et al., 2018 ), the ability to engage in plastic changes after WMT might be reduced in older age. This was already reflected in an early meta-analysis on moderators of memory training effects ( Verhaeghen et al., 1992 ). However, findings in contemporary cognitive intervention literature diverge and either report no significant relationship ( Willis and Caskie, 2013 ; Roheger et al., 2019 ), positive relationships (i.e., the older the individual, the more benefits) ( Brooks et al., 1999 ), or negative relationships (i.e., the younger the individual, the more benefits) ( Fairchild et al., 2013 ). In terms of differential prognostic effects for different training regimes (e.g., WMT vs. memory training), this will be further discussed below.
The only study investigating brain imaging parameters as predictors for WMT responsiveness strengthens the finding of our systematic review that age might be a negative predictor for positive training responsiveness: Heinzel et al. (2014a) found a more “youth-like” BOLD response pattern in healthy older adults to be predictive of increased working memory performance after training. This youth-like response pattern is reflected in a higher load-dependent working memory network Delta score, indicating that both high working memory network efficiency (represented by decreased activation during low-level tasks) and high working memory network capacity (represented by increased activation during high-level tasks) are related to plasticity ( Barulli and Stern, 2013 ). This BOLD response pattern has also been discussed as a biomarker for cognitive reserve ( Stern, 2009 ). Against this backdrop, one could hypothesize that cognitive reserve and brain reserve constitute higher-order predictors for WMT success and are operationalized by several different proxies within the existing prognostic research approaches ( Stern et al., 2018 ).
Within the cognitive reserve framework, it is not uncommon to find education alone as a proxy for this construct ( Stern, 2002 ; Valenzuela and Sachdev, 2006 ; Stern et al., 2018 ). In our systematic review, we found a tendency of education being a negative predictor of WMT responsiveness. In cognitive intervention research, it is discussed that cognitive interventions might be able to diminish the cognitive reserve disadvantage of less-educated older adults ( Clark et al., 2016 ; Mondini et al., 2016 ), thereby leading to more training-related gains. As this might appear counterintuitive at first, it is important here to differentiate between brain reserve and lifetime proxies of cognitive reserve such as education, occupational attainment, and leisure time activities ( Stern et al., 2019 ). A higher cognitive reserve is commonly associated with less cognitive deficits, given the same brain pathology ( Wilson et al., 2013 ; Hoenig et al., 2019 ). It follows that two individuals with similar cognitive functioning but different educational backgrounds might also differ in their brain pathology, i.e., the individual with higher education might already show a higher level of brain pathology compared to the individual with lower education, which in turn comes down to lower levels of brain reserve for individuals with higher education. Therefore, for the individual with lower education, even though the lifetime cognitive reserve is lower, the brain reserve might be higher, which corresponds to a better hardware to adapt training benefits.
Only one study investigated a genetic factor as predictor for WMT responsiveness in healthy older adults ( Heinzel et al., 2014b ), revealing carriers of the Val/Val COMT genotype, which is associated with reduced prefrontal dopamine metabolism, to benefit less from WMT than carriers of any Met COMT genotype. The COMT genotype affects prefrontal dopamine metabolism, which is itself related to cognitive plasticity (higher prefrontal dopamine metabolism = more cognitive plasticity) ( Frias et al., 2005 ; Diamond, 2007 ). Furthermore, previous research indicated that advantageous dopamine-related genes are critically involved in working memory performance and the ability to benefit from WMT ( Brehmer et al., 2009 ; Bellander et al., 2011 ; Bäckman and Nyberg, 2013 ), which further strengthens the finding of Heinzel et al. (2014b) that these relationships are also present in healthy older adults.
We did not find a consistent influence of sex on responsiveness to WMT in healthy older adults, even though some kind of “sex-specific plasticity” and following sex-specific differences between training responsiveness to different cognitive domains are proposed in literature ( Beinhoff et al., 2008 ; Rahe et al., 2015 ; Roheger et al., 2019 ). Note, however, that sex as a prognostic factor for WMT responsiveness was investigated in two studies with direct training effects as dependent variable only. Therefore, no final conclusions can be drawn. Even though motivational factors and personality traits are discussed to play a significant role in predicting responsiveness to general cognitive interventions ( Colquitt et al., 2000 ; West et al., 2008 ; Studer-Luethi et al., 2012 ; Double and Birney, 2016 ; Kalbe et al., 2018 ), they were not yet investigated as prognostic factors within the WMT context.
Summarizing possible prerequisites for WMT responsiveness, we hypothesize that there has to be not only room for improvement (i.e., lower baseline performance) to engage in training-related cognitive flexibility but also sufficient “hardware” (e.g., age, intelligence, brain metabolism, genetic variation) to engage in training-related cognitive and neural plasticity. It needs to be highlighted again that the body of evidence (so far) is too weak to draw clear conclusions. Even though some findings fit well into the compensation vs. magnification account and the cognitive reserve framework, future studies of high methodological quality will have to replicate those findings.
Regarding dose of training as one training-related prognostic factor investigated in the context of WMT responsiveness, results were mixed and are in accordance with heterogeneous results in literature. For example, Teixeira-Santos et al. (2019) identified shorter compared to longer training durations to be beneficial for training outcome. However, they discuss this finding to be unexpected and influenced by confounding factors such as the type of outcome variable and highly heterogeneous training durations that impede comparability between studies. All of the included studies in our review implemented an adaptive training regime, where the task difficulty adapted to user performance. Four studies compared adaptive vs. non-adaptive WMT regimes, with adaptivity emerging as a positive predictor for training responsiveness. Adaptivity of trained task difficulty is discussed to contribute to the maintenance of training motivation and the avoidance of underchallenging and overstraining participants during training ( Weicker et al., 2016 ). However, some studies did not find beneficial effects of implementing individually adaptive training regimes ( von Bastian and Eschen, 2016 ).
Only one study within our systematic review used a multi-domain training. Zinke et al. (2014) included an executive control task next to several working memory tasks within their WMT regime. Executive control might, however, strongly be dependent on working memory ( Chai et al., 2018 ). Even though we cannot evaluate the contribution of single training tasks or the training of single domains to the overall prognostic effects, we conclude that this exception from targeted WMT does not constitute a danger for the validity of our findings regarding WMT responsiveness.
Working Memory Training vs. Memory Training
Just recently, a systematic review on prognostic factors of memory improvements after memory training using a similar systematic review technique has been published ( Roheger et al., 2020 ). Roheger et al. (2020) identified further methodological shortcomings of prognostic research in the context of memory training and, on a content-related level, more vulnerable individuals (e.g., lower baseline performance, higher age) to benefit most from memory training. They also identified several “hardware” factors (e.g., hippocampal volume, genetic variation in apolipoprotein-E-4) as prognostic factors. Primarily, however, the direction of age as a prognostic factor seems to differ between the two training regimes.
We hypothesize this difference to be due to the different cognitive training approaches investigated. Memory training, as investigated by Roheger et al. (2020) , can be referred to as a strategy-based training, whereas WMT can be referred to as a process-based training ( Lustig et al., 2009 ; Teixeira-Santos et al., 2019 ). Whereas strategy-based trainings focus on the application of specific strategies to a task where the target population typically does poorly, process-based trainings focus on tasks that load on a specific cognitive function, however, without explicit strategy training ( Lustig et al., 2009 ). Thereby, process-based trainings are believed to produce more transfer effects to untrained domains, as untrained cognitive functions might depend on the targeted cognitive domain ( Lustig et al., 2009 ; Teixeira-Santos et al., 2019 ). This difference in the conceptualization of memory training vs. WMTs, however, implicates different levels of cognitive demands that have to be met in order to benefit from the trainings. Given the higher cognitive demands of WMT, we hypothesize that younger individuals might benefit more, as their hardware potential to engage in neural and cognitive plasticity is higher. Older individuals, however, might be less able to engage in neural plasticity but might therefore rather benefit from strategy-based training approaches, optimizing their cognitive performance within a given structural constraint in terms of flexibility ( Lövdén et al., 2010 , 2012 ). In the framework of Lövdén et al. (2012 ), WMT gains equal practice gains that are related to plasticity and better fit the magnification model, whereas memory training gains equal instruction gains that are related to flexibility and better fit the compensation account. Further research is necessary to prove this concept, but we are convinced that these findings highlight the urgent need for personalized cognitive prevention and intervention methods to counteract cognitive decline at best for every individual.
Another systematic review and meta-analysis on prognostic factors and models of cognitive and behavioral changes after multidomain cognitive training in healthy older adults is still ongoing (preliminary Prospero ID 147531). Those findings, in combination with the findings of the present systematic review and of Roheger et al. (2020) , will further contribute to the understanding of which cognitive interventions yield best outcomes for which individual. Furthermore, the discussion around precision medicine in the context of cognitive interventions can be taken to a whole new level if one would not only consider the cognitive domain trained (or the combination of domains) but also the nature of the training tasks, the training setting (e.g., computerized vs . paper–pencil vs . mixed, home-based vs . individual vs . group settings), and its intensity. So far, however, the body of data is too small for subgroup analyses.
Strengths and Limitations
This systematic review is, to the authors' best knowledge, the first one to systematically assess prognostic factors and models for WMT responsiveness in healthy older adults on a single-person-within-study level rather than investigating moderating factors in a meta-analysis on a study-wide aggregated level as done in a recent meta-analysis on WMT in healthy older adults ( Teixeira-Santos et al., 2019 ). Further strengths include the applied methods following the PICOTS system to define our review question, the CHARMS checklist for data extraction, and the PRISMA guidelines for the reporting of systematic reviews ( Moher et al., 2009 ; Moons et al., 2014 ; Debray et al., 2017 ; Riley et al., 2019 ). One limitation is that, due to insufficient reporting quality throughout many of the included studies, the studies in their entirety were sometimes difficult to comprehend, information might be misinterpreted by the reviewers, and results should be interpreted cautiously. It follows that as already discussed above, due to methodological heterogeneity, we were not able to perform a quantitative meta-analysis but had to focus on the qualitative directionality of the prognostic effects, limiting the validity of our findings. Furthermore, the applied WMT regimes within our included studies were highly heterogeneous regarding training duration, training tasks, and training setting. Only a multi-level IPD meta-analysis might be able to appropriately investigate the interplay of training-related and individual characteristics to answer the question “who benefits most.” Additionally, the analyses to identify predictors of WMT responsiveness were conducted with data of the WMT groups only. Therefore, they did not control for effects in the control group ( Hingorani et al., 2013 ), which impedes disentangling predictors of WMT responsiveness from predictors of retest and practice effects ( Calamia et al., 2012 ). In this context, we need to admit that on the design stage of this systematic review, no comparator factor (C in PICOTS) was being considered as our aim was to systematically assess any approach to prognostic research on WMT responsiveness. Furthermore, even though the risk of bias assessment followed the QUIPS checklist ( Hayden et al., 2013 ) across six domains, the overall rating procedure across the items of one domain and across the six domains is not standardized by the developers.
To summarize, prognostic research within the evaluation of WMT regimes in healthy older adults is still underrepresented given the urgent need for personalized cognitive prevention and intervention methods to counteract cognitive decline. Given the methodological shortcomings of the included studies, no clear conclusions can be drawn, and emerging patterns of prognostic effects will have to survive sound methodological replication in future attempts to promote precision medicine approaches in the context of WMT. However, within the small body of evidence and despite the complex relationships between cognitive reserve, neural plasticity, and different proxies for these constructs, it seems that the requirements for both, flexibility and plasticity, have to be met. An IPD meta-analysis might be able to overcome the current research gaps regarding prognostic factors for WMT responsiveness in healthy older adults.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author/s.
Author Contributions
AO, MR, and EK conceptualized the presented work. MR conducted the systematic search. NS contributed to the systematic search. AO, MR, and A-KF conducted the title and abstract screening. AO and MR conducted the full text screening, extracted the data, and conducted the risk of bias assessment. AO drafted the first version of the manuscript. EK supervised the project during each stage of work. All authors revised the manuscript for intellectual content and approved the final version of the manuscript.
This publication was funded by the Brandau-Laibach-Stiftung, Germany. No additional funding by funding agencies in the public, commercial, or not-for-profit sectors was obtained.
Conflict of Interest
AO reports no conflicts of interest. MR has received a grant from the Brandau-Laibach Stiftung, and a grant from the German Ministry of Education and Research. A-KF has received a grant from the German Parkinson Society, and honoraria from ProLog Wissen GmbH, Cologne, Germany, pro audito Switzerland, Zürich, Switzerland, Seminar- und Fortbildungszentrum Rheine, Germany, and LOGOMANIA, Fendt & Sax GbR, Munich, Germany. A-KF was author of the cognitive training program NEUROvitalis but receives no corresponding honoraria. EK has received grants from the German Ministry of Education and Research, ParkinsonFonds Deutschland gGmbH, the German Parkinson Society and honoraria from Oticon GmbH, Hamburg, Germany, Lilly Pharma GmbH, Bad Homburg, Germany, Bernafon AG, Bern, Switzerland, and Desitin GmbH, Hamburg, Germany. EK was author of the cognitive training program NEUROvitalis but receives no corresponding honoraria.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.575804/full#supplementary-material
Aprahamian, I., Martinelli, J. E., Neri, A. L., and Yassuda, M. S. (2010). The accuracy of the Clock Drawing Test compared to that of standard screening tests for Alzheimer's disease: Results from a study of Brazilian elderly with heterogeneous educational backgrounds. Int. Psychoger. 22, 64–71. doi: 10.1017/S1041610209991141
PubMed Abstract | CrossRef Full Text | Google Scholar
Au, J., Sheehan, E., Tsai, N., Duncan, G. J., Buschkuehl, M., and Jaeggi, S. M. (2015). Improving fluid intelligence with training on working memory: a meta-analysis. Psychon. Bull. Rev . 22, 366–377. doi: 10.3758/s13423-014-0699-x
Bäckman, L., and Nyberg, L. (2013). Dopamine and training-related working-memory improvement. Neurosci. Biobehav. Rev. 37, 2209–2219. doi: 10.1016/j.neubiorev.2013.01.014
Baltes, P. B., and Lindenberger, U. (1988). On the range of cognitive plasticity in old age as a function of experience: 15 years of intervention research. Behav. Ther . 19, 283–300. doi: 10.1016/S0005-7894(88)80003-0
CrossRef Full Text | Google Scholar
Barulli, D., and Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn. Sci. 17, 502–509. doi: 10.1016/j.tics.2013.08.012
Beinhoff, U., Tumani, H., Brettschneider, J., Bittner, D., and Riepe, M. (2008). Gender-specificities in Alzheimer's disease and mild cognitive impairment. J. Neurol . 255, 117–122. doi: 10.1007/s00415-008-0726-9
Bellander, M., Brehmer, Y., Westerberg, H., Karlsson, S., Fürth, D., Bergman, O., et al. (2011). Preliminary evidence that allelic variation in the LMX1A gene influences training-related working memory improvement. Neuropsychologia 49, 1938–1942. doi: 10.1016/j.neuropsychologia.2011.03.021
Berkowitz, C., Mosconi, L., Scheyer, O., Rahman, A., Hristov, H., and Isaacson, R. (2018). Precision medicine for alzheimer's disease prevention. Healthcare 6:82. doi: 10.3390/healthcare6030082
Bewick, V., Cheek, L., and Ball, J. (2003). Statistics review 7: correlation and regression. Critical Care 7:451. doi: 10.1186/cc2401
Borella, E., Carbone, E., Pastore, M., De Beni, R., and Carretti, B. (2017b). Working memory training for healthy older adults: the role of individual characteristics in explaining short-and long-term gains. Front. Hum. Neurosci . 11:99. doi: 10.3389/fnhum.2017.00099
Borella, E., Carretti, B., Cantarella, A., Riboldi, F., Zavagnin, M., and De Beni, R. (2014). Benefits of training visuospatial working memory in young–old and old–old. Dev. Psychol . 50:714. doi: 10.1037/a0034293
Borella, E., Carretti, B., Meneghetti, C., Carbone, E., Vincenzi, M., Madonna, J. C., et al. (2017a). Is working memory training in older adults sensitive to music? Psychol. Res . 83, 1107–1123. doi: 10.1007/s00426-017-0961-8
Borella, E., Carretti, B., Riboldi, F., and De Beni, R. (2010). Working memory training in older adults: evidence of transfer and maintenance effects. Psychol. Aging 25:767. doi: 10.1037/a0020683
Borella, E., Carretti, B., Sciore, R., Capotosto, E., Taconnat, L., Cornoldi, C., et al. (2017c). Training working memory in older adults: Is there an advantage of using strategies? Psychol. Aging 32:178. doi: 10.1037/pag0000155
Borella, E., Carretti, B., Zanoni, G., Zavagnin, M., and De Beni, R. (2013). Working memory training in old age: an examination of transfer and maintenance effects. Arch. Clin. Neuropsychol. 28, 331–347. doi: 10.1093/arclin/act020
Brehmer, Y., Rieckmann, A., Bellander, M., Westerberg, H., Fischer, H., and Bäckman, L. (2011). Neural correlates of training-related working-memory gains in old age. Neuroimage 58, 1110–1120. doi: 10.1016/j.neuroimage.2011.06.079
Brehmer, Y., Westerberg, H., Bellander, M., Fürth, D., Karlsson, S., and Bäckman, L. (2009). Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett . 467, 117–120. doi: 10.1016/j.neulet.2009.10.018
Brooks, J. O., Friedman, L., Pearman, A. M., Gray, C., and Yesavage, J. A. (1999). Mnemonic training in older adults: effects of age, length of training, and type of cognitive pretraining. Int. Psychogeriatr. 11, 75–84. doi: 10.1017/S1041610299005608
Brum, P. S., Borella, E., Carretti, B., and Sanches Yassuda, M. (2018). Verbal working memory training in older adults: an investigation of dose response. Aging Ment. Health 24, 81–91. doi: 10.1080/13607863.2018.1531372
Burke, S. N., and Barnes, C. A. (2006). Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40. doi: 10.1038/nrn1809
Bürki, C. N., Ludwig, C., Chicherio, C., and de Ribaupierre, A. (2014). Individual differences in cognitive plasticity: an investigation of training curves in younger and older adults. Psychol. Re. 78, 821–835. doi: 10.1007/s00426-014-0559-3
Calamia, M., Markon, K., and Tranel, D. (2012). Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin. Neuropsychol . 26, 543–570. doi: 10.1080/13854046.2012.680913
Carretti, B., Borella, E., and De Beni, R. (2007). Does strategic memory training improve the working memory performance of younger and older adults? Exp. Psychol. 54, 311–320. doi: 10.1027/1618-3169.54.4.311
Carretti, B., Borella, E., Zavagnin, M., and de Beni, R. (2013). Gains in language comprehension relating to working memory training in healthy older adults. Int. J. Geriatr. Psychiatry 28, 539–546. doi: 10.1002/gps.3859
Carretti, B., Mammarella, I. C., and Borella, E. (2012). Age differences in proactive interference in verbal and visuo-spatial working memory. J. Cognit. Psychol. 24, 243–255. doi: 10.1080/20445911.2011.603695
Chai, W. J., Abd Hamid, A. I., and Abdullah, J. M. (2018). Working memory from the psychological and neurosciences perspectives: a review. Front. Psychol . 9:401. doi: 10.3389/fpsyg.2018.00401
Chiu, H.-L., Chu, H., Tsai, J.-C., Liu, D., Chen, Y.-R., Yang, H.-L., et al. (2017). The effect of cognitive-based training for the healthy older people: a meta-analysis of randomized controlled trials. PLoS ONE 12:e0176742. doi: 10.1371/journal.pone.0176742
Clark, D. O., Xu, H., Unverzagt, F. W., and Hendrie, H. (2016). Does targeted cognitive training reduce educational disparities in cognitive function among cognitively normal older adults? Int. J. Geriatr. Psychiatry 31, 809–817. doi: 10.1002/gps.4395
Colquitt, J. A., LePine, J. A., and Noe, R. A. (2000). Toward an integrative theory of training motivation: a meta-analytic path analysis of 20 years of research. J. Appl. Psychol. 85:678. doi: 10.1037/0021-9010.85.5.678
Cornoldi, C., Bassani, C., Berto, R., and Mammarella, N. (2007). Aging and the intrusion superiority effect in visuo-spatial working memory. Aging Neuropsychol. Cogn. 14, 1–21. doi: 10.1080/138255890969311
Dawson, N. V., and Weiss, R. (2012). Dichotomizing continuous variables in statistical analysis: a practice to avoid. Med. Decis. Making 32, 225–226. doi: 10.1177/0272989X12437605
De Beni, R., Borella, E., Carretti, B., Marigo, C., and Nava, L. (2008). BAC. Portfolio per la Valutazione del Benessere e delle Abilità Cognitive nell'età adulta e Avanzata [The Assessment of Well-being and Cognitive Abilities in Adulthood and Aging]. Firenze: Giunti, OS.
Google Scholar
Deary, I. J., Corley, J., Gow, A. J., Harris, S. E., Houlihan, L. M., Marioni, R. E., et al. (2009). Age-associated cognitive decline. Br. Med. Bull . 92, 135–152. doi: 10.1093/bmb/ldp033
Debray, T. P., Damen, J. A., Snell, K. I., Ensor, J., Hooft, L., Reitsma, J. B., et al. (2017). A guide to systematic review and meta-analysis of prediction model performance. BMJ 356:i6460. doi: 10.1136/bmj.i6460
Diamond, A. (2007). Consequences of variations in genes that affect dopamine in prefrontal cortex. Cereb. Cortex 17, i161–i170. doi: 10.1093/cercor/bhm082
Double, K. S., and Birney, D. P. (2016). The effects of personality and metacognitive beliefs on cognitive training adherence and performance. Pers. Individ. Dif . 102, 7–12. doi: 10.1016/j.paid.2016.04.101
Fairchild, J., Friedman, L., Rosen, A., and Yesavage, J. (2013). Which older adults maintain benefit from cognitive training? Use of signal detection methods to identify long-term treatment gains. Int. Psychogeriatr. 25, 607–616. doi: 10.1017/S1041610212002049
Fernandes, A., Malaquias, C., Figueiredo, D., da Rocha, E., and Lins, R. (2019). Why quantitative variables should not be recoded as categorical. J. Appl. Math. Phys. 7:1519. doi: 10.4236/jamp.2019.77103
Folstein, M.F., Folstein, S.E., White, T., and Messer, M.A. (2010). Mini Mental State Examination, 2nd Edn . Lutz: Psychological Assessment Resources, Inc.
Frias, C. M. d., Annerbrink, K., Westberg, L., Eriksson, E., Adolfsson, R., et al. (2005). Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J. Cogn. Neurosci . 17, 1018–1025. doi: 10.1162/0898929054475136
Gevins, A., and Cutillo, B. (1993). Spatiotemporal dynamics of component processes in human working memory. Electroencephal. Clin. Neurophys. 87, 128–143. doi: 10.1016/0013-4694(93)90119-G
Greenwood, P. M., and Parasuraman, R. (2010). Neuronal and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Front. Aging Neurosci . 2:150. doi: 10.3389/fnagi.2010.00150
Hayden, J. A., van der Windt, D. A., Cartwright, J. L., Côt,é, P., and Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Ann. Intern. Med . 158, 280–286. doi: 10.7326/0003-4819-158-4-201302190-00009
Heinzel, S., Lorenz, R. C., Brockhaus, W.-R., Wüstenberg, T., Kathmann, N., Heinz, A., et al. (2014a). Working memory load-dependent brain response predicts behavioral training gains in older adults. J. Neurosci. 34, 1224–1233. doi: 10.1523/JNEUROSCI.2463-13.2014
Heinzel, S., Riemer, T. G., Schulte, S., Onken, J., Heinz, A., and Rapp, M. A. (2014b). Catechol-O-methyltransferase (COMT) genotype affects age-related changes in plasticity in working memory: a pilot study. Biomed Res. Int . 2014:414351. doi: 10.1155/2014/414351
Hingorani, A. D., van der Windt, D. A., Riley, R. D., Abrams, K., Moons, K. G., Steyerberg, E. W., et al. (2013). Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 346:e5793. doi: 10.1136/bmj.e5793
Hoenig, M. C., Bischof, G. N., Onur, Ö. A., Kukolja, J., Jessen, F., Fliessbach, K., et al. (2019). Level of education mitigates the impact of tau pathology on neuronal function. Eur. J. Nucl. Med. Mol. Imaging 46, 1787–1795. doi: 10.1007/s00259-019-04342-3
Jaeggi, S. M., Buschkuehl, M., Jonides, J., and Perrig, W. J. (2008). Improving fluid intelligence with training on working memory. PNAS 105, 6829–6833. doi: 10.1073/pnas.0801268105
Kalbe, E., Bintener, C., Ophey, A., Reuter, C., Göbel, S., Klöters, S., et al. (2018). Computerized cognitive training in healthy older adults: baseline cognitive level and subjective cognitive concerns predict training outcome. Health 10:20. doi: 10.4236/health.2017.101003
Kliegel, M., Rott, C., d'Heureuse, V., Becker, G., and Schönemann, P. (2001). Demenz im höchsten Alter ist keine Notwendigkeit. Ergebnisse der Heidelberger Hundertjährigen-Studie. Z Gerontopsychol Psychiatr 14, 169–180. doi: 10.1024//1011-6877.14.4.169
Klingberg, T., Forssberg, H., and Westerberg, H. (2002). Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 14, 1–10. doi: 10.1162/089892902317205276
Li, S.-C. (2013). Neuromodulation and developmental contextual influences on neural and cognitive plasticity across the lifespan. Neurosci. Biobehav. Rev. 37, 2201–2208. doi: 10.1016/j.neubiorev.2013.07.019
López-Higes, R., Martín-Aragoneses, M. T., Rubio-Valdehita, S., Delgado-Losada, M. L., Montejo, P., Montenegro, M., et al. (2018). Efficacy of cognitive training in older adults with and without subjective cognitive decline is associated with inhibition efficiency and working memory span, not with cognitive reserve. Front. Aging Neurosci . 10:23. doi: 10.3389/fnagi.2018.00023
Lövdén, M., Bäckman, L., Lindenberger, U., Schaefer, S., and Schmiedek, F. (2010). A theoretical framework for the study of adult cognitive plasticity. Psychol. Bull . 136, 659–679. doi: 10.1037/a0020080
Lövdén, M., Brehmer, Y., Li, S.-C., and Lindenberger, U. (2012). Training-induced compensation versus magnification of individual differences in memory performance. Front. Hum. Neurosci . 6:141. doi: 10.3389/fnhum.2012.00141
Lustig, C., Shah, P., Seidler, R., and Reuter-Lorenz, P. A. (2009). Aging, training, and the brain: a review and future directions. Neuropsychol. Rev . 19, 504–522. doi: 10.1007/s11065-009-9119-9
Matysiak, O., Kroemeke, A., and Brzezicka, A. (2019). Working memory capacity as a predictor of cognitive training efficacy in the elderly population. Front. Aging Neurosci . 11:126. doi: 10.3389/fnagi.2019.00126
McAvinue, L. P., Golemme, M., Castorina, M., Tatti, E., Pigni, F. M., Salomone, S., et al. (2013). An evaluation of a working memory training scheme in older adults. Front. Aging Neurosci . 5:20. doi: 10.3389/fnagi.2013.00020
Melby-Lervåg, M., Redick, T. S., and Hulme, C. (2016). Working memory training does not improve performance on measures of intelligence or other measures of “far transfer” evidence from a meta-analytic review. Perspect. Psychol. Sci. 11, 512–534. doi: 10.1177/1745691616635612
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med . 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Mondini, S., Madella, I., Zangrossi, A., Bigolin, A., Tomasi, C., Michieletto, M., et al. (2016). Cognitive reserve in dementia: implications for cognitive training. Front. Aging Neurosci . 8:84. doi: 10.3389/fnagi.2016.00084
Moons, K. G., de Groot, J. A., Bouwmeester, W., Vergouwe, Y., Mallett, S., Altman, D. G., et al. (2014). Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 11:e1001744. doi: 10.1371/journal.pmed.1001744
Moons, K. G., Royston, P., Vergouwe, Y., Grobbee, D. E., and Altman, D. G. (2009). Prognosis and prognostic research: what, why, and how? BMJ 338:b375. doi: 10.1136/bmj.b375
Moreau, D., Kirk, I. J., and Waldie, K. E. (2016). Seven pervasive statistical flaws in cognitive training interventions. Front. Hum. Neurosci . 10:153. doi: 10.3389/fnhum.2016.00153
Nelson, H. (1982). National Adult Reading Test Manual . Windsor: NFER-Nelson.
PubMed Abstract | Google Scholar
Newsom, J. T. (2015). Longitudinal Structural Equation Modeling: A Comprehensive Introduction. Routledge: Taylor & Francis Group.
Noack, H., Lövdén, M., Schmiedek, F., and Lindenberger, U. (2009). Cognitive plasticity in adulthood and old age: gauging the generality of cognitive intervention effects. Restor. Neurol. Neurosci . 27, 435–453. doi: 10.3233/RNN-2009-0496
Oswald, W. D., Gunzelmann, T., Rupprecht, R., and Hagen, B. (2006). Differential effects of single versus combined cognitive and physical training with older adults: the SimA study in a 5-year perspective. Eur. J. Ageing 3:179. doi: 10.1007/s10433-006-0035-z
Paraskevoudi, N., Balci, F., and Vatakis, A. (2018). “Walking” through the sensory, cognitive, and temporal degradations of healthy aging. Ann. N. Y. Acad. Sci . 1426, 72–92. doi: 10.1111/nyas.13734
Parish, A., Kim, J., Lewallen, K. M., Miller, S., Myers, J., Panepinto, R., et al. (2019). Knowledge and perceptions about aging and frailty: an integrative review of the literature. Geriatr. Nurs. 40, 13–24. doi: 10.1016/j.gerinurse.2018.05.007
Park, D. C., and Bischof, G. N. (2013). The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci . 15, 109–119. doi: 10.1016/B978-0-12-380882-0.00007-3
Rahe, J., Liesk, J., Rosen, J. B., Petrelli, A., Kaesberg, S., Onur, O. A., et al. (2015). Sex differences in cognitive training effects of patients with amnestic mild cognitive impairment. Aging Neuropsychol. Cogn. 22, 620–638. doi: 10.1080/13825585.2015.1028883
Reitz, C. (2016). Toward precision medicine in Alzheimer's disease. Ann. Transl. Med. 4:107. doi: 10.21037/atm.2016.03.05
Riley, R. D., Hayden, J. A., Steyerberg, E. W., Moons, K. G., Abrams, K., Kyzas, P. A., et al. (2013). Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med . 10:e1001380. doi: 10.1371/journal.pmed.1001380
Riley, R. D., Moons, K. G., Snell, K. I., Ensor, J., Hooft, L., Altman, D. G., et al. (2019). A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 364:k4597. doi: 10.1136/bmj.k4597
Roheger, M., Folkerts, A.-K., Krohm, F., Skoetz, N., and Kalbe, E. (2020). Prognostic factors for change in memory test performance after memory training in healthy older adults: a systematic review and outline of statistical challenges. Diagn. Progn. Res . 4:7. doi: 10.1186/s41512-020-0071-8
Roheger, M., Meyer, J., Kessler, J., and Kalbe, E. (2019). Predicting short-and long-term cognitive training success in healthy older adults: who benefits? Aging Neuropsychol. Cogn . 27, 351–369. doi: 10.1080/13825585.2019.1617396
Sala, G., Aksayli, N. D., Tatlidil, K. S., Gondo, Y., and Gobet, F. (2019). Working memory training does not enhance older adults' cognitive skills: a comprehensive meta-analysis. Intelligence 77:101386. doi: 10.1016/j.intell.2019.101386
Salthouse, T. A. (1990). Working memory as a processing resource in cognitive aging. Dev. Rev. 10, 101–124. doi: 10.1016/0273-2297(90)90006-P
Schubert, T., Strobach, T., and Karbach, J. (2014). New directions in cognitive training: on methods, transfer, and application. Psychol. Res . 78, 749–755. doi: 10.1007/s00426-014-0619-8
Shulman, K. I. (2000). Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 15, 548–561. doi: 10.1002/1099-1166(200006)15:6<548::AID-GPS242>3.3.CO;2-L
Simon, S. S., Tusch, E. S., Feng, N. C., Hakansson, K., Mohammed, A. H., and Daffner, K. R. (2018). Is computerized working memory training effective in healthy older adults? Evidence from a multi-site, randomized controlled trial. J. Alzheimers Dis. 65, 931–949. doi: 10.3233/jad-180455
Smoleń, T., Jastrzebski, J., Estrada, E., and Chuderski, A. (2018). Most evidence for the compensation account of cognitive training is unreliable. Mem. Cognit . 46, 1315–1330. doi: 10.3758/s13421-018-0839-z
Soveri, A., Antfolk, J., Karlsson, L., Salo, B., and Laine, M. (2017). Working memory training revisited: a multi-level meta-analysis of n-back training studies. Psychon. Bull. Rev . 24, 1077–1096. doi: 10.3758/s13423-016-1217-0
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Stern, Y., Barnes, C. A., Grady, C., Jones, R. N., and Raz, N. (2019). Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging 83, 124–129. doi: 10.1016/j.neurobiolaging.2019.03.022
Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., et al. (2018). Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dement. doi: 10.1016/j.jalz.2018.07.219
Studer-Luethi, B., Jaeggi, S. M., Buschkuehl, M., and Perrig, W. J. (2012). Influence of neuroticism and conscientiousness on working memory training outcome. Pers. Individ. Dif . 53, 44–49. doi: 10.1016/j.paid.2012.02.012
Teixeira-Santos, A. C., Moreira, C. S., Magalhães, R., Magalhães, C., Pereira, D. R., Leite, J., et al. (2019). Reviewing working memory training gains in healthy older adults: A meta-analytic review of transfer for cognitive outcomes. Neurosci. Biobehav. Rev . 103, 163–177. doi: 10.1016/j.neubiorev.2019.05.009
Turner, M. L., and Engle, R. W. (1989). Is working memory capacity task dependent? J. Mem. Lang. 28, 127–154. doi: 10.1016/0749-596X(89)90040-5
Tusch, E. S., Alperin, B. R., Ryan, E., Holcomb, P. J., Mohammed, A. H., and Daffner, K. R. (2016). Changes in neural activity underlying working memory after computerized cognitive training in older adults. Front. Aging Neurosci . 8:255. doi: 10.3389/fnagi.2016.00255
Valenzuela, M. J., and Sachdev, P. (2006). Brain reserve and cognitive decline: a non-parametric systematic review. Psychol. Med . 36, 1065–1073. doi: 10.1017/S0033291706007744
Verhaeghen, P., Marcoen, A., and Goossens, L. (1992). Improving memory performance in the aged through mnemonic training: a meta-analytic study. Psychol. Aging 7:242. doi: 10.1037/0882-7974.7.2.242
von Bastian, C. C., and Eschen, A. (2016). Does working memory training have to be adaptive? Psychol. Res . 80, 181–194. doi: 10.1007/s00426-015-0655-z
Wechsler, D. (1981). Wechsler Adult Intelligence Scale-revised manual . New York, NY: Psychological Corporation.
Weicker, J., Hudl, N., Frisch, S., Lepsien, J., Mueller, K., Villringer, A., et al. (2018). WOME: theory-based working memory training—a placebo-controlled, double-blind evaluation in older adults. Front. Aging Neurosci . 10:247. doi: 10.3389/fnagi.2018.00247
Weicker, J., Villringer, A., and Thöne-Otto, A. (2016). Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 30, 190–212. doi: 10.1037/neu0000227
West, R. L., Bagwell, D. K., and Dark-Freudeman, A. (2008). Self-efficacy and memory aging: the impact of a memory intervention based on self-efficacy. Aging Neuropsychol. Cogn. 15, 302–329. doi: 10.1080/13825580701440510
Whitlock, L. A., McLaughlin, A. C., and Allaire, J. C. (2012). Individual differences in response to cognitive training: using a multi-modal, attentionally demanding game-based intervention for older adults. Comput. Human Behav . 28, 1091–1096. doi: 10.1016/j.chb.2012.01.012
Willis, S. L., and Caskie, G. I. (2013). Reasoning training in the ACTIVE study: how much is needed and who benefits? J. Aging Health 25 (Suppl. 8), 43S–64S. doi: 10.1177/0898264313503987
Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Schneider, J. A., and Bennett, D. A. (2013). Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. doi: 10.1212/WNL.0b013e31829c5e8a
Wolff, R. F., Moons, K. G., Riley, R. D., Whiting, P. F., Westwood, M., Collins, G. S., et al. (2019). PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med . 170, 51–58. doi: 10.7326/M18-1376
Zinke, K., Zeintl, M., Eschen, A., Herzog, C., and Kliegel, M. (2012). Potentials and limits of plasticity induced by working memory training in old-old age. Gerontology 58, 79–87. doi: 10.1159/000324240
Zinke, K., Zeintl, M., Rose, N. S., Putzmann, J., Pydde, A., and Kliegel, M. (2014). Working memory training and transfer in older adults: effects of age, baseline performance, and training gains. Dev. Psychol . 50:304. doi: 10.1037/a0032982
Keywords: prognostic review, systematic review, healthy aging, working memory training, training responsiveness, individual differences
Citation: Ophey A, Roheger M, Folkerts A-K, Skoetz N and Kalbe E (2020) A Systematic Review on Predictors of Working Memory Training Responsiveness in Healthy Older Adults: Methodological Challenges and Future Directions. Front. Aging Neurosci. 12:575804. doi: 10.3389/fnagi.2020.575804
Received: 24 June 2020; Accepted: 26 August 2020; Published: 14 October 2020.
Reviewed by:
Copyright © 2020 Ophey, Roheger, Folkerts, Skoetz and Kalbe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anja Ophey, anja.ophey@uk-koeln.de ; Elke Kalbe, elke.kalbe@uk-koeln.de
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Journal of Cognition

- Download PDF (English) XML (English)
- Alt. Display
- Collection: Theoretical review with commentaries: attention and working memory
Review Article
Working memory and attention – a conceptual analysis and review.
- Klaus Oberauer
There is broad agreement that working memory is closely related to attention. This article delineates several theoretical options for conceptualizing this link, and evaluates their viability in light of their theoretical implications and the empirical support they received. A first divide exists between the concept of attention as a limited resource, and the concept of attention as selective information processing. Theories conceptualizing attention as a resource assume that this resource is responsible for the limited capacity of working memory. Three versions of this idea have been proposed: Attention as a resource for storage and processing, a shared resource for perceptual attention and memory maintenance, and a resource for the control of attention. The first of these three is empirically well supported, but the other two are not. By contrast, when attention is understood as a selection mechanism, it is usually not invoked to explain the capacity limit of working memory – rather, researchers ask how different forms of attention interact with working memory, in two areas. The first pertains to attentional selection of the contents of working memory, controlled by mechanisms of filtering out irrelevant stimuli, and removing no-longer relevant representations from working memory. Within working memory contents, a single item is often selected into the focus of attention for processing. The second area pertains to the role of working memory in cognitive control. Working memory contributes to controlling perceptual attention – by holding templates for targets of perceptual selection – and controlling action – by holding task sets to implement our current goals.
- Working memory
- Cognitive Control
There is a broad consensus that working memory and attention are intimately linked ( Awh, Jonides, & Reuter-Lorenz, 1998 ; Baddeley, 1993 ; Chun, 2011 ; Cowan, 1995 ; Gazzaley & Nobre, 2012 ; Kane, Bleckley, Conway, & Engle, 2001 ; Kiyonaga & Egner, 2014 ; Oberauer, 2009 ; Olivers, 2008 ). But what is it that we agree upon? Both working memory and attention can be conceptualized in different ways, resulting in a broad array of theoretical options for linking them. The purpose of this review is to propose a map for organizing these theoretical options, delineate their implications, and to evaluate the evidence for each of them.
The meaning of the concept working memory (WM) depends on the theory in which the concept figures. The definitions reviewed by Cowan ( 2017 ) differ primarily in the substantive assumptions they include (e.g., whether or not WM consists of multiple storage modules, and to what extent it includes long-term memory). Beyond these differences in theoretical assumptions, however, there is a broad consensus on what the term working memory refers to: The mechanisms and processes that hold the mental representations currently most needed for an ongoing cognitive task available for processing.
The meanings of the term attention are more diverse, as they reflect distinctions not only of definitions but also of different referents of the term: Attention is not a unitary entity ( Chun, Golomb, & Turk-Browne, 2011 ). Conceptualizations of attention can be distinguished along several dimensions that provide a coordinate system for our conceptual map. A first distinction pertains to how attention is defined. One definition of attention characterizes it as a limited resource for information processing (e.g., Wickens, 1980 ). Another concept of attention is as a process of (or mechanism for) selection of information to be processed with priority (e.g., Chun et al., 2011 ; Desimone & Duncan, 1995 ). These two concepts of attention play different roles in theorizing about working memory, and I will discuss them in turn below.
A second distinction pertains to what we attend to. I find it useful to distinguish the possible objects of attention along two dimensions (see Table 1 ). 1 First, we can distinguish between attention to our currently perceived environment (e.g., attention to visual objects or auditory streams) from attention to information currently not perceived, such as attention to remembered episodes or concepts that we think about. 2 Second, we can distinguish between attention to things and events in the world around us on the one hand, and attention to our own goals and (mental or overt) actions on the other. The latter form of attention includes selection of our current goal or task set and shielding it from distraction ( Kane & Engle, 2003 ; Monsell, 2003 ), selection of one of several possible actions ( Pashler, 1994 ), and monitoring of our actions and their outcomes ( Yeung, Botvinick, & Cohen, 2004 ).
A Taxonomy of Attention.
| Controlled | Automatic | |||
|---|---|---|---|---|
| Things/Events | Goals/Actions | Things/Events | Goals/Actions | |
| Perceptual | Selective attention to locations, visual objects, events, features. | Selective attention to ongoing actions, monitoring of action outcomes | Capture of attention by salient stimuli, to stimuli learned to be relevant, or to stimuli in the focus of attention of WM | Capture of attention by errors or unexpected difficulties. |
| Non-Perceptual | Attention to items in WM. | Attention to intended actions: Selection of task sets, response selection. | Involuntary retrieval from long-term memory; intrusive thoughts. | Involuntary retrieval of task sets associated to current stimulus; involuntary selection of response (e.g., Stroop, flanker task) |
Note: Descriptions pertaining to attention as selection/prioritization are printed in regular font; descriptions pertaining to attention as a resource in italics.
A third distinction pertains to the forces that determine what we attend to – this is the distinction between controlled and automatic deployment of attention ( Shiffrin & Schneider, 1977 ). Attention is controlled when it is directed according to our current goals. The influence of current goals on attention is often referred to as “top-down”. Attention is automatic to the extent that its direction is influenced by forces independent of our current goals – these include the “bottom-up” attraction of attention by perceived properties of the stimuli (e.g., their “salience”) as well as influences of our learning history on what we attend to, for instance when attention is drawn to information that we have learned to be relevant ( Awh, Belopolsky, & Theeuwes, 2012 ; Theeuwes, 2018 ).
The concept of executive attention is often used when discussing the relation between attention and working memory. Executive attention is a term that is notoriously poorly defined ( Jurado & Rosselli, 2007 ). It is used on the one hand to refer to attention directed to one’s own goals and (mental or overt) actions, including response selection ( Szmalec, Vandierendonck, & Kemps, 2005 ), action planning, protecting the pursuit of our current goal from distractions and temptations, as well as switching from one task to another. On the other hand, executive attention is also used to refer to the top-down control of attention, including attention to things and events in the environment – for keeping our attention on the relevant stimuli or features and avoiding distraction by irrelevant ones, as in the Stroop task and the flanker task. As such, the term executive attention is used to denote one pole on each of two dimensions in my proposed taxonomy, one pertaining to the objects of attention (things and events in the world vs. our own goals and actions), the other pertaining to what determines the orientation of attention (controlled vs. automatic). The first meaning assigns executive attention a function in controlling our thoughts and actions (including what we attend to) whereas the second states that executive attention is itself controlled. One way to perhaps bring together the two meanings is by assuming that we attend to (i.e., select, assign resources to) our own goals and actions – including the action of attending to some object – in order to control them. Nevertheless, I find the term executive attention disquietingly ambiguous, and therefore will use instead the terms attention to (cognitive) action and controlled attention to refer to the two aspects of executive attention, respectively.
I organize the review by the two definitions of attention – as a resource or as a selection mechanism – because they have different implications for how attention and working memory are related. Within each section I will discuss the different objects of attention, and the different modes of control.
Attention as a Resource
The idea of attention as a resource is that the cognitive system has a limited resource that can be used for carrying out so-called attention-demanding processes. The resource is assumed to be a continuous quantity that can be split arbitrarily and allotted to different processes, depending on task demands. Processing efficiency (i.e., speed, accuracy) is a positive monotonic function of the amount of resource assigned to a process ( Navon & Gopher, 1979 ). The assumption that WM capacity reflects a limited resource has a long tradition ( Anderson, Reder, & Lebiere, 1996 ; Case, 1972 ; Just & Carpenter, 1980 ; Ma, Husain, & Bays, 2014 ). Authors linking WM to an attentional resource are endorsing the view that the limited capacity of WM reflects a limited resource, and that this resource also serves some (or all) functions commonly ascribed to attention. Three versions of this idea can be distinguished by which functions the attentional resource is assumed to be needed for: (1) storage and processing of information (e.g., Just & Carpenter, 1992 ), (2) perceptual attention and memory maintenance (e.g., Ester, Fukuda, May, Vogel, & Awh, 2014 ; Kiyonaga & Egner, 2014 ), or (3) the control of attention (e.g., Allen, Baddeley, & Hitch, 2006 ; Baddeley, 1993 , 1996 ; Lavie, 2005 ).
Attention for Storage and Processing
Many theorists discussing the relation between working memory and attention characterize attention as a limited resource for maintaining representations in an “active”, available state ( Cowan, 2005 ). Often this resource is assumed to be shared between “storage” and “processing” ( Case, Kurland, & Goldberg, 1982 ; Cowan et al., 2005 ; Just & Carpenter, 1992 ). According to this view, the same attentional resource is required for keeping representations available and for carrying out certain basic cognitive processes such as selecting a response to a stimulus. A prediction from this theory is that attention-demanding cognitive processes compete with concurrent storage ( Z. Chen & Cowan, 2009 ).
There are two variants of this theoretical idea. One is that a share of the resource needs to be continuously assigned to a representation to keep it in WM ( Case et al., 1982 ). The other is that attention is required directly only for processing, not storage. In this view attention indirectly contributes to memory maintenance because it is needed for refreshing WM representations, which would otherwise decay ( Barrouillet, Bernardin, & Camos, 2004 ). Barrouillet and colleagues further specify the resource required for refreshing as the limited resource for so-called central processes, such as response selection ( Barrouillet, Bernardin, Portrat, Vergauwe, & Camos, 2007 ). Dual-task studies with variants of the PRP (psychological refractory period) paradigm have established a strong capacity limit on central processes ( Pashler, 1994 ), which has been explained by a limited central-attentional resource ( Navon & Miller, 2002 ; Tombu & Jolicoeur, 2003 ).
Theorists linking WM to attention as resource commonly assume that there is a single, content-general attentional resource. It follows that storage and processing compete with each other whether or not they share any contents. This assumption leads to the prediction of dual-task costs when WM storage and processing demands from very different contents are combined with each other. There is considerable evidence confirming this prediction ( Chein, Moore, & Conway, 2011 ; Morey & Bieler, 2012 ; Saults & Cowan, 2007 ; Vergauwe, Barrouillet, & Camos, 2010 ), lending support to the notion that WM capacity is limited by an attentional resource. There is also evidence that storage and processing compete for central processing capacity: The extent to which maintenance in WM is impaired by concurrent processing is a monotonic function of cognitive load , defined as the proportion of time during which central attention is engaged by the processing demand ( Barrouillet et al., 2007 ).
One problem for the assumption of a shared resource for storage and processing is that, although a memory load reduces the efficiency of concurrent response-selection tasks, that dual-task cost diminishes substantially over the first few seconds of the retention interval ( Jolicoeur & Dell’Acqua, 1998 ; Thalmann, Souza, & Oberauer, 2019 ; Vergauwe, Camos, & Barrouillet, 2014 ), and is often not observed at all when there is an unfilled interval of a few seconds between encoding of the memory set and commencement of the processing task ( Hazeltine & Witfall, 2011 ; Klapp, Marshburn, & Lester, 1983 ; Oberauer, Demmrich, Mayr, & Kliegl, 2001 ). This observation has already led Klapp and colleagues ( 1983 ) to question the idea of a shared resource for storage and processing: To uphold this idea we would have to assume that the resource demand of maintenance dwindles to a negligible level within a few seconds. This would be compatible with the assumption that a central processing resource is required for short-term consolidation of information in working memory ( Jolicoeur & Dell’Acqua, 1998 ; Nieuwenstein & Wyble, 2014 ; Ricker & Hardman, 2017 ) but not with the assumption that a resource is needed for maintenance throughout the retention interval.
As mentioned above, the assumption of shared resources for storage and processing comes in two variants: The first, traditional one is that a representation needs a share of the resource assigned to it to be in WM, and the same resource is needed for carrying out cognitive operations. The second variant is that maintenance processing such as refreshing share a limited resource with other cognitive operations ( Barrouillet et al., 2004 ). The second variant rests on the premise that without refreshing the representations in WM decay – only on that assumption does the processing resource assigned to refreshing become essential for WM maintenance. The decay assumption, however, is probably not true, at least for verbal materials ( Oberauer & Lewandowsky, 2013 , 2014 ).
The first variant has a conceptual problem: Simultaneous maintenance and processing compete for a shared resource only until the processing task is completed – after that, the full resource can be re-assigned to the representations in WM. Why then should memory performance suffer from a concurrent processing task although memory is tested only after the processing task is done? (for a more detailed treatment see Oberauer, Farrell, Jarrold, & Lewandowsky, 2016 ). The problem is illustrated by a study that, according to the authors, reveals the neuronal basis of resource sharing: Watanabe and Funahashi ( 2014 ) recorded from multiple neurons in the lateral pre-frontal cortex (LPFC) while monkeys did a spatial attention task, a spatial WM task, or a dual-task combination of the two. The two tasks recruited largely overlapping LPFC neurons, which showed spatial selectivity when each task was done alone. While both tasks were done simultaneously, the LPFC neurons lost most of their spatial selectivity, and collectively their firing rate pattern contained less information about the attended location and the remembered location during that period. After the attention task was completed, however, the information about the location in memory was “reawakened” in the firing pattern of the LPFC neurons, reaching the same strength as in the single-task condition. The authors did observe a (small) performance decrement in the dual-task relative to the single-task condition, but that dual-task cost is not explained by their neural data – looking at the neural data, we would expect no detrimental effect on memory by the concurrent attention task.
To conclude, the assumption of a shared resource for memory retention and central processes has received much empirical support. At the same time, it is challenged by the finding that dual-task costs on processing speed tend to vanish over time, and – depending on the version endorsed – the lack of evidence for decay, and the problem of how to explain that the competition between processing and storage affects memory performance after the competition has ended.
Attention for Perception and Memory
A resource shared between “storage” and “processing” spans both sides of the distinction between attention to things and events (i.e., the information to be stored), and attention to goals and actions (i.e., to the task sets guiding the processing operations). We can also ask whether the same resource applies to both sides of another distinction, the one between perceptual attention and attention to not-perceived objects. Most task paradigms for studying WM require retention of information in the absence of perceptual input. There is evidence, however, that the limited capacity of WM applies not only to information in memory but equally to information still in view. Tsubomi, Fukuda, Watanabe, and Vogel ( 2013 ) measured the contralateral delay activity (CDA), a neural marker of the number of objects a person holds in visual WM ( Luria, Balaban, Awh, & Vogel, 2016 ; Vogel & Machizawa, 2004 ) while participants attended to a variable number of color patches still in view, or attempted to remember them after their offset. In both cases, the CDA amplitude increased with set size up to about 3 items and then levelled off. Individual CDA amplitudes correlated with performance on a test of one randomly selected item regardless of whether that item remained in view until the time of test or had to be retained in memory for a second.
The study of Tsubomi et al. ( 2013 ) shows striking similarities between the capacity limits for attending to perceptual stimuli and for maintaining stimuli in memory (see also Ester et al., 2014 ). Still, these two functions could rely on separate resources that happen to bear similarities to each other. If the same limited resource underlies perceptual attention and maintenance in WM, then demanding both at the same time should incur a substantial dual-task cost, such that when the load of one task is increased, performance on the other suffers. The evidence for this prediction is mixed. Fougnie and Marois ( 2006 ) found load-dependent dual-task costs when combining a visual WM task with a visual attention task (simultaneous tracking of multiple moving objects, or monitoring multiple parallel streams of rapidly presented visual stimuli for a target) but these costs were less than the cost of combining two visual WM tasks. Souza and Oberauer ( 2017 ) found only negligible dual-task costs when inserting a visual attention task (monitoring a stimulus for a subtle brightness change) in the retention interval of a visual WM task. Several studies investigated dual-task costs between WM and visual search. These dual-task costs increase with the load on each of the two tasks – as expected on the assumption of a shared resource – only when the contents of WM were spatial locations (for a review see Woodman & Chun, 2006 ). To conclude, although attending to perceptual information and maintaining information in WM after it disappeared from the environment have much in common, the evidence that they share a limited resource is not yet convincing.
Controlled Attention
The concept of attention as a limited resource is often linked specifically to controlled attention, whereas automatic attention is thought not to be resource demanding ( Schneider & Shiffrin, 1977 ; Shiffrin & Schneider, 1977 ). There are two ways in which this link can be spelled out: (a) Attention that is allocated in a controlled manner – according to “top down” influences from our current goals – underlies a resource limit but attention that is automatically attracted to some information independent of its relevance for our current goal does not underlie that resource limit. Stated in this way we face the awkward conclusion that allocating attention to the same object (e.g., a red traffic light in a street scene, or a word we hold in WM) does or does not rely on a limited resource depending on what forces led attention to that object. The same cognitive function – prioritizing processing of the attended information – would be resource consuming or not depending on how it was invoked.
In my view, a less awkward interpretation is: (b) Paying attention to an object does not require a resource per se – rather the process of controlling attention in a top-down manner consumes the limited resource. This interpretation reflects how Shiffrin and Schneider ( 1977, p. 156 ) explain why controlled processes are capacity limited: These processes need to be controlled by continuously paying attention to them, and attention cannot be allocated to more than one process at a time. In other words, the attentional resource imposes a bottleneck on the control processes, not on the controlled processes. The limitation is on how many different (cognitive or overt) actions we can attend to at the same time in order to control them. For instance, in visual search, perceptual attention can be drawn to some stimuli automatically, and theoretically there is no limit on how many such forces exert their pull in parallel. Perceptual attention can also be directed in a controlled manner – by attending to the action of deploying attention to visual stimuli – and this control process is limited to one action at a time. The limitation does not rest with the controlled attention – a limit on how many visual stimuli can be attended at the same time – but with the controlling attention.
This conception of an attentional resource differs from the preceding two. The notion of a resource for storage and processing and the idea of a shared attentional resource for perception and memory share the assumption that the resource is allocated to representations of objects and events that we perceive or hold in WM. In contrast, the “attentional control” idea assumes a resource for the control of what we attend to, and more generally, of what we think and do. These conceptualizations have different implications when we apply them to WM. For instance, consider a situation in which WM receives an overload of information, some of which is relevant and some of which is irrelevant. Examples of this scenario are the complex-span paradigm ( Daneman & Carpenter, 1980 ), in which to-be-remembered items alternate with stimuli to be processed but not retained, or the filtering paradigm ( Vogel, McCollough, & Machizawa, 2005 ), in which participants see an array of visual stimuli and need to remember a pre-defined subset (e.g., only the red objects). According to theories assuming a limited resource allocated to representations in WM, attention limits how much of the given information can be retained, and a separate parameter determines the filtering efficiency, that is, the extent to which the cognitive system manages to keep the distractor information out of WM, so that it does not consume part of the valuable storage resource. These theories predict that individuals with lower WM capacity maintain a smaller amount of both relevant and irrelevant information, but their proportion, reflecting filtering efficiency, should be independent of WM capacity. According to the controlled-attention view, by contrast, the attentional resource determines the filtering efficiency. Hence, individuals with lower WM capacity retain the same amount of information as those with higher capacity, but people differing in WM capacity differ in the ratio of relevant to irrelevant information that they retain.
Paradoxes lurk when we try to combine the two notions of attentional resources, assuming that the same limited resource is required for both storage and control: According to this fusion version of the attentional-resource idea, keeping some irrelevant piece of information out of WM, or removing it from WM, consumes attentional resource (because it is an act of control over what we attend to) and at the same time frees up attentional resource (because it reduces the amount of information that is held in WM). In the same manner, stopping a cognitive process costs attentional resource but at the same time frees up attentional resource. With such a conception, it becomes virtually impossible to say whether some cognitive process – such as filtering or deleting information from WM – renders a net cost or a net gain in resource. As a consequence, the theory becomes untestable. This problem needs to be kept in mind when attempts are made to reconcile the two versions of attentional-resource theories of WM (e.g., Cowan, Fristoe, Elliott, Brunner, & Saults, 2006 ). 3
If WM and the control of attention share a limited resource, we should expect substantial dual-task costs when an attention-control demand is combined with WM maintenance. Evidence for such a dual-task cost comes from studies demonstrating that a load on WM increases people’s susceptibility to distraction, for instance by the irrelevant stimuli in a flanker task ( Kelley & Lavie, 2011 ; Lavie, Hirst, de Fockert, & Viding, 2004 ). Interpretation of this result is complicated by the observation that only a verbal WM load increases the flanker effect – a visual WM load has the opposite effect ( Konstantinou, Beal, King, & Lavie, 2014 ; Konstantinou & Lavie, 2013 ). Konstantinou et al. ( 2014 ) explain this dissociation by assuming that visual WM contents place a load on a visual perceptual resource, and increasing the load on perceptual resources has been shown to reduce flanker interference ( Lavie, 2005 ). In contrast, verbal WM relies on rehearsal for maintenance, and rehearsal competes for a shared attentional-control resource with the control of visual attention. The latter assumption is at odds with the position of most other resource theorists, who assume that rehearsal requires little, if any such resource ( Baddeley, 1986 ; Camos, Lagner, & Barrouillet, 2009 ; Cowan, 2001 ). Other studies provide further evidence that a load on WM can both increase and decrease people’s distractability by a flanker stimulus during a perceptual comparison task: When the category of stimuli held in WM matched that of the targets of the comparison task (but not that of the flankers), the flanker compatibility effect increased, but when the WM contents matched the category of the flankers, and not the targets, then the flanker compatibility effect decreased under load compared to no load ( Kim, Kim, & Chun, 2005 ; Park, Kim, & Chun, 2007 ). Taken together, there is no convincing evidence that loading WM depletes a resource needed for the control of attention.
We can also ask whether concurrent demands on the control of attention impair performance in a WM task. This appears not to be the case. The effect of concurrent processing on memory is larger when the processing task requires more attention control (e.g., task switching vs. task repetition, incongruent vs. neutral Stroop trials), but that effect is entirely accounted for by the longer duration of response selection in the more difficult conditions ( Barrouillet, Portrat, & Camos, 2011 ; Liefooghe, Barrouillet, Vandierendonck, & Camos, 2008 ). Hence, the dual-task cost of concurrent processing for memory is a function of the demand on central attention for action selection, not the demand on the control of attention. Moreover, Lawrence, Myerson, Oonk, and Abrams ( 2001 ) found that when people had to make saccades to irrelevant locations during the retention interval, memory performance is impaired, in particular for spatial information. That effect was equally large for reflexive saccades towards a suddenly appearing target and for controlled anti-saccades away from a target, contrary to the assumption that the control of attention in the anti-saccade condition competes for WM resources. Bunting, Cowan, and Colflesh ( 2008 ) used a manual analog of the anti-saccade task as distractor activity during the retention interval, and found significantly worse performance in the anti-press than the pro-press condition in only 3 out of 12 experimental conditions.
A second prediction from the assumption that WM maintenance and controlled attention share a resource is that measures of the efficiency of the two should be correlated across individuals. This prediction has been tested with regard to two forms of control over the contents of WM ( Hasher, Zacks, & May, 1999 ): Filtering irrelevant stimuli at encoding so that they never enter WM, and removal of no-longer relevant stimuli from WM after they have been encoded. Support for the prediction comes from studies measuring filtering efficiency in visual change-detection tasks through the effect of irrelevant stimuli on the CDA ( Vogel et al., 2005 ). Individual differences in filtering efficiency are strongly correlated with accuracy in change detection ( Luria et al., 2016 ). However, when Mall, Morey, Wolff, and Lehnert ( 2014 ) measured filtering efficiency through behavioral indicators – the performance gain from being able to ignore half the stimuli in the array, and the proportion of time people fixated on locations of irrelevant stimuli during encoding and retention – they found no correlation with people’s WM capacity, measured through complex-span tasks. One possible interpretation is that controlled attention (as indexed by filtering) and WM maintenance share a resource that is not domain general but rather specific to visual stimuli. Removal efficiency has been measured through the speed with which people remove to-be-updated information from WM in an updating paradigm ( Ecker, Lewandowsky, & Oberauer, 2014 ). Whereas this first study showed no correlation of removal efficiency with WM capacity, a subsequent study measuring removal efficiency through a larger set of updating tasks observed a small positive correlation ( Singh, Gignac, Brydges, & Ecker, 2018 ). This result could reflect a shared resource for WM maintenance and attentional control. Alternatively, it could mean that people who efficiently remove no-longer relevant information from WM are better at reducing interference from that information in WM, which improves their ability to retrieve the relevant information ( Oberauer, Lewandowsky, Farrell, Jarrold, & Greaves, 2012 ).
Other research investigated the correlation between WM capacity and measures of attentional control outside the context of WM tasks, for instance the ability to attend to relevant and ignore irrelevant stimuli or features in perceptual decision tasks (e.g., the Stroop, flanker, or Simon task), the ability to suppress a strong action tendency (e.g., moving the eyes away from a suddenly appearing stimulus in the anti-saccade task), or the ability to stop an already prepared action (i.e., the stop-signal paradigm). Numerous studies have found positive correlations between WM capacity and these measures of attention control (e.g., Chuderski, 2014 ; McVay & Kane, 2012 ; Shipstead, Lindsey, Marshall, & Engle, 2014 ; Unsworth, 2015 ; Unsworth, Fukuda, Awh, & Vogel, 2014 ), whereas a few others failed to find such a relationship ( Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009 ; Wilhelm, Hildebrandt, & Oberauer, 2013 ). Additional support comes from findings of a positive correlation between WM capacity and people’s self-reported mind wandering in response to thought probes during a cognitive task ( McVay & Kane, 2009 , 2012 ; Randall, Oswald, & Beier, 2014 ).
Taken together, the evidence for a close relation between WM and the control of attention is mixed. The most convincing evidence comes from correlational studies linking WM capacity to indicators of attention control from tasks without a memory demand. There is some evidence that WM capacity is also correlated with the efficiency of controlling the contents of WM through filtering and removal, but it is yet too weak and inconsistent to draw strong conclusions. This correlational evidence, however, can be explained without invoking the notion of a shared resource, as I’ll discuss below (in the section “How is WM related to the control of attention and action?”). The experimental evidence from dual-task costs speaks against competition between WM maintenance and attention control for a shared resource.
I have considered three theoretical options for spelling out the idea of WM as relying on an attentional resource: (1) a shared resource for “storage” and “processing”, (2) a shared resource for perceptual attention and WM, and (3) a shared resource for attention control and WM. Of these three, the first option has received the most convincing empirical support, but it also suffers from empirical challenges, and from the conceptual problem of explaining how the competition for resources between storage and processing can have an impact on memory performance after the competition is over. I do not see these challenges as fatal – it is probably still too early to announce the “demise” ( Klapp et al., 1983 ) of the idea that WM is limited by an attentional resource – but theorists working with this concept should aim to address these challenges. In the remainder of this article I discuss the relation of WM to attention from the perspective that attention is the selection and prioritization of information, which does not entail a commitment to a limited resource.
Attention as Selection
A different perspective on the relation between WM and attention emerges when attention is defined not as a resource but as a mechanism for selecting and prioritizing representations. In this perspective, attention does not explain the capacity limit of WM. Rather, we should consider WM as an instance of attention – specifically, WM is attention to memory representations. Holding a set of representations in WM means selecting them from among all the representations that our mind is capable of, thereby rendering them available as input for cognitive operations. As such, WM meets the definition of attention as a mechanism of selection ( Oberauer, 2009 ). In this perspective, the relationship between the concept of WM and the concept of attention is not an empirical but a conceptual one.
Nevertheless, we can ask several empirical questions about how WM is related to attention as a selection mechanism: (1) How is information selected into WM? (2) How is information selected within WM? (3) What is the relation between attention to memory and attention to perceived stimuli – are they the same, and if not, how do they influence each other? (4) How is WM related to the control of attention and action? I next address these questions in turn.
How is Information Selected into Working Memory?
Information can be selected to be brought into WM from perception or from long-term memory. This selection is to a large extent controlled: People are very good, though not perfect, at letting only relevant information into WM. Moreover, people also have control over which information to keep in WM and which to remove.
Filtering Perceptual Information. With regard to perceived information, perceptual attention arguably plays an important role in selecting which stimuli are encoded into WM. Stimuli that are known to be irrelevant from the start, and are easy to discriminate from relevant stimuli, can be filtered out very effectively ( Baddeley, Papagno, & Andrade, 1993 ), though not always perfectly ( Ueno, Allen, Baddeley, Hitch, & Saito, 2011 ; Vogel et al., 2005 ); children and older adults seem to have more difficulty with filtering irrelevant stimuli at encoding ( Sander, Werkle-Bergner, & Lindenberger, 2011 ). A question discussed in the context of visual WM is whether people can selectively encode relevant features but not irrelevant features of the same visual object. Some experiments show that relevant and irrelevant features of the same object have similar behavioral effects on memory performance ( Marshall & Bays, 2013 ) and attentional capture ( Gao et al., 2016 ; see the section on effects of WM on perceptual attention for an explanation of this effect). However, one fMRI study found that the relevant but not the irrelevant feature of a visual object could be reconstructed from the pattern of BOLD activity during the retention interval ( Yu & Shim, 2017 ). Logie, Brockmole, and Jaswal ( 2011 ) have tested the effects of changes in irrelevant features on change-detection accuracy and found that such changes impair performance for retention intervals up to about 2 s but not thereafter. They propose that irrelevant features are initially encoded and subsequently removed from WM. This could explain why irrelevant features are not detectable in the sluggish BOLD signal that aggregates information over several seconds.
Filtering could be accomplished by perceptual selection – not attending to the irrelevant stimuli – but it could also be a separate selection step, such that a stimulus, even though selected for perceptual attention, is not encoded into WM. The latter possibility would imply that perceptual attention might be necessary, but is not sufficient for encoding them into WM. Evidence for this possibility comes from several sources. A series of experiments by H. Chen and Wyble ( 2015a , 2015b ) used stimuli as attentional cues for a perceptual decision task, and after several trials inserted a surprise memory test for a feature of the cue. Although they have arguably attended to the cue because it was relevant for the decision task, people had poor memory for its features only a few seconds after its disappearance, suggesting that the stimulus, or at least the feature probed in the memory test, was not encoded into WM. When people expected the memory test, their performance was much better. In a related experiment H. Chen, Swan, and Wyble ( 2016 ) had participants visually track several moving target objects among distractors. To avoid confusing the targets with distractors participants had to continuously attend to them while they moved. Yet, in a surprise memory test they had little memory for the target’s colors.
A second source of evidence suggesting that attention is not sufficient to encode stimuli into WM comes from some of my experiments ( Oberauer, 2018 ): Participants saw six words presented one by one in different screen locations; each word was followed by a cue to remember or forget it. The cue appeared only after word offset so that people had to attend to each word in case they would have to remember it. I also varied the time interval between each forget cue and the onset of the next word to manipulate how much time people had to remove a to-be-forgotten word from WM. The to-be-forgotten words had no effect on memory performance regardless of the cue-word interval, implying that they did not contribute at all to the load on WM.
These findings could mean that information, although attended, is not encoded into WM. Alternatively, the visual stimuli of Chen and Wyble, or the to-be-forgotten words in my experiments, could be encoded into WM but then removed very quickly so that their accessibility, and their effect on WM load, was not measurable even a few seconds later (see the section below on Removal). Perhaps neurophysiological markers of WM load with high temporal resolution, such as the CDA, could be leveraged to distinguish between these possibilities.
One limitation for efficient filtering (or removal) arises when people have to process the distracting material. When participants in my experiments ( Oberauer, 2018 ) had to make a judgment on each word while it was on the screen, they could not entirely prevent encoding to-be-forgotten words into WM, though they were still able to diminish their effect on WM load relative to to-be-remembered words. Marshall and Bays ( 2013 ) found that comparing two stimuli during the retention interval of a visual WM task impaired WM performance as much as adding two more stimuli to the memory set, suggesting that encoding of these stimuli into WM could not be prevented at all.
Selective Retrieval from Long-Term Memory. Much of the information we process in WM comes from long-term memory. For the WM system to work effectively, it has to retrieve information from long-term memory selectively, so that only information useful for the current task enters WM ( Oberauer, 2009 ). A demonstration of the effectiveness of this gating mechanism comes from experiments investigating the effect of previously acquired long-term memories on WM performance ( Oberauer, Awh, & Sutterer, 2017 ). We had participants learn 120 associations between everyday objects and randomly selected colors. In a subsequent WM test they had to maintain three object-color conjunctions on each trial, and reproduce each object’s color by selecting it on a color wheel. Some of the objects in the WM test were objects for which they had learned an associated color before. These objects could reoccur in the WM test with their learned color – in which case retrieving the associated color should facilitate WM performance – whereas others reoccurred with a new random color – in which case retrieving the color from long-term memory should interfere with WM performance. We found evidence for proactive facilitation, but against proactive interference, implying that information from long-term memory is used if and only if the information in WM was so poor that drawing on long-term memory could only make things better.
Removal of Information from WM. The selection of which information to hold in WM is also controlled after encoding: Information no longer relevant must be rapidly removed so that it does not clutter WM ( Hasher et al., 1999 ). There is a body of evidence showing that people can selectively remove no-longer relevant information from WM (for a review see Lewis-Peacock, Kessler, & Oberauer, 2018 ).
Removing an entire memory set when replacing it with a new one is a seamless and rapid process, though – as filtering – it is not perfect: Traces of the old memory set remain in WM, creating some mild proactive interference when items in the two sets are similar to each other ( Ralph et al., 2011 ; Tehan & Humphreys, 1998 ), and a congruency benefit when the two sets partially overlap, sharing the same items in the same contexts ( Oberauer, Souza, Druey, & Gade, 2013 ). Removal of a single item from the current memory set has been isolated experimentally as a process involved in WM updating ( Ecker, Oberauer, & Lewandowsky, 2014 ). By contrast, removal is much less efficient when it comes to removing more than one item from a memory set but less than all of them: People find it difficult to remove a random subset of several items from a memory set. For instance, when informed, after encoding a list of six words, that the words in positions 2, 3, and 5 could be forgotten, there was no evidence that they did so – successful removal of a subset of three words was found only when they were already clearly marked as a separate subset at encoding ( Oberauer, 2018 ). In sum, the efficiency of removal is limited by the ability to discriminate between to-be-maintained and to-be-removed contents of WM.
To conclude, the WM system is equipped with very efficient – though not perfect – mechanisms for controlling its contents through filtering perceptual input, selectively retrieving information from LTM, and removing no-longer relevant materials. Through these selection processes the cognitive system manages to usually have only the most relevant information for the current goal in WM.
How is Information selected within WM?
Selecting information to be held in WM is a form of selection, but it not necessarily selection of one piece of information at the exclusion of all others: We often hold multiple separate items in WM simultaneously. Sometimes we have to select a single item from the set currently held in WM as the input for a process, or as the object of mental manipulation. Our ability to select individual items from the set currently held in WM points to a selection mechanism that I refer to as the focus of attention in WM ( Oberauer, 2002 ; Oberauer & Hein, 2012 ). Evidence for the operation of such a narrow selection mechanism within WM comes from three observations: (1) In short-term recognition tests the last-presented item in a list is accessed at a faster rate than preceding items, and this has been interpreted as showing that the last-encoded item remains in the focus of attention (for a review McElree, 2006 ). (2) When an item in WM is needed as input for a cognitive operation (e.g., adding or subtracting a number from a particular digit in WM), or when one item needs to be selected as the object of an updating operation (e.g., replacing an item in WM by a new stimulus), then operating on the same WM item again in the next step takes less time than selecting another item from the memory set for the next operation. This item-switch cost (or item-repetition benefit) has been explained by assuming that the object of a cognitive operation remains in the focus of attention after the operation has been completed, and therefore does not need to be selected again when the same object is required for the next operation ( Garavan, 1998 ; Oberauer, 2003 ). (3) After encoding a set of stimuli into WM, a retro-cue presented one to several seconds into the retention interval can guide attention to one item and thereby improve memory performance when that item is tested – often at the expense of performance when another item is tested ( Griffin & Nobre, 2003 ; Landman, Spekreijse, & Lamme, 2003 ; for a review see Souza & Oberauer, 2016 ).
Whereas most of these empirical demonstrations come from situations in which a single item in WM needs to be selected, it has been argued that the focus of attention can hold more than one item ( Gilchrist & Cowan, 2011 ). From the perspective of attention as selection, this should be feasible to the extent that selecting multiple items simultaneously does not undercut the purpose of selection. For instance, if the task is to update one out of several digits in WM through an arithmetic operation, selecting more than that one digit into the focus of attention would only lead to confusion – but if the task is to add two digits in WM together, selecting both of them into the focus of attention at the same time is arguably useful because then they could be used simultaneously as retrieval cues for the relevant arithmetic fact ( Oberauer, 2013 ). Another situation in which it is functional to select two items into the focus simultaneously is when two tasks must be carried out simultaneously, one on each item, and the two items are sufficiently different to not risk cross-talk between the two tasks ( Göthe, Oberauer, & Kliegl, 2016 ; Oberauer & Bialkova, 2011 ).
Using the retro-cue paradigm, neuroscience research has revealed a distinction between attended and unattended information in WM 4 : Whereas the attended information can be decoded from neural signals such as the pattern of BOLD activity over voxels, or the pattern of EEG activity over electrodes, the unattended information cannot – it remains neurally silent, but can be brought back into a neurally active state later by a retro-cue drawing attention to it ( LaRocque, Lewis-Peacock, Drysdale, Oberauer, & Postle, 2013 ; Lewis-Peacock, Drysdale, Oberauer, & Postle, 2011 ; Sprague, Ester, & Serences, 2016 ) or by an uninformative strong input to the cortex ( Rose et al., 2016 ; Wolff, Jochim, Akyürek, & Stokes, 2017 ). One recent study, however, paints a more differentiated picture: Decoding of orientations maintained in VWM from fMRI signals in visual cortex was again good for attended and absent for unattended items, but decoding from signals in parietal cortex (IPS and frontal eye fields) was equally good for both attended and unattended items – though much weaker than decoding of attended items in visual cortex ( Christophel, Iamshchinina, Yan, Allefeld, & Haynes, 2018 ).
Behavioral evidence shows that retro-cues can be used to select not just individual items but also subsets of several items within WM ( Oberauer, 2001 , 2005 ), and selection of a subset can be followed by selection of an item within that subset ( Oberauer, 2002 ). Therefore, we can distinguish three levels of selection in WM: (1) Selecting information to be in WM, constituting the current memory set, (2) selecting a subset of the memory set, and (3) selecting a single item from that subset. I have referred to these three levels as (1) the activated part of long-term memory, (2) the region of direct access, and (3) the focus of attention, respectively (see Oberauer, 2009 , for a detailed discussion of the 3-level framework and evidence supporting it; and Oberauer et al., 2013 , for a computational implementation). It is currently not clear whether more than one WM representation is neurally active (i.e., decodable from neural activity during the retention interval) at the same time, so we do not know whether the state of being neurally active characterizes the second or the third level of selection. One possibility is that during WM maintenance multiple representations – those in the direct-access region – are active at the same time, such that their pattern of neural activity is superimposed. Another possibility is that only one item – the one in the focus of attention – is neurally active at any time. If the focus of attention circulates among the items in WM, it would still be possible to decode several items from neural activation patterns ( Emrich, Rigall, LaRocque, & Postle, 2013 ) because the temporal resolution of decoding from BOLD signals is lower than the speed at which the focus of attention shifts from one item to another (i.e., about 300 ms; Oberauer, 2003 ).
Univariate neural correlates of WM load, most notably the amplitude of the CDA ( Vogel & Machizawa, 2004 ) and the BOLD activation in the inter-parietal sulcus (IPS) ( Todd & Marois, 2004 , 2005 ; Xu & Chun, 2006 ), imply that at least some form of persistent neural activity increases with the number of items maintained in WM. These neural measures, however, do not carry information about the content of WM, and therefore we do not know whether they reflect neurally active representations or some neural activity reflecting control processes that are involved in maintaining items selected. Another open question is whether these univariate measures of WM load reflect the first or the second level of selection – to find out we need studies that track these neural indicators of WM load while a retro-cue asks participants to select a subset of the current memory set: Does the neural marker track the set size of the subset or of the entire memory set? One study asking this question found that BOLD activation in IPS reflects the size of the entire memory set before the retro-cue but the size of the cued subset afterwards ( Lepsien, Thornton, & Nobre, 2011 ), suggesting that IPS activation reflects the second level of selection, the direct-access region. In that study, however, participants were not asked to still maintain the not-cued subset in memory, so we don’t know whether they maintained it (at the third selection level, the activated part of LTM) or just removed it from WM.
A somewhat speculative hypothesis on how to reconcile all these findings is that univariate markers of WM load track the amount of information selected at the second level (i.e., the direct-access region). This information is maintained in WM through temporary bindings between contents and contexts through which they are accessible, probably in parietal cortex. These bindings are neurally silent – either because they are implemented through rapid synaptic plasticity ( Mongillo, Barak, & Tsodyks, 2008 ) or because they are implemented in a pattern of neural activity that bears no similarity to the bound contents, such as a circular convolution of each content with its context ( Eliasmith, 2013 ; Plate, 2003 ), so that they cannot be identified through decoding of the WM contents. However, neural activity patterns corresponding to the contents of the direct-access region could be re-activated during the retention interval by feeding non-specific activation into the contexts that act as retrieval cues for these contents, so that they could (faintly) be decoded from parietal cortical areas ( Bettencourt & Xu, 2016 ; Christophel et al., 2018 ). This non-specific activation could be spontaneous noise in the neural network ( Oberauer & Lin, 2017 ), or an attentional mechanism that selectively activates all contexts to which the contents of the direct-access region are bound. The content (or contents) selected for the third level of selection, the focus of attention, is represented in a neurally active fashion, probably in the prefrontal cortex ( Bichot, Heard, DeGennaro, & Desimone, 2015 ; Mendoza-Halliday & Martinez-Trujillo, 2017 ), and this representation re-activates the corresponding sensory representation in those sensory cortical areas involved in its initial processing, so that the information in the focus of attention can be decoded from neural activity in those areas.
A prediction from this hypothesis is that when two to-be-remembered stimuli are presented sequentially, univariate markers such as the CDA should add up to reflect the combined load of both stimuli, whereas the decodability of the first stimulus should be substantially impaired by the encoding of the second, because the focus of attention abandons the first to encode the second stimulus. Evidence for the first assumption comes from studies showing that the CDA reflects the combined load of two successively presented parts of a memory set ( Feldmann-Wüstefeld, Vogel, & Awh, 2018 ; Ikkai, McCollough, & Vogel, 2010 ); the second prediction remains to be tested.
What is the Relation between WM and Perceptual Attention?
An extreme position would be that WM and perceptual attention are the same: By virtue of attending to a perceived stimulus, it is selected into WM. Maintaining stimuli in WM that are no longer present in the environment differs from perceptual attention only in the absence of the physical stimulus. The cognitive state is still the same, with the only difference that the representation in WM is arguably weaker and less precise due to the lack of informative sensory input. This extreme position is attractive due to its parsimony, but it is almost certainly wrong. We have already seen that perceptual attention to stimuli during the retention interval of a visual WM task leads to less interference than adding the same stimuli to WM ( Fougnie & Marois, 2006 ). I have also discussed instances where stimuli were attended to and yet they leave hardly any trace in WM (H. Chen et al., 2016 ; H. Chen & Wyble, 2015a , 2015b ; Oberauer, 2018 ). Moreover, single-cell recordings from monkey LPFC neurons showed partial but not complete overlap between the neurons responding selectively to a feature while it is perceptually attended and those doing so while the feature is being held in WM ( Mendoza-Halliday & Martinez-Trujillo, 2017 ). If we accept that perceptual attention and WM are different entities, we can meaningfully ask how they causally affect each other.
How does perceptual attention affect WM? Some authors have argued that perceptual attention can be used to rehearse visual or spatial WM contents. The evidence for this idea is mixed. Some studies found a correlation between spontaneous eye movements during the retention interval – which presumably track visual attention – and recall success for sequences of spatial locations ( Tremblay, Saint-Aubin, & Jalberg, 2006 ), but no such correlation was found for change detection in visual arrays ( Williams, Pouget, Boucher, & Woodman, 2013 ). Directing people to attend to individual items in a visual array improves memory for those items relative to not-attended items in the array ( Souza, Rerko, & Oberauer, 2015 ; Souza, Vergauwe, & Oberauer, 2018 ). However, it is not clear whether this effect relies on perceptual attention. Engaging perceptual attention by a secondary task during the retention interval (i.e., detection of a slight brightness change in the fixation cross) impaired performance in a visual change-detection task ( Williams et al., 2013 ), but had at best a negligible effect on errors in a visual continuous-reproduction task, whereas engaging central attention impaired continuous reproduction more severely ( Souza & Oberauer, 2017 ).
As discussed above in the section on Filtering, perceptual attention is probably necessary but not sufficient for encoding of stimuli into WM. Yet, filtering is not perfect, so that attended information is sometimes encoded into WM to some extent even when this is not desired. To the extent that this happens, we can expect that distractors presented during the retention interval of a WM task interfere with the to-be-remembered information, thereby impairing memory performance.
Evidence for such interference comes from studies of spatial WM. Van der Stigchel, Merten, Meeter, and Theeuwes ( 2007 ) found that recall of locations is biased towards the location of a suddenly appearing irrelevant stimulus on the screen, suggesting that this stimulus was inadvertently encoded into WM. Lawrence, Myerson, and Abrams ( 2004 ) had participants identify and compare two symbols during the retention interval of a WM task, which either appeared at fixation or in the periphery (left or right of fixation). When the symbols appeared in the periphery, spatial (but not verbal) WM performance was impaired more than for centrally displayed symbols. This suggests that attending to additional locations entails encoding these locations into WM to some degree, thereby interfering with memory for other locations. The interfering effect was stronger when participants were instructed to move their eyes to the peripheral symbols than when they were instructed to maintain fixation, in line with other findings showing that processing distractors enforces stronger encoding into WM than merely attending to them ( Oberauer, 2018 ). Both studies unfortunately lack a control condition in which irrelevant stimuli are presented but not attended, so it is not clear how much perceptual attention contributes to their encoding into WM.
Does attending to a stimulus in the environment distract the focus of attention from information in WM? Two observations indicate that it might not: The beneficial effect of a retro-cue directing the focus of attention to one item in WM is not diminished by a subsequent task engaging perceptual attention ( Hollingworth & Maxcey-Richard, 2013 ; Rerko, Souza, & Oberauer, 2014 ). Likewise, the object-repetition benefit in a spatial WM updating task was not diminished by requiring people to focus visual attention on a stimulus in the periphery in between updating steps ( Hedge, Oberauer, & Leonards, 2015 ). However, the retro-cue effect probably arises in part from strengthening of the cued item’s binding to its context, and this effect lasts after the focus of attention has moved away from the cued item ( Rerko et al., 2014 ; Souza et al., 2015 ). The same could be true for the object-repetition benefit: The item to be updated is selected into the focus of attention, and this strengthens the item’s binding to its context as a side effect, leaving that item temporarily more accessible than other items even if the focus of attention moves away from it. Evidence suggesting that attending to perceptual stimuli does distract the focus of attention comes from studies using multivariate neural signals to read out the information in the pattern of neural activity. The decodability of a single item in WM is drastically diminished – at least temporarily – by the onset of an irrelevant stimulus, or just by the person attending to a location in anticipation of a stimulus, during the retention interval ( Bettencourt & Xu, 2016 ; van Moorselaar et al., 2017 ). However, in these studies the irrelevant stimulus hardly affected memory performance. Therefore, an alternative possibility is that the content of the focus of attention is represented in pre-frontal cortex ( Bichot et al., 2015 ), and the corresponding sensory representations are merely epiphenomenal, so that the elimination of the latter does not imply a distraction of the focus of attention in WM.
To conclude, surprisingly little can be said with confidence: Perceptual attention to stimuli often – but not always – leads to them being encoded into WM to some extent, so that they interfere with similar information. The use of perceptual attention for rehearsal has not been demonstrated convincingly. Whether the focus of attention can stay on an item in WM while perceptual attention engages with a different stimulus in the environment is still unclear.
How does information in WM affect perceptual attention? It appears plausible that holding some information in WM tends to draw perceptual attention to similar information in the environment, thereby facilitating its processing. Initial evidence for that assumption comes from experiments by Awh et al. ( 1998 ): Holding the spatial location of an object in WM facilitates processing of other stimuli appearing in the same location during the retention interval. A subsequent similar study taking additional measures to discourage eye movements, however, failed to replicate this finding ( Belopolsky & Theeuwes, 2009 ).
A more specific version of the same idea is the assumption that the item held in the focus of attention in WM – usually a single item – functions as a “search template”, guiding perceptual attention to matching stimuli ( Olivers, Peters, Houtkamp, & Roelfsema, 2011 ). This idea has received considerable empirical support from studies of the “attentional capture” effect in visual search: When people are asked to hold an item in WM – for instance a color, or just a color word – and carry out a visual search task during the retention interval, attention is drawn to stimuli in the search display matching the item in WM ( Soto, Hodsoll, Rotshtein, & Humphreys, 2008 ). When more than one item is held in WM and one of them is retro-cued, then only the retro-cued item causes attentional capture ( Mallett & Lewis-Peacock, 2018 ; van Moorselaar, Battistoni, Theeuwes, & Olivers, 2014 ; van Moorselaar, Theeuwes, & Olivers, 2014 ). This finding provides further evidence for the special functional status of representations in the focus of attention (i.e., the third level of selection).
How is WM related to the control of attention and action?
Some theorists argue for a close relation of WM specifically to controlled attention ( Kane et al., 2001 ; McVay & Kane, 2009 ; Unsworth et al., 2014 ). The evidence for this link comes primarily from correlations between measures of WM capacity and controlled attention (reviewed above in the section on resources for attention control). There are at least two interpretations of this correlation. One is that people with high ability to control their attention are good at keeping irrelevant contents out of WM ( Hasher & Zacks, 1988 ), either by filtering them out at encoding ( Vogel et al., 2005 ) or by removing them once they are no longer relevant ( Oberauer et al., 2012 ), and therefore they make better use of their WM capacity. This account has difficulties explaining why measures of controlled attention were found to correlate substantially also with measures of (visual) WM in which no irrelevant stimuli were presented, and no contents need to be removed from WM ( Unsworth et al., 2014 ).
A second explanation, which I believe to be more promising, implies the reverse direction of causality. It starts from the assumption that the main function of WM is to hold representations that control what we think and do, including what we direct our attention to ( Oberauer, 2009 ). For instance, in visual search perceptual attention can be controlled by holding a template of the search target in the focus of attention in WM ( Olivers et al., 2011 ). Selection of responses to stimuli in accordance with the currently relevant task goal is accomplished by holding a task set – a representation of the relevant stimulus categories, the response options, and the mapping between them – in WM ( Monsell, 2003 ; Oberauer et al., 2013 ). In both cases, control could also rely on representations in long-term memory. For the case of visual search, Woodman, Carlisle, and Reinhart ( 2013 ) present strong evidence that search targets that repeat across successive trials are held in WM only for the first few trials, after which search is controlled by target representations in long-term memory. The finding that search becomes more efficient with practice when the same set of stimuli is consistently used as targets or distractors further underscores the role of long-term memory in controlling perceptual attention in search tasks ( Shiffrin & Schneider, 1977 ). For the case of response selection, practicing a task with consistent stimulus-response mappings leads to long-term learning of these mappings, greatly improving task performance. Representations in WM are necessary for control when we want to do something new – searching for a new target, or carrying out a new task that we just learned from instruction. WM representations are particularly important when the new action is inconsistent with one that we have learned – for instance, searching for a target that used to consistently figure as distractor, or switching from one task to another that maps the same stimuli to new responses. In these cases, WM provides a medium for building and maintaining new representations that control our cognitive processes and actions, if necessary countermanding our long-term knowledge. On these assumptions, the correlation between WM capacity and performance in controlled-attention tasks arises because people with better WM capacity have better (i.e., more robust, more precise) representations in WM of the (cognitive or overt) action they intend to carry out, such as search templates and task sets.
To conclude, I argue that WM plays a crucial role in controlling attention and action by holding the representations that guide attention and action. The control process consists of selecting these representations into WM – once they are established in WM, they have their influence on attention and action automatically: Perceptual attention is “captured” by stimuli matching the content of the focus of attention even when this is only detrimental to performance in the current task ( Foerster & Schneider, 2018 ; Gao et al., 2016 ); newly instructed tasks, once implemented as task sets in WM, function like a “prepared reflex”, influencing response selection even when they are currently not relevant ( Meiran, Liefooghe, & De Houwer, 2017 ).
Conclusions
Attention is closely related to WM. Unpacking this relationship reveals many different ways in which the WM-attention link can be spelled out. A first divide is between theoretical ideas about attention as a resource on the one hand, and about attention as a mechanism for selecting and prioritizing information on the other. The first approach entails the theoretical commitment that a limited attentional resource is at least in part responsible for the capacity limit of WM. This assumption has considerable empirical support but also significant weaknesses (for a review see Oberauer et al., 2016 ), so that researchers should not endorse it as a default. The second approach does not imply a commitment to any assumptions about WM or attention, and therefore offers a more neutral starting point for asking how the two are related. From the theoretical considerations and the evidence reviewed here I conclude that the following assertions about specific relations between attention and WM are justified:
- By virtue of holding a selected subset of all available representations in memory, WM is by definition a form of attention.
- The selection of information to be held in WM is a form of controlled attention: The selection of stimuli to be encoded into WM is controlled by a filtering mechanism set according to our intentions; the retrieval of long-term memory information into WM is gated to admit only information relevant for our current goals, and information no longer relevant for our current goal is removed from WM.
- Attending to a perceived stimulus probably facilitates encoding of that stimulus into WM, but does not mandate it. Even attended information can be, to a large extent, filtered out.
- Within the contents of WM the focus of attention can be directed to individual items, or subsets of items, selected for manipulating them, or as input for processes (e.g., mental arithmetic, visual search).
- Control of attention and action relies on representations in WM that guide attention and action, such as search templates and task sets, especially when these are new and in conflict with knowledge in long-term memory. Once established in WM, these representations control attention and action independently of our intentions.
Unsurprisingly, there are also many things we don’t know. Table 2 presents a non-exhaustive list of open questions that I believe future research should address with high priority. I hope that this effort will lead to an increasingly more precise and nuanced picture of how WM is related to attention.
Open Questions.
| Topic | Question |
|---|---|
| Relation of central attention to WM | Under which circumstances – in particular, for how long into the retention interval – does an attention-demanding processing task compete with maintenance in WM? |
| Relation of perceptual attention and WM | Is the capacity limit of perceptual attention caused by the same limiting factors as the capacity limit of WM? |
| To what extent does perceptual attention to a stimulus lead to its encoding into WM even without the intention to encode it? | |
| The focus of attention in WM | Is the focus of attention in WM the same as the focus of perceptual attention, so that directing attention to a perceived stimulus diverts the focus from its current content in WM, and vice versa? |
| Is the distinction between WM contents in and outside of the focus of attention a qualitative difference or merely a quantitative difference (in degree of memory strength or activation)? | |
| How many distinct items can be selected simultaneously into the focus of attention so that they guide perceptual attention? Some have argued that it is only one item at a time ( ); others argue for more than one ( ) | |
| The role of neurally active representations | Are all contents of WM represented in a neurally active manner that allows decoding of their contents from neural signals, or only a selected subset of WM contents – maybe only a single item at a time? |
| Are neurally active representations in sensory cortex functionally important for maintenance in WM, or merely an epiphenomenon arising from back-projection of WM representations into sensory areas? | |
| Relation between WM and the control of attention | Under which conditions does a concurrent load on WM impair the control of attention in conflict tasks (e.g., flanker, Stroop tasks)? |
| What causal relation underlies the correlation between WM capacity and measures of attention control (e.g., filtering in visual WM tasks; anti-saccade performance, mind wandering)? | |
I will use the term object (of attention) in a broad sense, referring to every entity that we can pay attention to (e.g., physical objects, events, people, concepts and ideas, goals and actions, …).
Chun et al. ( 2011 ) refer to this distinction as “internal” vs. “external” attention. I find this terminology misleading: The memory of a tree is not more internal than the perception of a tree: Both are internal representations of external objects.
Another paradoxical implication of the fusion account is that, once the resource is completely absorbed for storage purposes, there is no resource left for control processes clearing irrelevant material from WM, and once an ongoing process monopolizes the entire attentional resource, there is no way of stopping it. A meta-control process is necessary to ensure that there is always enough resource left for control processes. If the meta-control process needs a share of the resource for itself, we are on the way to an infinite regress.
The term “unattended” is to be understood relative to the “attended” content of WM. At the same time, all contents of WM are prioritized over all other memory representations, and as such are attended, though on a broader level of selection.
Ethics and Consent
This article reports no original research, so no ethics approval is required.
Funding Information
The work on this article was supported by a grant from the Swiss National Science Foundation (SNSF, grant number 100014_135002). Thanks to Peter Shepherdson and Claudia von Bastian for their comments on a previous version of this manuscript.
Competing Interests
The author has no competing interests to declare.
Allen, R. J., Baddeley, A. D., & Hitch, G. J. (2006). Is the binding of visual features in working memory resource-demanding? Journal of Experimental Psychology: General , 135, 298–313. DOI: https://doi.org/10.1037/0096-3445.135.2.298
Anderson, J. R., Reder, L. M., & Lebiere, C. (1996). Working memory: Activation limits on retrieval. Cognitive Psychology , 30, 221–256. DOI: https://doi.org/10.1006/cogp.1996.0007
Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Sciences , 16, 437–443. DOI: https://doi.org/10.1016/j.tics.2012.06.010
Awh, E., Jonides, J., & Reuter-Lorenz, P. A. (1998). Rehearsal in spatial working memory. Journal of Experimental Psychology: Human Perception and Performance , 24, 780–790. DOI: https://doi.org/10.1037/0096-1523.24.3.780
Baddeley, A. D. (1986). Working memory . Oxford: Clarendon Press.
Baddeley, A. D. (1993). Working memory or working attention? In: A. Baddeley, & L. Weiskrantz (Eds.), Attention: Selection, awareness, and control , (pp. 152–170). Oxford: Clarendon.
Baddeley, A. D. (1996). Exploring the central executive. Quarterly Journal of Experimental Psychology , 49A, 5–28. DOI: https://doi.org/10.1080/713755608
Baddeley, A. D., Papagno, C., & Andrade, J. (1993). The sandwich effect: The role of attentional factors in serial recall. Journal of Experimental Psychology: Learning, Memory and Cognition , 19, 862–870. DOI: https://doi.org/10.1037/0278-7393.19.4.862
Barrouillet, P., Bernardin, S., & Camos, V. (2004). Time constraints and resource sharing in adults’ working memory spans. Journal of Experimental Psychology: General , 133, 83–100. DOI: https://doi.org/10.1037/0096-3445.133.1.83
Barrouillet, P., Bernardin, S., Portrat, S., Vergauwe, E., & Camos, V. (2007). Time and cognitive load in working memory. Journal of Experimental Psychology: Learning, Memory & Cognition , 33, 570–585. DOI: https://doi.org/10.1037/0278-7393.33.3.570
Barrouillet, P., Portrat, S., & Camos, V. (2011). On the law relating processing to storage in working memory. Psychological Review , 118, 175–192. DOI: https://doi.org/10.1037/a0022324
Belopolsky, A. V., & Theeuwes, J. (2009). No functional role of attention-based rehearsal in maintenance of spatial working memory representations. Acta Psychologica , 132, 124–135. DOI: https://doi.org/10.1016/j.actpsy.2009.01.002
Bettencourt, K. C., & Xu, Y. (2016). Decoding the content of visual short-term memory under distraction in occipital and parietal areas. Nature Neuroscience , 19, 150–157. DOI: https://doi.org/10.1038/nn.4174
Bichot, N. P., Heard, M. T., DeGennaro, E. M., & Desimone, R. (2015). A Source for Feature-Based Attention in the Prefrontal Cortex. Neuron , 88(4), 832–844. DOI: https://doi.org/10.1016/j.neuron.2015.10.001
Bunting, M. F., Cowan, N., & Colflesh, G. J. H. (2008). The deployment of attention in short-term memory tasks: Trade-offs between immediate and delayed deployment. Memory & Cognition , 36, 799–812. DOI: https://doi.org/10.3758/MC.36.4.799
Camos, V., Lagner, P., & Barrouillet, P. (2009). Two maintenance mechanisms of verbal information in working memory. Journal of Memory and Language , 61, 457–469. DOI: https://doi.org/10.1016/j.jml.2009.06.002
Case, R. (1972). Validation of a neo-Piagetian mental capacity construct. Journal of Experimental Child Psychology , 14, 287–302. DOI: https://doi.org/10.1016/0022-0965(72)90051-3
Case, R., Kurland, M., & Goldberg, J. (1982). Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology , 33, 386–404. DOI: https://doi.org/10.1016/0022-0965(82)90054-6
Chein, J. M., Moore, A. B., & Conway, A. R. A. (2011). Domain-general mechanisms of complex working memory span. NeuroImage , 54, 550–559. DOI: https://doi.org/10.1016/j.neuroimage.2010.07.067
Chen, H., Swan, G., & Wyble, B. (2016). Prolonged focal attention without binding: Trackng a ball for half a minute without remembering its color. Cognition , 147, 144–148. DOI: https://doi.org/10.1016/j.cognition.2015.11.014
Chen, H., & Wyble, B. (2015a). Amnesia for object attributes: Failure to report attended information that had just reached conscious awareness. Psychological Science , 26, 203–210. DOI: https://doi.org/10.1177/0956797614560648
Chen, H., & Wyble, B. (2015b). The location but not the attributes of visual cues are automatically encoded into working memory. Vision Research , 107, 76–85. DOI: https://doi.org/10.1016/j.visres.2014.11.010
Chen, Z., & Cowan, N. (2009). How verbal memory loads consume attention. Memory & Cognition , 37, 829–836. DOI: https://doi.org/10.3758/MC.37.6.829
Christophel, T. B., Iamshchinina, P., Yan, C., Allefeld, C., & Haynes, J.-D. (2018). Cortical specialization for attended versus unattended working memory. Nature Neuroscience , 21, 494–496. DOI: https://doi.org/10.1038/s41593-018-0094-4
Chuderski, A. (2014). The relational integration task explains fluid reasoning above and beyond other working memory tasks. Memory & Cognition , 42, 448–463. DOI: https://doi.org/10.3758/s13421-013-0366-x
Chun, M. M. (2011). Visual working memory as visual attention sustained internally over time. Neuropsychologia , 49(6), 1407–1409. DOI: https://doi.org/10.1016/j.neuropsychologia.2011.01.029
Chun, M. M., Golomb, J. D., & Turk-Browne, N. B. (2011). A taxonomy of external and internal attention. Annual Review of Psychology , 62, 73–101. DOI: https://doi.org/10.1146/annurev.psych.093008.100427
Cowan, N. (1995). Attention and memory: An integrated framework . New York: Oxford University Press.
Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences , 24, 87–185. DOI: https://doi.org/10.1017/S0140525X01003922
Cowan, N. (2005). Working memory capacity . New York: Psychology Press.
Cowan, N. (2017). The many faces of working memory and short-term storage. Psychonomic Bulletin & Review , 24, 1158–1170. DOI: https://doi.org/10.3758/s13423-016-1191-6
Cowan, N., Elliott, E. M., Saults, J. S., Morey, C. C., Mattox, S., Hismjatullina, A., & Conway, A. R. A. (2005). On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology , 51, 42–100. DOI: https://doi.org/10.1016/j.cogpsych.2004.12.001
Cowan, N., Fristoe, N., Elliott, E. M., Brunner, R. P., & Saults, J. S. (2006). Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition , 34, 1754–1768. DOI: https://doi.org/10.3758/BF03195936
Daneman, M., & Carpenter, P. A. (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior , 19, 450–466. DOI: https://doi.org/10.1016/S0022-5371(80)90312-6
Desimone, R., & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience , 18, 193–222. DOI: https://doi.org/10.1146/annurev.ne.18.030195.001205
Ecker, U. K. H., Lewandowsky, S., & Oberauer, K. (2014). Removal of information from working memory: A specific updating process. Journal of Memory and Language , 74, 77–90. DOI: https://doi.org/10.1016/j.jml.2013.09.003
Ecker, U. K. H., Oberauer, K., & Lewandowsky, S. (2014). Working memory updating involves item-specific removal. Journal of Memory & Language , 74, 1–15. DOI: https://doi.org/10.1016/j.jml.2014.03.006
Eliasmith, C. (2013). How to build a brain: A neural architecture for biological cognition . New York, NY: Oxford University Press. DOI: https://doi.org/10.1093/acprof:oso/9780199794546.001.0001
Emrich, S. M., Rigall, A. C., LaRocque, J. J., & Postle, B. R. (2013). Distributed patterns of activity in sensory cortex reflect the precision of multiple items maintained in visual short-term memory. Journal of Neuroscience , 33, 6516–6523. DOI: https://doi.org/10.1523/JNEUROSCI.5732-12.2013
Ester, E. F., Fukuda, K., May, L. M., Vogel, E. K., & Awh, E. (2014). Evidence for a fixed capacity limit in attending multiple locations. Cognitive, Affective, & Behavioral Neuroscience , 14, 62–77. DOI: https://doi.org/10.3758/s13415-013-0222-2
Feldmann-Wüstefeld, T., Vogel, E. K., & Awh, E. (2018). Contralateral delay activity indexes working memory storage, not the current focus of spatial attention. Journal of Cognitive Neuroscience , 30(8), 1185–1196. DOI: https://doi.org/10.1162/jocn_a_01271
Foerster, R. M., & Schneider, W. X. (2018). Involuntary top-down control by search-irrelevant features: Visual working memory biases attention in an object-based manner. Cognition , 172, 37–45. DOI: https://doi.org/10.1016/j.cognition.2017.12.002
Fougnie, D., & Marois, R. (2006). Distinct capacity limits for attention and working memory. Psychological Science , 17, 526–534. DOI: https://doi.org/10.1111/j.1467-9280.2006.01739.x
Gao, Z., Yu, S., Zhu, C., Shui, R., Weng, X., Li, P., & Shen, M. (2016). Object-based encoding in visual working memory: Evidence from memory-driven attentional capture. Scientific Reports , 6. DOI: https://doi.org/10.1038/srep22822
Garavan, H. (1998). Serial attention within working memory. Memory & Cognition , 26, 263–276. DOI: https://doi.org/10.3758/BF03201138
Gazzaley, A., & Nobre, A. C. (2012). Top-down modulation: bridging selective attention and working memory. Trends in Cognitive Sciences , 16, 129–135. DOI: https://doi.org/10.1016/j.tics.2011.11.014
Gilchrist, A. L., & Cowan, N. (2011). Can the focus of attention accommodate multiple, separate items? Journal of Experimental Psychology: Learning, Memory, and Cognition , 37, 1484–1502. DOI: https://doi.org/10.1037/a0024352
Göthe, K., Oberauer, K., & Kliegl, R. (2016). Eliminating dual-task costs by minimizing crosstalk between tasks: The role of modality and feature pairings. Cognition , 150, 92–108. DOI: https://doi.org/10.1016/j.cognition.2016.02.003
Griffin, I. C., & Nobre, A. C. (2003). Orienting attention to locations in internal representations. Journal of Cognitive Neuroscience , 15, 1176–1194. DOI: https://doi.org/10.1162/089892903322598139
Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view. In: G. H. Bower (Ed.), The Psychology of Learning and Motivation , 22, (pp. 193–225). New York: Academic Press. DOI: https://doi.org/10.1016/S0079-7421(08)60041-9
Hasher, L., Zacks, R. T., & May, C. P. (1999). Inhibitory control, circadian arousal, and age. In: D. Gopher, & A. Koriat (Eds.), Attention and Performance , (pp. 653–675). Cambridge, MA: MIT Press.
Hazeltine, E., & Witfall, T. (2011). Searching working memory for the source of dual-task costs. Psychological Research , 75, 466–475. DOI: https://doi.org/10.1007/s00426-011-0343-6
Hedge, C., Oberauer, K., & Leonards, U. (2015). Selection in spatial working memory is independent of perceptual selective attention, but they interact in a shared spatial priority map. Attention, Perception & Psychophysics , 77, 26653–22668. DOI: https://doi.org/10.3758/s13414-015-0976-4
Hollingworth, A., & Beck, V. M. (2016). Memory-based attention capture when multiple items are maintained in visual working memory. Journal of Experimental Psychology: Human Perception and Performance , 42, 911–917. DOI: https://doi.org/10.1037/xhp0000230
Hollingworth, A., & Maxcey-Richard, A. M. (2013). Selective maintenance in visual working memory does not require sustained visual attention. Journal of Experimental Psychology: Human Perception and Performance , 39, 1047–1058. DOI: https://doi.org/10.1037/a0030238
Ikkai, A., McCollough, A. W., & Vogel, E. K. (2010). Contralateral delay activity provides a neural measure of the number of representations in visual working memory. Journal of Neurophysiology , 103, 1963–1968. DOI: https://doi.org/10.1152/jn.00978.2009
Jolicoeur, P., & Dell’Acqua, R. (1998). The demonstration of short-term consolidation. Cognitive Psychology , 36, 138–202. DOI: https://doi.org/10.1006/cogp.1998.0684
Jurado, M. B., & Rosselli, M. (2007). The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review , 17, 213–233. DOI: https://doi.org/10.1007/s11065-007-9040-z
Just, M. A., & Carpenter, P. A. (1980). A theory of reading: From eye fixations to comprehension. Psychological Review , 87, 329–354. DOI: https://doi.org/10.1037/0033-295X.87.4.329
Just, M. A., & Carpenter, P. A. (1992). A capacity theory of comprehension: Individual differences in working memory. Psychological Review , 99, 122–149. DOI: https://doi.org/10.1037/0033-295X.99.1.122
Kane, M. J., Bleckley, M. K., Conway, A. R. A., & Engle, R. W. (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General , 130, 169–183. DOI: https://doi.org/10.1037/0096-3445.130.2.169
Kane, M. J., & Engle, R. W. (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General , 132(47–70). DOI: https://doi.org/10.1037/0096-3445.132.1.47
Kelley, T. A., & Lavie, N. (2011). Working Memory Load Modulates Distractor Competition in Primary Visual Cortex. Cerebral Cortex , 21(3), 659–665. DOI: https://doi.org/10.1093/cercor/bhq139
Keye, D., Wilhelm, O., Oberauer, K., & van Ravenzwaaij, D. (2009). Individual differences in conflict-monitoring: Testing means and covariance hypothesis about the Simon and the Eriksen Flanker task. Psychological Research-Psychologische Forschung , 73(6), 762–776. DOI: https://doi.org/10.1007/s00426-008-0188-9
Kim, S.-Y., Kim, M.-S., & Chun, M. M. (2005). Concurrent working memory load can reduce distraction. Proceedings of the National Academy of Sciences , 102, 16524–16529. DOI: https://doi.org/10.1073/pnas.0505454102
Kiyonaga, A., & Egner, T. (2014). Working memory as internal attention: Toward an integrative account of internal and external selection processes. Psychonomic Bulletin & Review . DOI: https://doi.org/10.3758/s13423-012-0359-y
Klapp, S. T., Marshburn, E. A., & Lester, P. T. (1983). Short-term memory does not involve the “working memory” of information processing: The demise of a common assumption. Journal of Experimental Psychology: General , 112, 240–264. DOI: https://doi.org/10.1037/0096-3445.112.2.240
Konstantinou, N., Beal, E., King, J.-R., & Lavie, N. (2014). Working memory load and distraction: Dissociable effects of visual maintenance and cognitive control. Attention, Perception & Psychophysics , 76, 1985–1997. DOI: https://doi.org/10.3758/s13414-014-0742-z
Konstantinou, N., & Lavie, N. (2013). Dissociable roles of different types of working memory load in visual detection. Journal of Experimental Psychology: Human Perception and Performance , 39, 919–924. DOI: https://doi.org/10.1037/a0033037
Landman, R., Spekreijse, H., & Lamme, V. A. F. (2003). Large capacity storage of integrated objects before change blindness. Vision Research , 43(149–164). DOI: https://doi.org/10.1016/S0042-6989(02)00402-9
LaRocque, J. J., Lewis-Peacock, J. A., Drysdale, A. T., Oberauer, K., & Postle, B. R. (2013). Decoding attended information in short-term memory: An EEG study. Journal of Cognitive Neuroscience , 25(1), 127–142. DOI: https://doi.org/10.1162/jocn_a_00305
Lavie, N. (2005). Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences , 9, 75–82. DOI: https://doi.org/10.1016/j.tics.2004.12.004
Lavie, N., Hirst, A., de Fockert, J. W., & Viding, E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General , 133, 339–354. DOI: https://doi.org/10.1037/0096-3445.133.3.339
Lawrence, B. M., Myerson, J., & Abrams, R. A. (2004). Interference with spatial working memory: An eye movement is more than a shift of attention. Psychonomic Bulletin & Review , 11, 488–494. DOI: https://doi.org/10.3758/BF03196600
Lawrence, B. M., Myerson, J., Oonk, H. M., & Abrams, R. A. (2001). The effects of eye and limb movements on working memory. Memory , 9, 433–444. DOI: https://doi.org/10.1080/09658210143000047
Lepsien, J., Thornton, I., & Nobre, A. C. (2011). Modulation of working-memory maintenance by directed attention. Neuropsychologia , 49, 1569–1577. DOI: https://doi.org/10.1016/j.neuropsychologia.2011.03.011
Lewis-Peacock, J. A., Drysdale, A. T., Oberauer, K., & Postle, B. R. (2011). Neural evidence for a distinction between short-term memory and the focus of attention. Journal of Cognitive Neuroscience , 24, 61–79. DOI: https://doi.org/10.1162/jocn_a_00140
Lewis-Peacock, J. A., Kessler, Y., & Oberauer, K. (2018). The removal of information from working memory. Annals of the New York Academy of Science , 1424, 33–44. DOI: https://doi.org/10.1111/nyas.13714
Liefooghe, B., Barrouillet, P., Vandierendonck, A., & Camos, V. (2008). Working memory costs of task switching. Journal of Experimental Psychology: Learning, Memory, and Cognition , 34, 478–494. DOI: https://doi.org/10.1037/0278-7393.34.3.478
Logie, R. H., Brockmole, J. B., & Jaswal, S. (2011). Feature binding in visual short-term memory is unaffected by task-irrelevant changes of location, shape, and color. Memory & Cognition , 39, 24–36. DOI: https://doi.org/10.3758/s13421-010-0001-z
Luria, R., Balaban, H., Awh, E., & Vogel, E. K. (2016). The contralateral delay activity as a neural measure of visual working memory. Neuroscience and Biobehavioral Reviews , 62, 100–108. DOI: https://doi.org/10.1016/j.neubiorev.2016.01.003
Ma, W. J., Husain, M., & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience Reviews , 17, 347–356. DOI: https://doi.org/10.1038/nn.3655
Mall, J. T., Morey, C. C., Wolff, M. J., & Lehnert, F. (2014). Visual selective attention is equally functional for individuals with low and high working memory capacity: Evidence from accuracy and eye movements. Attention, Perception & Psychophysics . DOI: https://doi.org/10.3758/s13414-013-0610-2
Mallett, R., & Lewis-Peacock, J. A. (2018). Behavioral decoding of working memory items inside and outside the focus of attention. Annals of the New York Academy of Sciences , 1424(1), 256–267. DOI: https://doi.org/10.1111/nyas.13647
Marshall, L., & Bays, P. M. (2013). Obligatory encoding of task-irrelevant features depletes working memory resources. Journal of Vision , 13, 1–13. DOI: https://doi.org/10.1167/13.2.21
McElree, B. (2006). Accessing recent events. In: B. H. Ross (Ed.), The Psychology of Learning and Motivation , 46, (pp. 155–200). San Diego: Academic Press. DOI: https://doi.org/10.1016/S0079-7421(06)46005-9
McVay, J. C., & Kane, M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition , 35, 196–204. DOI: https://doi.org/10.1037/a0014104
McVay, J. C., & Kane, M. J. (2012). Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General , 141, 302–320. DOI: https://doi.org/10.1037/a0025250
Meiran, N., Liefooghe, B., & De Houwer, J. (2017). Powerful instructions: Automaticity without practice. Current Directions in Psychological Science , 26, 509–514. DOI: https://doi.org/10.1177/0963721417711638
Mendoza-Halliday, D., & Martinez-Trujillo, J. C. (2017). Neuronal population coding of perceived and memorized visual features in the lateral prefrontal cortex. nature Communications , 8, 15471. DOI: https://doi.org/10.1038/ncomms15471
Mongillo, G., Barak, O., & Tsodyks, M. (2008). Synaptic theory of working memory. Science , 319, 1543–1546. DOI: https://doi.org/10.1126/science.1150769
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences , 7, 134–140. DOI: https://doi.org/10.1016/S1364-6613(03)00028-7
Morey, C. C., & Bieler, M. (2012). Visual short-term memory always requires general attention. Psychonomic Bulletin & Review , 20, 163–170. DOI: https://doi.org/10.3758/s13423-012-0313-z
Navon, D., & Gopher, D. (1979). On the economy of the human-processing system. Psychological Review , 86, 214–255. DOI: https://doi.org/10.1037/0033-295X.86.3.214
Navon, D., & Miller, J. (2002). Queuing or sharing? A critical evaluation of the single-bottleneck notion. Cognitive Psychology , 44, 193–251. DOI: https://doi.org/10.1006/cogp.2001.0767
Nieuwenstein, M., & Wyble, B. (2014). Beyond a mask and against the bottleneck: Retroactive dual-task interference during working memory consolidation of a masked visual target. Journal of Experimental Psychology: General , 143, 1409–1427. DOI: https://doi.org/10.1037/a0035257
Oberauer, K. (2001). Removing irrelevant information from working memory: A cognitive aging study with the modified Sternberg task. Journal of Experimental Psychology-Learning Memory and Cognition , 27(4), 948–957. DOI: https://doi.org/10.1037/0278-7393.27.4.948
Oberauer, K. (2002). Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition , 28(3), 411–421. DOI: https://doi.org/10.1037/0278-7393.28.3.411
Oberauer, K. (2003). Selective attention to elements in working memory. Experimental Psychology , 50(4), 257–269. DOI: https://doi.org/10.1026//1618-3169.50.4.257
Oberauer, K. (2005). Control of the contents of working memory – A comparison of two paradigms and two age groups. Journal of Experimental Psychology: Learning, Memory, and Cognition , 31(4), 714–728. DOI: https://doi.org/10.1037/0278-7393.31.4.714
Oberauer, K. (2009). Design for a working memory. Psychology of Learning and Motivation: Advances in Research and Theory , 51, 45–100. DOI: https://doi.org/10.1016/S0079-7421(09)51002-X
Oberauer, K. (2013). The focus of attention in working memory – from metaphors to mechanisms. frontiers in human neuroscience , 7. DOI: https://doi.org/10.3389/fnhum.2013.00673
Oberauer, K. (2018). Removal of irrelevant information from working memory: Sometimes fast, sometimes slow, and sometimes not at all. Annals of the New York Academy of Science , 1424, 239–255. DOI: https://doi.org/10.1111/nyas.13603
Oberauer, K., Awh, E., & Sutterer, D. W. (2017). The role of long-term memory in a test of visual working memory: Proactive facilitation but no proactive interference. Journal of Experimental Psychology: Learning, Memory and Cognition , 43, 1–22. DOI: https://doi.org/10.1037/xlm0000302
Oberauer, K., & Bialkova, S. (2011). Serial and parallel processes in working memory after practice. Journal of Experimental Psychology: Human Perception and Performance , 37(2), 606–614. DOI: https://doi.org/10.1037/a0020986
Oberauer, K., Demmrich, A., Mayr, U., & Kliegl, R. (2001). Dissociating retention and access in working memory: An age-comparative study of mental arithmetic. Memory & Cognition , 29(1), 18–33. DOI: https://doi.org/10.3758/BF03195737
Oberauer, K., Farrell, S., Jarrold, C., & Lewandowsky, S. (2016). What limits working memory capacity? Psychological Bulletin , 142, 758–799. DOI: https://doi.org/10.1037/bul0000046
Oberauer, K., & Hein, L. (2012). Attention to information in working memory. Current Directions in Psychological Science , 21, 164–169. DOI: https://doi.org/10.1177/0963721412444727
Oberauer, K., & Lewandowsky, S. (2013). Evidence against decay in verbal working memory. Journal of Experimental Psychology: General , 142, 380–411. DOI: https://doi.org/10.1037/a0029588
Oberauer, K., & Lewandowsky, S. (2014). Further evidence against decay in working memory. Journal of Memory and Language , 73, 15–30. DOI: https://doi.org/10.1016/j.jml.2014.02.003
Oberauer, K., Lewandowsky, S., Farrell, S., Jarrold, C., & Greaves, M. (2012). Modeling working memory: An interference model of complex span. Psychonomic Bulletin & Review , 19, 779–819. DOI: https://doi.org/10.3758/s13423-012-0272-4
Oberauer, K., & Lin, H.-Y. (2017). An interference model of visual working memory. Psychological Review , 124, 21–59. DOI: https://doi.org/10.1037/rev0000044
Oberauer, K., Souza, A. S., Druey, M., & Gade, M. (2013). Analogous mechanisms of selection and updating in declarative and procedural working memory: Experiments and a computational model. Cognitive Psychology , 66, 157–211. DOI: https://doi.org/10.1016/j.cogpsych.2012.11.001
Olivers, C. N. L. (2008). Interactions between visual working memory and visual attention. Frontiers in Bioscience , 13, 1182–1191. DOI: https://doi.org/10.2741/2754
Olivers, C. N. L., Peters, J., Houtkamp, R., & Roelfsema, P. R. (2011). Different states in visual working memory: When it guides attention and when it does not. Trends in Cognitive Sciences , 15, 327–334. DOI: https://doi.org/10.1016/j.tics.2011.05.004
Park, S., Kim, M.-S., & Chun, M. M. (2007). Concurrent working memory load can facilitate selective attention: Evidence for specialized load. Journal of Experimental Psychology: Human Perception and Performance , 33(1062–1075). DOI: https://doi.org/10.1037/0096-1523.33.5.1062
Pashler, H. (1994). Dual-task interference in simple tasks: Data and theory. Psychological Bulletin , 116, 220–244. DOI: https://doi.org/10.1037/0033-2909.116.2.220
Plate, T. A. (2003). Convolution-based memory models. In: L. Nadel (Ed.), Encyclopedia of cognitive science , (pp. 824–828). London: Nature Publishing Group.
Ralph, A., Walters, J. N., Stevens, A., Fitzgerald, K. J., Tehan, G., Surprenant, A. M., Turcotte, J., et al. (2011). Immunity to proactive interference is not a property of the focus of attention in working memory. Memory & Cognition , 39, 217–230. DOI: https://doi.org/10.3758/s13421-010-0030-7
Randall, J. G., Oswald, F. L., & Beier, M. E. (2014). Mind-wandering, cognition, and performance: A theory-driven meta-analysis of attention regulation. Psychological Bulletin , 140, 1411–1431. DOI: https://doi.org/10.1037/a0037428
Rerko, L., Souza, A. S., & Oberauer, K. (2014). Retro-cue benefits in working memory without sustained attention. Memory & Cognition , 42, 712–728. DOI: https://doi.org/10.3758/s13421-013-0392-8
Ricker, T. J., & Hardman, K. O. (2017). The nature of short-term consolidation in visual working memory. Journal of Experimental Psychology: General . DOI: https://doi.org/10.1037/xge0000346
Rose, N. S., LaRocque, J. J., Riggall, A. C., Gosseries, O., Starrett, M. J., Meyering, E. E., & Postle, B. R. (2016). Reactivation of latent working memories with transcranial magnetic stimulation. Science , 354, 1136–1139. DOI: https://doi.org/10.1126/science.aah7011
Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Binding and strategic selection in working memory: A lifespan dissociation. Psychology and Aging , 26, 612–624. DOI: https://doi.org/10.1037/a0023055
Saults, J. S., & Cowan, N. (2007). A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General , 136, 663–684. DOI: https://doi.org/10.1037/0096-3445.136.4.663
Schneider, W., & Shiffrin, R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review , 84, 1–66. DOI: https://doi.org/10.1037/0033-295X.84.1.1
Shiffrin, R. M., & Schneider, W. (1977). Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychological Review , 84, 127–190. DOI: https://doi.org/10.1037/0033-295X.84.2.127
Shipstead, Z., Lindsey, D. R. B., Marshall, R. L., & Engle, R. E. (2014). The mechanisms of working memory capacity: Primary memory, secondary memory, and attention control. Journal of Memory and Language , 72, 116–141. DOI: https://doi.org/10.1016/j.jml.2014.01.004
Singh, K. A., Gignac, G. E., Brydges, C. R., & Ecker, U. K. H. (2018). Working memory capacity mediates the relationship between removal and fluid intelligence. Journal of Memory and Language , 101, 18–36. DOI: https://doi.org/10.1016/j.jml.2018.03.002
Soto, D., Hodsoll, J., Rotshtein, P., & Humphreys, G. W. (2008). Automatic guidance of attention from working memory. Trends in Cognitive Sciences , 12, 342–348. DOI: https://doi.org/10.1016/j.tics.2008.05.007
Souza, A. S., & Oberauer, K. (2016). In search of the focus of attention in working memory: 13 years of the retro-cue effect. Attention, Perception & Psychophysics . DOI: https://doi.org/10.3758/s13414-016-1108-5
Souza, A. S., & Oberauer, K. (2017). The contributions of visual and central attention to visual working memory. Attention, Perception & Psychophysics , 79, 1897–1916. DOI: https://doi.org/10.3758/s13414-017-1357-y
Souza, A. S., Rerko, L., & Oberauer, K. (2015). Refreshing memory traces: Thinking of an item improves retrieval from visual working memory. Annals of the New York Academy of Sciences , 1339, 20–31. DOI: https://doi.org/10.1111/nyas.12603
Souza, A. S., Vergauwe, E., & Oberauer, K. (2018). Where to attend next: Guiding refreshing of visual, spatial, and verbal representations in working memory. Annals of the New York Academy of Science . DOI: https://doi.org/10.1111/nyas.13621
Sprague, T. C., Ester, E. F., & Serences, J. T. (2016). Restoring latent visual working memory representations in human cortex. Neuron , 91, 694–707. DOI: https://doi.org/10.1016/j.neuron.2016.07.006
Szmalec, A., Vandierendonck, A., & Kemps, E. (2005). Response selection involves executive control: Evidence from the selective interference paradigm. Memory & Cognition , 33, 531–541. DOI: https://doi.org/10.3758/BF03193069
Tehan, G., & Humphreys, M. S. (1998). Creating proactive interference in immediate recall: Building a DOG from a DART, a MOP, and a FIG. Memory & Cognition , 26, 477–489. DOI: https://doi.org/10.3758/BF03201157
Thalmann, M., Souza, A. S., & Oberauer, K. (2019). Revisiting the attentional demands of rehearsal in working-memory tasks. Journal of Memory and Language , 105, 1–18. DOI: https://doi.org/10.1016/j.jml.2018.10.005
Theeuwes, J. (2018). Visual selection: Usually fast and automatic; seldom slow and volitional. Journal of Cognition , 1, 1–15. DOI: https://doi.org/10.5334/joc.13
Todd, J. J., & Marois, R. (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature , 428, 751–753. DOI: https://doi.org/10.1038/nature02466
Todd, J. J., & Marois, R. (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, Affective, & Behavioral Neuroscience , 5, 144–155. DOI: https://doi.org/10.3758/CABN.5.2.144
Tombu, M., & Jolicoeur, P. (2003). A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception and Performance , 29, 3–18. DOI: https://doi.org/10.1037/0096–1523.29.1.3
Tremblay, S., Saint-Aubin, J., & Jalberg, A. (2006). Rehearsal in serial memory for visual-spatial information: Evidence from eye movements. Psychonomic Bulletin & Review , 13, 452–457. DOI: https://doi.org/10.3758/BF03193869
Tsubomi, H., Fukuda, K., Watanabe, K., & Vogel, E. K. (2013). Neural limits to representing objects still within view. Journal of Neuroscience , 33, 8257–8263. DOI: https://doi.org/10.1523/JNEUROSCI.5348-12.2013
Ueno, T., Allen, R. J., Baddeley, A. D., Hitch, G. J., & Saito, S. (2011). Disruption of visual feature binding in working meemory. Memory & Cognition , 39, 12–23. DOI: https://doi.org/10.3758/s13421-010-0013-8
Unsworth, N. (2015). Consistency of attentional control as an important cognitive trait: A latent variable analysis. Intelligence , 49, 110–128. DOI: https://doi.org/10.1016/j.intell.2015.01.005
Unsworth, N., Fukuda, K., Awh, E., & Vogel, E. K. (2014). Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognitive Psychology , 71, 1–26. DOI: https://doi.org/10.1016/j.cogpsych.2014.01.003
Van der Stigchel, S., Merten, H., Meeter, M., & Theeuwes, J. (2007). The effects of a task-irrelevant visual event on spatial working memory. Psychonomic Bulletin & Review , 14, 1066–1071. DOI: https://doi.org/10.3758/BF03193092
van Moorselaar, D., Battistoni, E., Theeuwes, J., & Olivers, C. N. L. (2014). Rapid influences of cued visual memories on attentional guidance. Annals of the New York Academy of Sciences . DOI: https://doi.org/10.1111/nyas.12574
van Moorselaar, D., Foster, J. J., Sutterer, D. W., Theeuwes, J., Olivers, C. N. L., & Awh, E. (2017). Spatially selective alpha oscillations reveal moment-by-moment trade-offs between working memory and attention. Journal of Cognitive Neuroscience , 30, 256–266. DOI: https://doi.org/10.1162/jocn_a_01198
van Moorselaar, D., Theeuwes, J., & Olivers, C. N. L. (2014). In competition for the attentional template: Can multiple items within visual working memory guide attention? Journal of Experimental Psychology: Human Perception and Performance , 40, 1450–1464. DOI: https://doi.org/10.1037/a0036229
Vergauwe, E., Barrouillet, P., & Camos, V. (2010). Do mental processes share a domain-general resource? Psychological Science , 21, 384–390. DOI: https://doi.org/10.1177/0956797610361340
Vergauwe, E., Camos, V., & Barrouillet, P. (2014). The impact of storage on processing: How is information maintained in working memory? Journal of Experimental Psychology: Learning, Memory, and Cognition , 40, 1072–1095. DOI: https://doi.org/10.1037/a0035779
Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature , 428, 748–751. DOI: https://doi.org/10.1038/nature02447
Vogel, E. K., McCollough, A. W., & Machizawa, M. G. (2005). Neural measures reveal individual differences in controlling access to working memory. Nature , 438(24), 500–503. DOI: https://doi.org/10.1038/nature04171
Watanabe, K., & Funahashi, S. (2014). Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nature Neuroscience , 17, 601–611. DOI: https://doi.org/10.1038/nn.3667
Wickens, C. D. (1980). The structure of attentional ressources. In: R. S. Nickerson (Ed.), Attention & Performance , VIII, (pp. 239–257). Hillsdale, N.J.: Erlbaum.
Wilhelm, O., Hildebrandt, A., & Oberauer, K. (2013). What is working memory capacity, and how can we measure it? frontiers in Psychology , 4. DOI: https://doi.org/10.3389/fpsyg.2013.00433
Williams, M., Pouget, P., Boucher, L., & Woodman, G. F. (2013). Visual-spatial attention aids the maintenance of object representations in visual working memory. Memory & Cognition , 41, 698–715. DOI: https://doi.org/10.3758/s13421-013-0296-7
Wolff, M. J., Jochim, J., Akyürek, E. G., & Stokes, M. G. (2017). Dynamic hidden states underlying working-memory-guided behavior. Nature Neuroscience , 20, 864–871. DOI: https://doi.org/10.1038/nn.4546
Woodman, G. F., Carlisle, N. B., & Reinhart, R. M. G. (2013). Where do we store the memory representations that guide attention? Journal of Vision , 13, 1–17. DOI: https://doi.org/10.1167/13.3.1
Woodman, G. F., & Chun, M. M. (2006). The role of working memory and long-term memory in visual search. Visual Cognition , 14, 808–830. DOI: https://doi.org/10.1080/13506280500197397
Xu, Y., & Chun, M. M. (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature , 440, 91–94. DOI: https://doi.org/10.1038/nature04262
Yeung, N., Botvinick, M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review , 111, 931–959. DOI: https://doi.org/10.1037/0033-295X.111.4.931
Yu, Q., & Shim, W. M. (2017). Occipital, parietal, and frontal cortices selectively maintain task-relevant features of multi-feature objects in visual working memory. NeuroImage , 157, 97–107. DOI: https://doi.org/10.1016/j.neuroimage.2017.05.055
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Working Memory: A Selective Review
Affiliation.
- 1 a Private Practice , Bettendorf , Iowa.
- PMID: 27191213
- DOI: 10.1080/21622965.2016.1167491
The purpose of this paper is to provide a selective overview of the evolution of the concept and assessment of working memory, and how its assessment has been confused with the assessment of some components of attention. A literature search using PsychNet Gold was conducted using the terms working memory. In addition, the writer reviewed recommendations from a sampling of recent neuropsychology texts in regard to the assessment of attention and working memory, as well as the two most recent editions of the Wechsler Memory Scale. It is argued that many clinicians have an incomplete understanding of the relationship between attention and working memory, and often conflate the two in assessment and treatment. Suggestions were made for assessing these abilities.
Keywords: Wechsler Memory Scale; assessment; attention; short term memory; working memory.
PubMed Disclaimer
Similar articles
- French neuropsychological procedure consensus in epilepsy surgery. Brissart H, Planton M, Bilger M, Bulteau C, Forthoffer N, Guinet V, Hennion S, Kleitz C, Laguitton V, Mirabel H, Mosca C, Pécheux N, Pradier S, Samson S, Tramoni E, Voltzenlogel V, Denos M, Boutin M. Brissart H, et al. Epilepsy Behav. 2019 Nov;100(Pt A):106522. doi: 10.1016/j.yebeh.2019.106522. Epub 2019 Oct 15. Epilepsy Behav. 2019. PMID: 31627076 Review.
- Attention allocation: Relationships to general working memory or specific language processing. Archibald LM, Levee T, Olino T. Archibald LM, et al. J Exp Child Psychol. 2015 Nov;139:83-98. doi: 10.1016/j.jecp.2015.06.002. Epub 2015 Jun 18. J Exp Child Psychol. 2015. PMID: 26094240
- The external-internal loop of interference: two types of attention and their influence on the learning abilities of mice. Sauce B, Wass C, Smith A, Kwan S, Matzel LD. Sauce B, et al. Neurobiol Learn Mem. 2014 Dec;116:181-92. doi: 10.1016/j.nlm.2014.10.005. Epub 2014 Oct 24. Neurobiol Learn Mem. 2014. PMID: 25452087 Free PMC article.
- Substantive validity of working memory measures in major cognitive functioning test batteries for children. Leffard SA, Miller JA, Bernstein J, DeMann JJ, Mangis HA, McCoy EL. Leffard SA, et al. Appl Neuropsychol. 2006;13(4):230-41. doi: 10.1207/s15324826an1304_4. Appl Neuropsychol. 2006. PMID: 17362143 Review.
- The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Abramovitch A, Abramowitz JS, Mittelman A. Abramovitch A, et al. Clin Psychol Rev. 2013 Dec;33(8):1163-71. doi: 10.1016/j.cpr.2013.09.004. Epub 2013 Sep 29. Clin Psychol Rev. 2013. PMID: 24128603
- The effects of caffeine and d-amphetamine on spatial span task in healthy participants. Kassim FM, Lim JHM, Slawik SV, Gaus K, Peters B, Lee JWY, Hepple EK, Rodger J, Albrecht MA, Martin-Iverson MT. Kassim FM, et al. PLoS One. 2023 Jul 13;18(7):e0287538. doi: 10.1371/journal.pone.0287538. eCollection 2023. PLoS One. 2023. PMID: 37440493 Free PMC article. Clinical Trial.
- Transfer Effect of Cognitive Advantages in Visual Working Memory Capacity: Evidence from Elite Football Players. Wang X, Liu Z, Zhang H, Ji C. Wang X, et al. Behav Sci (Basel). 2023 Jun 2;13(6):464. doi: 10.3390/bs13060464. Behav Sci (Basel). 2023. PMID: 37366716 Free PMC article.
- Blunted Cardiovascular Reactivity Predicts Worse Performance in Working Memory Tasks. Magnúsdóttir BB, Gylfason HF, Jóhannsdóttir KR. Magnúsdóttir BB, et al. Brain Sci. 2023 Apr 11;13(4):649. doi: 10.3390/brainsci13040649. Brain Sci. 2023. PMID: 37190615 Free PMC article.
- Bumetanide induces post-traumatic microglia-interneuron contact to promote neurogenesis and recovery. Tessier M, Garcia MS, Goubert E, Blasco E, Consumi A, Dehapiot B, Tian L, Molinari F, Laurin J, Guillemot F, Hübner CA, Pellegrino C, Rivera C. Tessier M, et al. Brain. 2023 Oct 3;146(10):4247-4261. doi: 10.1093/brain/awad132. Brain. 2023. PMID: 37082944 Free PMC article.
- Mutation of the attractin gene impairs working memory in rats. Liu MQ, Xue C, Li XH, Ding HQ, Zhang MY, Chen K, Li Y, Gao SZ, Xu XJ, Zhang WN. Liu MQ, et al. Brain Behav. 2023 Feb;13(2):e2876. doi: 10.1002/brb3.2876. Epub 2023 Jan 9. Brain Behav. 2023. PMID: 36621889 Free PMC article.
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Taylor & Francis
Other Literature Sources
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Visual memory, the long and the short of it: A review of visual working memory and long-term memory
- Tutorial Review
- Published: 23 April 2018
- Volume 80 , pages 1035–1056, ( 2018 )
Cite this article

- Mark W. Schurgin 1
26k Accesses
47 Citations
5 Altmetric
Explore all metrics
The majority of research on visual memory has taken a compartmentalized approach, focusing exclusively on memory over shorter or longer durations, that is, visual working memory (VWM) or visual episodic long-term memory (VLTM), respectively. This tutorial provides a review spanning the two areas, with readers in mind who may only be familiar with one or the other. The review is divided into six sections. It starts by distinguishing VWM and VLTM from one another, in terms of how they are generally defined and their relative functions. This is followed by a review of the major theories and methods guiding VLTM and VWM research. The final section is devoted toward identifying points of overlap and distinction across the two literatures to provide a synthesis that will inform future research in both fields. By more intimately relating methods and theories from VWM and VLTM to one another, new advances can be made that may shed light on the kinds of representational content and structure supporting human visual memory.

Similar content being viewed by others

Noisy and hierarchical visual memory across timescales
Introduction to the special issue on visual working memory.
Mapping visual working memory models to a theoretical framework
Explore related subjects.
- Artificial Intelligence
Avoid common mistakes on your manuscript.
Introduction
Human visual memory is impressive and fundamental to cognition and remains one of the core areas of human cognition that artificial intelligence (AI) systems have yet to be able to replicate (Andreopoulos & Tsotsos, 2013 ; DiCarlo, Zoccolan, & Rust, 2012 ; Pinto, Cox, & DiCarlo, 2008 ). We can recognize past objects we have seen when given incredibly brief exposures (Biederman & Ju, 1988 ; Potter, 1976 ; Potter & Levy, 1969 ), despite drastic image-level differences across encounters (through variability in factors such as orientation and lighting: Cox & Dicarlo, 2008 ; Cox, Meier, Oertelt, & DiCarlo, 2005 ; Dicarlo & Cox, 2007 ; Rust & Stocker, 2010 ; Wallis & Bülthoff, 2001 ), and even when we have not seen an object for months, years, or even decades (i.e., many people can distinctly remember what subway tokens or an Atari game system look like). The exact computational and neural explanations of this ability remain unknown and are likely key to understanding disorders that impair visual memory and recognition, such as dementia.
The majority of research on visual memory has taken a compartmentalized approach, focusing exclusively on memory over shorter or longer durations, that is, on working or episodic long-term memory, respectively. The purpose of the present review is simply to supply a primer that spans the two areas, with readers in mind who may only be familiar with one or the other. Through this survey, I aim to identify points of overlap and distinction across the two literatures to provide a synthesis that will inform future research investigating the major questions remaining on human visual memory.
Distinguishing visual working memory and visual episodic long-term memory
Memories for visual information are typically distinguished as belonging to either visual working memory (VWM) or visual episodic long-term memory (VLTM). VWM is considered an online system that retains and manipulates information over the short term (Baddeley & Hitch, 1974 ; Cowan, 2008 ; Ma, Husain, & Bays, 2014 ; Vogel, Woodman, & Luck, 2006 ), whereas VLTM is typically defined as the passive storage of visual information over longer periods of time (Brady, Konkle, & Alvarez, 2011 ; Cowan, 2008 ; Squire, 2004 ). Further distinguishing these two systems is that they appear to rely on distinct neural substrates. For example, VWM tends to be associated with activity in the occipital and parietal cortex (Harrison & Tong, 2009 ; Pessoa, Gutierrez, Bandettini, & Ungerleider, 2002 ; Todd & Marois, 2004 ), whereas VLTM is associated with the use of the medial temporal lobe and hippocampus (Squire, 2004 ; Yassa et al., 2011 ; Yonelinas, Aly, Wang, & Koen, 2010 ).
Broadly speaking, it is intuitive to distinguish different memory systems, especially VWM and VLTM, by the time scale over which the memory takes place. However, while time may constrain systems in different ways, it may not be sufficient to understand all possible differences and similarities between them. A more holistic approach may be to distinguish these systems in terms of their functions. If we know the function of a system, we can clearly identify the specific challenges facing such functions, even if we do not know the specific solutions to these challenges a system may choose to employ.
The function of VWM
When establishing the function of VWM, researchers early on defined it as a space divided between storage and other processing demands (Baddeley & Hitch, 1974 ). It is thought to be the combination of multiple processes, providing an interface between perception, short-term memory, and other mechanisms such as attention (Cowan, 2008 ). VWM’s most notable characteristic is that it has a limited capacity (Awh, Barton, & Vogel, 2007 ; Vogel, Woodman, & Luck, 2001 ), and it is thought to be a core cognitive process underlying a wide range of behaviors (Baddeley, 2003 ; Ma et al., 2014 ). Given these descriptions, the function of VWM may be best described as supporting complex cognitive behaviors that require temporarily storing and manipulating information in order to produce actions.
VWM representations must therefore be in a format that is easily amendable and malleable, while remaining general enough to support a wide range of behaviors: it needs to output representations that can be taken as input by relevant processes. For example, an important role of VWM is to bridge gaps in our perception (both spatially and temporally) created by eye movements. Indeed, VWM has been shown to store features of saccade targets for subsequent comparisons (Hollingworth, Richard, & Luck, 2008 ), and the contents of VWM influence even simple saccades (Hollingworth, Matsukura, & Luck, 2013 ). In order to act as this bridge, representations in VWM must be content neutral (i.e., not specific to any one feature or class of objects) and also malleable (in order to integrate and compare information across time).
The function of VLTM
VLTM is typically described in terms of episodic recognition, which is commonly defined as the conscious recognition of visual events (Brady et al., 2011 ; Squire, 2004 ). This is a generally vague description that does not elucidate the function of VLTM. However, we can find insight into the function of VLTM from the study of object recognition. The goal of any object recognition system is to identify an object based on some previous input. Clearly, when VLTM studies utilize images of real-world objects in their tasks, the situation is analogous to an object recognition task. When referring to object recognition here, I mean so explicitly in the context of episodic long-term memories for objects, as opposed to other kinds of learning utilized by long-term memory, such as semantic or categorical information about those objects.
The fundamental challenge facing object recognition is the need for the system to demonstrate both tolerance and discrimination. Footnote 1 When we see an object again, we never encounter that same object exactly the same way twice. We see things briefly, in different contexts, with differences in lighting, viewpoint, or orientation that make direct comparisons of the perceptual input to our long-term memory representations next to impossible (Cox & Dicarlo, 2008 ; Cox et al., 2005 ; Dicarlo & Cox, 2007 ; Rust & Stocker, 2010 ; Wallis & Bulthoff, 1999 ). In order to recognize objects over the long term, our representations need to demonstrate tolerance. By tolerance, I am referring to our ability to recognize the same object across different encounters.
When perceiving or recognizing objects over the short term, your visual system has multiple cues to assist in acquiring tolerance. For example, if you toss a ball and watch it move through the air, the image hitting your retina transforms considerably over the ball’s journey. However, despite drastic changes in the appearance of the ball from moment to moment, you are still able to recognize that the ball is the same at each point in time. This is because your visual system can exploit expectations of spatiotemporal continuity (where an object is in space and time) and as a consequence can demonstrate tolerance to these changes (Kahneman, Treisman, & Gibbs, 1992 ; Scholl & Flombaum, 2010 ; Schurgin & Flombaum, 2018 ). However, over the long term, spatiotemporal continuity and other cues used to facilitate tolerance are unavailable, and thus tolerance must be reflected within long-term memory representations themselves.
While tolerance allows us to identify previous experiences despite considerable changes across encounters, there is a potential danger of overtolerance. One could imagine that if a representation is too tolerant, then it risks an observer mistaking every object she encounters as one that she’s seen before. To address this problem, our representations must also demonstrate a certain level of discrimination. In contrast to tolerance, discrimination refers to our ability to distinguish similar but distinct inputs from one another.
For example, imagine one day my coworker and I are having lunch in a break room and we each have an apple. We place our lunches beside each other and then both exit the room to grab some napkins. When we return, there are two apples on the table that are quite similar in appearance, but one apple is mine and the other is my coworker’s. In order to know which is mine, I must have a representation that is discriminating. Despite the similarities between both apples, I should be able to identify which apple belongs to me.
Similar to issues created by overtolerance, if a memory system is too discriminating then it would operate poorly since an observer would likely fail to recognize any previously seen input unless it was exactly the same as when it was first encountered. Given the variability in our visual inputs described earlier (e.g., due to differences in size, lighting, orientation, etc.), as well as the general assumption that our visual experience is noisy, this is simply an impossible threshold to meet. As a result, there is a natural tension that VLTM must manage between tolerance and discrimination. This is the function of VLTM—to manage this tension in order to recognize a previous visual experience given new input.
Core concepts of VLTM
As discussed previously, VLTM is typically defined as the passive storage system of visual episodic memory. The function of this system is to identify a past visual experience given some previous input, while balancing the need for both tolerance and discrimination. Having defined VLTM and identified its functions, we can expand our understanding of VLTM by establishing what core concepts researchers have identified in the field.
Familiarity and recollection
When trying to understand the nature of VLTM, researchers have focused on investigating the kinds of information VLTM may utilize. One particularly influential distinction in VLTM (and episodic long-term memory research generally) has been recollection and familiarity. Recollection refers to an observer accessing specific details about a previously experienced item. For example, let us say you happen to have gone to a friend’s house for a party the other night and met a variety of new people. In particular, you happened to meet a man named Bob, with whom you talked at length about the Chicago Cubs. The next day, you enter a coffee shop and as you get in line you happen to see that Bob is in front of you. Despite seeing Bob after a long delay, and in a completely new context, you remember all the specific details associated with him—his funky hairstyle, the color of his eyes, along with all the details of when you last met (i.e., your conversation about the Cubs, etc.).
In contrast to recollection, familiarity refers to an observer knowing an item is old or new, without having specific details associated with that memory—“familiarity is the process of recognizing an item on the basis of its perceived memory strength but without retrieval of any specific details about the study episode” (Diana, Yonelinas, & Ranganath, 2007 , p. 379). This notion of familiarity is quite common, and perhaps something we have all experienced. Given the example above, consider an alternate universe where you see Bob the next day in the coffee shop. While you recognize him as someone you know, you cannot remember any of the specific details related to this knowledge, including where you met him or what context you know him from. This is familiarity.
Dual-process signal-detection model
One model used to explain recollection and familiarity processes is the dual-process signal-detection (DPSD) model (Yonelinas et al., 2010 ). The DPSD model specifies that familiarity is primarily a signal-detection process of discriminating between two Gaussian distributions of memory-match strength that differ on average between old and new items, where familiarity occurs when a signal exceeds a decision criterion (see Fig. 1 ). Therefore, your ability to be familiar with something you have seen before depends on the strength of the memory signal and an individual’s decision criterion (how liberal or conservative that individual is being with their decisions). Experiences that are very different (i.e., new) should produce very little to no overlap, so familiarity should not occur. But if given visual inputs that are highly similar (even if you have not necessarily encountered them before), this will likely result in a familiarity signal.
Illustration of the dual-process signal-detection (DPSD) and continuous dual-process (CDP) models. In the DPSD model, familiarity and recollection are two distinct but parallel processes. Familiarity is a signal-detection process of discriminating between two Gaussian distributions between old and new items, where familiarity occurs when the signal exceeds a decision criterion (i.e., participants “know” they saw the item). Recollection is a threshold-based process where signal strength passes a certain threshold and is either recollected or not. In the diagram, both Stimuli A and B pass the threshold and would thus be recollected with the same amount of detail, regardless that each stimulus may illicit different amounts of memory-match signal strength. Stimuli C does not pass the threshold and would thus not be recollected. In the CDP model, both familiarity and recollection vary continuously and operate using signal-detection-based processes. These processes are interactive and are combined during decision-making, resulting in a single distribution for studied items. In the simplest version of the model, familiarity occurs when the memory-match signal exceeds a lower decision criterion (i.e., “know”), whereas recollection occurs when the signal exceeds a higher decision criterion (i.e., participants “remember” the details of the item)
The DPSD model defines recollection as a threshold-based process. In order to recollect an item, an observer must collect enough information. Once enough information is collected, it has passed the “threshold,” and specific details will then be recollected. These classifications are exclusive and are an “all-or-nothing” process. It does not matter if an observer collects more information as long as the threshold has been passed. However, if an observer does not accumulate enough information, the threshold is not passed and recollection will not occur. Figure 1 schematizes this process. For example, let us imagine you have a memory associated with your favorite coffee mug, which has a certain recollection threshold. If you see your coffee mug clearly on your desk, then you receive a strong memory signal (Stimulus A), so the threshold is passed and your memory is recollected. The next day you see the coffee mug on your desk, but it is in a different place and is partially occluded (Stimulus B). While the perceptual information and signal provided is not as strong, you still gain enough information that it passes the threshold and your memory is recollected with the same amount of detail as the day before. However, later in the week, you see your coffee mug from very far away (Stimulus C). While there is some overlapping perceptual information here and you do receive some memory signal, it is not enough to pass your threshold and so you do not recollect the memory.
It is important to note that the example described above is provided to illustrate how a threshold process might operate (specifically within one potential visual context). While sensory overlap may drive recollection automatically in some cases, it can operate in many other circumstances as well. Indeed, in many studies of recollection, subjects are instructed to attempt recall, with varying levels of success. In these studies, a subject’s success or failure to recall information may depend on higher or lower thresholds relative to the memories being recalled.
The DPSD model is based on the claim that familiarity and recollection are two distinct but parallel processes. Despite their distinction from one another, humans likely utilize both processes in a variety of long-term memory tests and behaviors. Familiarity or recollection would both be sufficient to recognize something you have seen before. For example, in order to recognize someone you have previously met, you could rely on familiarity (i.e., a feeling that you have seen that person before) or recollection (i.e., specific details related to that person). Along similar lines, many common long-term memory tests ask observers to classify images as ones they saw during encoding ( old ) or as completely new images. An observer could use either familiarity or recollection to correctly classify an image as old .
Continuous dual-process model
Numerous studies exist in support of the DPSD, extending past humans (Bowles et al., 2010 ; Yonelinas et al., 2010 ) to research involving rodents (Fortin, Wright, & Eichenbaum, 2004 ) and monkeys (Miyamoto et al., 2014 ). However, this is not the only computational approach to understanding the possible distinction between familiarity and recollection. For example, Dede, Squire, and Wixted ( 2014 ) suggested these processes are better described computationally by the continuous dual-process (CDP) model. In this model, familiarity operates exactly as defined in the DPSD model. However, unlike the DPSD, the CDP model proposes that recollection can vary continuously using the same signal-detection process as familiarity. This means recollection is not an all-or-nothing process, and that different memories may illicit different memory-match signals that will vary in detail. Furthermore, during memory decision-making, familiarity and recollection are combined and are thus interactive. Since both familiarity and recollection can vary in confidence and accuracy, observers can combine both sources of information to inform subsequent performance. As a result, the CDP model has only a single distribution for studied items, reflecting variable information from both familiarity and recollection. In the simplest version of this model, familiarity occurs when the memory-match signal exceeds a lower decision criterion (i.e., participants “know” they saw an item), whereas recollection occurs when the signal exceeds a higher decision criterion (i.e., participants “remember” specific details of the item; Dunn, 2004 ; Wixted & Stretch, 2004 ; see Fig. 1 ).
In contrast, the DPSD model assumes these two processes are independent but operate in parallel. As a result, they should not interact. For example, if recollection is successful, then an observer would not use any familiarity information at test, as recollection would provide all the evidence needed to make a decision. However, if recollection failed, then an observer would rely solely on the strength of the familiarity signal.
Both the CDP and DPSD models have similar predictions in terms of performance in recognition tasks, but vary according to the types of systematic errors they would produce. By modeling systematic errors, Dede et al. ( 2014 ) found support that human data was better fit by the CDP model than a DPSD equivalent. This demonstrates that the CDP model is consistent with previous research in support of the DPSD model, but may more accurately capture the specific computations used in recognition decision-making. It also alters the previous distinctions between familiarity and recollection processes by suggesting an interactive relationship, rather than one that is orthogonal.
Potential limitations
It is unclear if researchers intend for the familiarity/recollection dichotomy to designate different types of representations, or different affordances from a single VLTM representation. In general, a feature of these models that leaves them difficult to interpret is that they are content neutral; the same models are applied in the same way regardless of what is to be remembered, and without any claims about how those things are described in symbolic or activation terms.
For example, it is possible that familiarity and recollection each reflect memories with different contents, something like a gist (familiarity) and a more detailed representation (recollection). Yonelinas et al. ( 2010 ) appear to endorse this kind of view, as they argue that recollection and familiarity are separate processes that make independent contributions to recognition memory. This is an intuitive conclusion under the DPSD model, as it specifies familiarity is a signal-detection-based process, whereas recollection is a threshold-based process, and thus likely differ in their content and format. This would suggest that individuals are able to make accurate familiarity-based judgments even when detailed representations fail to consolidate.
But under this view, we should then want specific accounts of the contents and formats in each representation. What goes into a gist? One possibility is that it is simply categorical or semantic knowledge for something you have seen before. Such knowledge would be able to interact with more detailed representations, allowing for observers to utilize both types of information when making memory judgments. However, defining gist in this way seems problematic, since almost any information related to a category should therefore create some familiarity signal.
By defining recollection and familiarity as exclusive processes, this also creates potential limitations in the kinds of information gist and more detailed representations can provide. Can an observer have familiarity with some aspects of an object and recollect others? Given a DPSD framework, this is not possible. Familiarity is recognition without any specific details, and recollection is an all-or-none process. What happens then when an observer confuses a previously seen object with a similar-looking item? Does this mean that they incorrectly recollected that object? Or does it mean that they relied only on familiarity to make their judgment? It is not clear in the DPSD model what information an observer may be relying on when making such errors.
In contrast, the CDP model would be able to account for such differences, as recollection can vary continuously (and is thus not all-or-none) and is also integrated into familiarity-based information when making a decision. Given that both familiarity and recollection can vary continuously, this creates more flexibility for different kinds of memory performance. It is possible for observers to have a strong familiarity signal (and more confidently report they “know” they saw an item), or other experiences where recollection occurred but the signal was weak, resulting in an observer reporting they “know” they saw an item rather than they “remember” all the details related to that item (Ingram, Mickes, & Wixted, 2012 ). For example, an observer could recollect varying amounts of specific details related to an object, which is not possible under the DPSD model.
However, the CDP model has its own limitations. The CDP provides a framework to explain the nature of memory signals but does not explain their underlying representations. The information and structure contained in memory remains unspecified (both in the CDP and DPSD). Again, it would be useful to specify the contents and formats of the representation in some detail to understand how it produces the response patterns taken to indicate familiarity and recollection.
Pattern separation and pattern completion
Another way researchers have tried to understand the nature of VLTM is to take an approach informed by neuroanatomy. The idea is that certain brain areas related to memory have specific properties that may make them amenable to completing different types of processes. Pattern completion and pattern separation is one such model seeking to explain distinctions in memory, but is also supported by brain localization properties.
Pattern completion is the process by which incomplete or degraded signals are filled in based on previously stored representations (Yassa & Stark, 2011 ). After you see an object, the next time you encounter that object the image hitting your retina is not going to be exactly the same (due to differences in viewpoint, orientation, lighting, etc.). Pattern completion is the process that would assist in VLTM being tolerant to variability in inputs related to the same object across encounters.
Pattern separation is the process that reduces the overlap between similar inputs in order to reduce interference at later recall (Yassa & Stark, 2011 ; see Fig. 2 ). This process supports the discriminatory ability of VLTM. It seeks to parse overlapping signals into distinct representations in order to assist in the individual recall of both items.
Illustration of pattern separation and pattern completion processes. Pattern separation is the process by which two overlapping signals are recognized as distinct from one another. Pattern completion is the process by which two overlapping signals are recognized as being from the same source and are combined into a single representation
This model was in part designed to address the issue that we want to be able to remember certain events as related but other events as distinct (i.e., tolerance and discrimination). For example, even when an observer is given different stimuli, considerable overlap might exist between the inputs into the system, causing different items to be incorrectly identified as related to one another (i.e., the problem of saturation). One possible way to address this issue would be to make the input representation very sparse, with a large and distributed network of neurons creating multiple potential pathways of activation for inputs. The hippocampus, which previous examples have shown is critical to episodic and recognition memory, has a region with such properties—the dentate gyrus (DG). This is consistent with pattern separation, which seeks to separate overlapping representations as distinct from one another.
Another issue this model sought to address is to explain how we remember certain events as being related to one another. Specifically, what is the process that recognizes two partially overlapping signals as arising from the same source? One possible solution would be to have a set of pathways that feeds back into itself to fill in potential missing information, so that partial or incomplete signals can be recognized. Another area of the hippocampus, CA3, has such properties, through recurrent collaterals that form a feedback loop (i.e., the axons of neurons in the circuit circle back to the inputs [dendrites] of neighboring axons), consistent with pattern completion computations (Yassa & Stark, 2011 ).
Even though there can be many different computations in support of distinctions in memory, the framework of pattern separation and pattern completion is made more viable by taking into account the specific properties of brain areas that may implement such processes. In fact, recent evidence using rats in a spatial maze has shown that the CA3 demonstrated coherent responses during conflict (when the global cues of the maze were rotated but local information remained the same), consistent with pattern completion, whereas the DG demonstrated disrupted responses during such conflict, consistent with pattern separation (Neunuebel & Knierim, 2014 ). While the contents of these processes are not specified, they provide a useful example for how a distinction supported by brain localization properties may inform our understanding of memory.
Unfortunately, many aspects of pattern separation and pattern completion remain unspecified. This results in a host of potential limitations. Often, this framework is referred to as a “neurocomputational” approach, but the computations of these processes are never defined. There are many possible ways one could design a system to parse or complete the same overlapping inputs that would result in vastly different outputs. In addition, the content and format of these inputs are not specified. This information would greatly affect the kinds of computations one might use to address these types of processes. While the specific representational content and format of pattern separation and completion remains elusive, researchers have begun using new approaches to further define these processes. For example, Schapiro, Turk-Browne, Botvinick, and Norman ( 2017 ) used hippocampal neural network modeling to make more specific assumptions for how pattern separation and completion may be instantiated. Although these assumptions remain primarily in neural terms, further specificity in the neural pathways and areas involved in these processes may help inform their potential representational content and format.
Pattern separation and pattern completion are also limited by their focus to explain memory-based processes exclusively on properties of the hippocampus. Pattern completion shares similarities with familiarity, as both processes could be used to explain how an observer might recognize a previously seen image with degraded input. But pattern completion is specified to occur in the CA3 region of the hippocampus. Thus, it is unable to explain why amnesic patients with brain damage to the hippocampus can still demonstrate unimpaired associative (or familiarity-based) memory performance (Gabrieli, Fleischman, Keane, Reminger, & Morrell, 1995 ).
Theoretical overlap and conclusion
Theories of familiarity and recollection bear some resemblance to pattern separation and completion. For example, familiarity refers to some sort of gist knowledge, when an observer knows they have seen something before but without specific details associated with that memory. And pattern completion is the process through which two overlapping signals are recognized as arising from the same source. One way the gist knowledge of familiarity could be expressed is through a mechanism such as pattern separation—recognizing overlap between signals to recognize an item as previously seen, without necessarily extracting specific details associated with a previous memory.
Along similar lines, recollection refers to an observer accessing specific details about a previously experienced item. This process is critical for our discriminatory ability to distinguish similar-looking items from ones we have seen before. Indeed, in order to assess recollection-based memory many times researchers give participants lures (very similar-looking items) at test and assess their ability to discriminate these lures. Pattern separation describes exactly the process through which a lure may be distinguished as being distinct from a previously studied item. Thus, while pattern separation has distinct functions and neural predictions in comparison to recollection, there is clearly overlap between these two theories of memory.
Regardless of the potential similarities between these models, and despite disagreements in the field as to which better explains VLTM, there is a critical consensus among these models that there are different kinds of information VLTM may rely on. This generally involves some sort of gist information associated with a previous item (familiarity, pattern completion), as well as more specific visual details related to that same item (recollection, pattern separation). Both kinds of information likely affect decision-making in a variety of VLTM tasks. As a result, it is important to keep in mind that some tasks may rely on one process or kind of information more than another.
Common methods in VLTM
Old/similar/new judgment.
One way to measure the discriminatory ability of VLTM is to utilize a paradigm often referred to as the old/similar/new judgment. Participants first view and encode images of objects, typically while doing an incidental encoding cover task (e.g., would this object fit in a shoe box?). At test, participants serially view objects that were present in the encoding task (old images), objects similar but not identical to ones in the encoding task (similar images), and completely new objects that were not present during encoding (new images). Participants then judge whether the images they see are old, similar, or new (see Fig. 3 ).
Illustration of typical VLTM methods. In a general incidental encoding task, participants see a stream of images of real-world objects and make some judgment about those objects (i.e., indoor/outdoor, does this fit in a shoe box?). After, they may be tested using several methods. Using old/similar/new judgment, participants are shown objects that were exactly the same as encoding (old), similar but not identical to images at encoding (similar) and completely new images (new), and are asked to classify them accordingly. In two-alternative forced-choice tests, they are shown two images, one they have seen before and either a completely new (old–new comparison) or similar looking object (old–similar comparison) and are asked to judge which of the two images they have previously seen. In delayed-estimation tasks, participants are initially shown a grayscale image of a previously seen object and are asked to report its color using a color wheel. (Color figure online)
The primary purpose of this task is to evaluate the discriminatory ability of VLTM, and how memories may acquire different levels of discriminability. For example, correctly classifying a similar item likely requires an observer to have more specific details in memory (i.e., recollection-based) than correctly identifying an old item, which could be accomplished using a gist-based or familiarity-based process (Kensinger, Garoff-Eaton, & Schacter, 2006 ; Kim & Yassa, 2013 ; Schurgin & Flombaum, 2017 ; Schurgin, Reagh, Yassa, & Flombaum, 2013 ; Stark, Yassa, Lacy, & Stark, 2013 ).
A potential limitation of old/similar/new judgments is that it is not clear what constitutes a false alarm for similar items. In contrast, when using a simpler old/new procedure researchers can obtain an unbiased measure of memory discriminability ( d' ) by taking the difference between the normalized proportion of hits (correctly classifying an old item is old ) and the normalized proportion of false alarms (incorrectly classifying a new item as old ; Green & Swets, 1966 ). However, what constitutes a false alarm for a similar judgment? Is it classifying an old item as similar? Or a new item as similar? Or a similar item as old? Without a clear understanding of what constitutes a false alarm, it is difficult to normalize responses given to similar items for potential biases. While different analyses have attempted to address this issue, research suggests analyzing old/similar/new responses using a signal-detection-based framework ( d a ) may provide an accurate, unbiased measure of memory performance for similar items (see Loiotile & Courtney, 2015 ).
How does emotion affect visual memory?
One study that utilized this method sought to investigate how negative emotional context may affect the likelihood of remembering an item’s specific visual details. Participants first completed an incidental encoding task, where they were exposed to hundreds of images of real-world objects (for 250 ms or 500 ms) and had to judge whether each object would fit in a shoebox. Half of the images were rated as being negative and arousing, and the other objects were rated as neutral. Two days after incidental encoding, participants were then given a surprise test. Participants viewed old images (exactly the same as encoding), similar images (similar but not identical), and completely new images relative to encoding. They were told to classify them accordingly (old/similar/new; Kensinger et al., 2006 ).
At test, it was observed that for old items, negative emotional context led to an increase in correct classification. This was true for items presented both for 250 ms and 500 ms, but was stronger as encoding time increased. However, for similar items there was no main effect of emotional context or encoding time. As a result of the main effect of emotional context for old images, the researchers concluded that negatively arousing content increased the likelihood that visual details of an object would be remembered (Kensinger et al., 2006 ). However, given the lack of an effect for similar images, it would be more accurate to conclude that emotion may have enhanced certain aspects of visual memory (i.e., for old images).
How do familiarity and recollection contribute to responses?
This method has also been used to investigate the relative contributions that familiarity and recollection processes may have in the behavioral responses of old, similar, and new judgments. In the study, participants completed a two-stage recognition test. In the first phase, participants saw 128 images of real-world objects on a computer screen for 2 seconds each, and were asked to report whether an object was an “indoor” or “outdoor” object. In the second phase, participants were given a surprise test where they viewed old images (exactly the same as encoding), similar images (similar but not identical), and completely new images. They were told to classify the images as either old, similar, or new. Additionally, after indicating what category an image belonged to, participants were then instructed to indicate whether they “remember” seeing the same image in the study session or if they just “know” that they have seen the same image without any conscious recollection of its original presentation (Kim & Yassa, 2013 ). It is theorized that “remember” judgments reflect recollection-based processes, whereas “know” judgments reflect familiarity-based processes, although there exist several criticisms that these are not true indices of these processes but rather reflect subjective states of awareness or differences in confidence (Yonelinas, 2002 ).
As expected, at test they found very different accuracy for classifying old (70% correct), similar (53% correct), and new (74% correct) images. When analyzing these responses according to whether a participant reported they “remembered” (recollection based) or “knew” (familiarity based), a few interesting patterns emerged. When judging old items correctly, observers made primarily “remember” responses, suggesting that correct classifications of old items was primarily driven by recollection. For similar items, there was a slight trend to report “remember” rather than “know” both when the item was judged as old or similar. This suggests that observers can classify similar items with or without recollection, and that incorrectly identifying similar items (i.e., misclassifying them as old) is not simply driven by familiarity signals (Kim & Yassa, 2013 ).
Source localization judgments
One approach to evaluate the strength of memory and further distinguish potential errors is to use a paradigm combining old/new judgments with source localization. At encoding, participants view images of objects typically presented in one of four quadrants in the display. Then, at test, participants are shown old, similar, and new images in the center of the screen they must classify as old (previously seen images) or new (similar or new images), and indicate which of the four quadrants the object originally appeared in (Cansino, Maquet, Dolan, & Rugg, 2002 ; Reagh & Yassa, 2014 ; see Fig. 4 ).
Illustration of typical localization VLTM methods. In a general incidental encoding task, participants see a stream of images of real-world objects and make some judgment about those objects (i.e., Does this fit in a shoe box?). Critically, every image is presented in one of four (or more) possible quadrants. After, they may be tested using several methods. Using old/new and localization tasks, participants are shown objects that were exactly the same as encoding (old) and similar-looking or completely new images (new), and are asked to classify them accordingly. If they classify an object as old, they are then asked to indicate which of the four quadrants the image originally appeared in. In classification tasks, participants are shown either repetitions (old image, same location), object lures (similar images, same location), spatial lures (old image, different location), or new images. They are asked to classify the images accordingly
The underlying assumption of source localization manipulations is that more episodic information is retrieved on trials when the source judgment was successful than on trials when it was not. This is similar to the logic behind the remember/know procedure discussed previously. When an observer makes a correct classification and source judgment, the assumption is that this indicates a recollection-like memory, whereas if an observer makes a correct classification of an image but an incorrect source judgment, this may indicate a familiarity-based memory. However, unlike the remember/know procedure, source localization judgments do not rely on the observer’s own introspection in order to classify memory quality. Thus, the aim of the paradigm is to evaluate what percentage of responses using old/new judgments may rely on memories that contain more or less information, and what brain areas may be involved in these processes.
What brain areas and behaviors are involved in source judgments?
Cansino et al. ( 2002 ) were interested in using this method to investigate what brain regions may be involved in different memory processes beyond simple item recognition tasks. To accomplish this, participants first viewed images of real-world objects and were asked to judge whether the objects were natural or artificial. Critically, each image was presented in one of four quadrants in the display. After completing the task, participants were then administered a surprise test where previously shown images (old images) were mixed with completely new images. These images were shown in the center of the screen. Participants had to judge whether each image was old or new. They were instructed to press a single key if an image was new, and if an image was old participants indicated which position the image was presented during encoding using one of four keys. If a participant did not know which quadrant an old image originated from, they were instructed to guess.
At test it was found that when classifying previously seen (old) items, observers correctly identified 87% of items presented. However, 60.7% of these responses contained correct source responses, and 26.3% of responses contained incorrect source responses. This suggests that even when classifying old and new objects in a typical retrieval task, memories for these items likely contain additional information beyond simply categorical or familiarity-based knowledge. Additionally, they observed via collected fMRI data that when recognizing an old object with a correct versus incorrect source judgment, there was greater activity observed in the right hippocampus and left prefrontal cortex (Cansino et al., 2002 ). This suggests that memories containing more information may elicit greater memory signals and decision-making coordination.
Potential neural correlates of “what” and “where” memory
Source localization judgments have also been used to explore possible dissociations between object (what) and spatial (where) memories and their potential neural correlates. In the experiment, participants first completed an encoding task where images of real-world objects were presented in one of 31 possible locations on the screen for 3 seconds each. They were instructed to first judge whether the object was an indoor or outdoor object, and then whether the object appeared on the left or right relative to the center of the screen. Afterward, participants were given a surprise test with four possible trial types: repeated images (old images in the same location), lure images (similar images in the original object’s location), spatial lure images (old images in a slightly different location), or new images (not shown during encoding). Participants were instructed to indicate whether an image showed no change, object change, location change, or new (Reagh & Yassa, 2014 ).
Behaviorally, there was no difference in lure discrimination whether it was an object trial (i.e., similar image) or spatial trial (i.e., old image in slightly different location). This effect was consistent across both high-similarity and low-similarity stimuli. Neuroimaging data were also collected via fMRI and demonstrated unique differences based on lure type. It was observed that the lateral entorhinal cortex (LEC) was more engaged during object lure discrimination than during spatial lure discrimination, whereas the opposite pattern was observed in the medial entorhinal cortex (MEC). Additionally, the perirhinal cortex (PRC) was more active during correct rejections of object than spatial lures, whereas the parahippocampal cortex (PHC) was more active during correct rejections of spatial than object lures. Regardless of lure type, the dentate gyrus (DG) and subregion CA3 demonstrated greater activity during lure discrimination. Overall, this suggests two parallel but interacting networks in the hippocampus and related regions for managing object identity and spatial interference (Reagh & Yassa, 2014 ).
Two-alternative forced-choice test (2AFC)
A paradigm referred to as the two-alternative forced-choice (2AFC) test has been primarily used to study the capacity of visual episodic long-term memory. In a typical test, observers see two objects on the screen during test, one that they have seen before and another object they have not encountered previously. The other may be completely novel (old–new comparison) or a similarly related lure (old–similar comparison; Brady, Konkle, Alvarez, & Oliva, 2008 ; Brady, Konkle, Oliva, & Alvarez, 2009 ; Konkle, Brady, Alvarez, & Oliva, 2010b ). The logic of this test is that it can tap into even “weak” memories that other methods may fail to reveal. It makes sense that a 2AFC judgment is easier than other kinds of responses because it is a binary response.
Typically, 2AFC is conceptualized using a signal-detection-theory framework (Green & Swets, 1966 ; Loiotile & Courtney, 2015 ). The logic is that observers have a memory representation that creates a normally distributed signal in a “memory strength” space. At test, when observers are shown an old item, that item elicits a normally distributed memory-match signal, which due to noise and other factors will vary in strength. If an observer was simply shown the old object, depending on the decision criteria this may result in incorrectly identifying an old object as new . However, by giving observers a foil in the 2AFC task, whether that foil is a completely new or similar-looking object, this gives observers a second normally distributed signal to assist in the comparison process. This second signal should be centered around a lower memory-match signal (i.e., zero) than the old image. As a result, observers can simply pick the max between the two items to correctly identify the old image (Macmillan & Creelman, 2004 ; see Fig. 5 ). This framework demonstrates from a modeling perspective why 2AFC tasks should be easier and are able to provide better performance for items that may otherwise fail to be remembered or classified correctly in other types of memory tasks—it is always easier to pick the max of two things. Footnote 2 Moreover, this performance is higher by a fixed amount, suggesting 2AFC taps into the same underlying memory signal as old/new testing procedures (Macmillan & Creelman, 2004 ).
Visualization of 2AFC logic. At test, observers are shown both an old and new image, which elicit their own normally distributed memory-match signals. While the old item signal may vary in the strength of its memory signal, it is more likely to be higher than the signal of the new item. By simply picking the item with the maximum signal, observers are likely to choose the old item. Thus, with the same underlying memory strength, 2AFC test procedures produce better memory performance compared to testing procedures where observers are only shown the old image
In addition to traditional 2AFC tasks, which involve an old item paired with a new or similar-looking item, researchers have also expanded on the number of potential options, creating 3AFC and 4AFC-type tasks. Generally, these tasks involve the addition of multiple similar-looking lures at test, in order to further evaluate the ability to discriminate between different kinds of lures. A potential limitation of 3AFC or 4AFC tasks is that adding lures may create interference and increase task difficulty, such as through adding increased decision noise (Holdstock et al., 2002 ). Additionally, when varying the kinds of lures available at test (such as providing two similar lures, or a similar lure and a completely new image), this creates conditions where the information available to an observer is not equivalent (Guerin, Robbins, Gilmore, & Schacter, 2012 ). This means performance across different testing conditions cannot be directly compared with one another.
The capacity of VLTM
Brady et al. ( 2008 ) sought to investigate the capacity of VLTM using a 2AFC method. Participants were presented 2,500 images of real-world objects for 3 seconds each and told to remember all the details of each image. After completing this study portion, participants were then given a 2AFC task where they saw two images on the screen. One was a previously encountered image from the previous session, whereas the other was either a novel image, an exemplar of an object they had previously encountered, or an image of an object they had previously encountered in a new state (i.e., changed orientation). Participants were instructed to indicate which of the two images they had previously encountered. Overall, performance was quite high, with significantly better accuracy for novel comparisons (92% correct), a replication of previous work by Standing ( 1973 ) showing incredibly high performance for novel test comparisons even when encoding 10,000 items into VLTM (see also Shepard 1967 ). However, quite surprisingly they observed extremely accurate performance for state and exemplar comparisons (87%–88% correct; Brady et al., 2008 ). These results demonstrate that even when given very brief exposure of images, humans are able to remember thousands of objects (seemingly with no limit) with extremely high accuracy. Furthermore, they suggest that human’s VLTM representations contain visual information necessary to assist in making difficult state and exemplar comparisons, beyond simply categorical or semantic knowledge of previous encounters.
Similar results have been found not just for objects but for the visual memory of scenes as well. Konkle et al. ( 2010a ) demonstrated that after studying thousands of images of scenes, participants were able to recognize 96% of the previously seen images in a novel comparison test. Again, they also observed that performance for test comparisons with a similar foil was quite high, with participants 84% correct even when they studied four of the same exemplar (a potential source of interference) during encoding. This suggests that the incredibly large capacity and high-fidelity representations observed in visual long-term memory are not isolated to a specific stimulus class (i.e., objects or scenes), but rather appear to be general properties of the system.
Given the logic of 2AFC discussed previously, one could assume if participants had been given a single image of an object at test and told to discriminate whether it was old or new, performance would be worse (despite the same underlying memory strength). Thus, the results discussed above are best described as a potential upper bound of VLTM performance. Under different testing procedures, performance will likely differ. However, these results still demonstrate that under potentially ideal testing conditions, visual long-term memory not only has a massive capacity but also contains representations with visual-rich and detailed information.
Delayed estimation (continuous report)
In order to estimate the fidelity of VLTM (i.e., the amount of information in memory) experiments have utilized a delayed estimation paradigm. At encoding participants observe objects embedded in unique colors. Then, at subsequent test, observers see grayscale versions of objects they observed previously and use a color wheel to indicate its original color. By taking the error in degrees between the response and the true value, researchers can create a distribution of long-term color memory responses and measure the standard deviation of the distribution to understand the fidelity of that representation (Brady, Konkle, Gill, Oliva, & Alvarez, 2013 ).
The precision of information in VLTM representations
Brady et al. ( 2013 ) used this method to understand the precision of color memory representations across VWM and VLTM. In the study, researchers gave participants two separate tasks. In the VWM condition, participants saw three real-world objects simultaneously for 3 seconds, arranged in a circle around fixation. Participants were instructed to remember the color of all the objects. After a 1-second delay, one of the objects reappeared in grayscale, and participants could alter the color of the image using their mouse and were told to click the mouse when it matched the original color. In the VLTM condition, participants first underwent a study block viewing images sequentially for 1 second each, with a 1-second blank interval between images. Similar to the VWM condition, participants were instructed to remember the color of the object.
After the study block, the color of the items was tested one at a time in a randomly chosen sequence, and participants reported the image color using the same response mechanism used in the short-term memory condition. The precision of participants’ memory representations was determined by calculating the distribution of the degree error of each response in color space (with larger mistakes represented by greater degree error). In the VWM condition, at Set Size 3 (and above), participants’ precision was 17.8 degrees, which did not significantly differ from the precision observed in the VLTM condition, 19.3 degrees (Brady et al., 2013 ). Therefore, it appears the precision of color representations across VWM and VLTM have equivalent limits, suggesting they may share or be constrained by similar processes.
Interactions between VLTM and perception
Delayed estimation has also been used to investigate the potential role long-term memories may have when biasing new perceptual information. In a series of experiments by Fan, Hutchinson, and Turk-Browne ( 2016 ), participants completed a set of initial exposure trials, where they encountered unique shapes embedded in specific colors. Each shape was shown for half a second, and after a brief delay (1.5 seconds) an achromatic version of the shape reappeared, and participants reported the color of the image using their mouse. During the initial exposure session, participants encountered the same shape three times on separate trials (randomly assorted), always in the same color. As a result, each unique shape was associated with a specific color in long-term memory.
After completing the initial exposure trials, participants were then given final test trials. These final test trials were similar, except now each shape was shown in an unrelated color, and participants had to judge the appearance of the new colors. Researchers found that participants’ responses for the final test trials were best characterized as a mixture of the original and current-color representations, suggesting participants had anchored their responses to their representations in long-term memory. Moreover, this anchoring effect increased when perceptual input became more degraded (for example, by shortening the stimulus presentation during the final test trials). These results demonstrate that while perceptual judgments do reflect the current state of the environment, they can be affected by previous experience and long-term memory (Fan et al., 2016 ).
Core concepts of VWM
As previously discussed, VWM is typically thought of as the interface of multiple processes including perception, short-term memory, and attention (Baddeley & Hitch, 1974 ; Cowan, 2008 ). Due to this conception, researchers have generally described the function of VWM as supporting complex cognitive behaviors that require temporarily storing and manipulating information in order to produce actions (Baddeley, 2003 ; Ma et al., 2014 ). In particular, a large body of research over the past decade has focused on the capacity limitations of VWM. As a result, many of the models proposed to explain VWM have focused on this limitation.
Fixed slot model
When trying to understand the inherent capacity limitations of VWM, a particularly influential model has been the fixed slot model. It suggests that VWM can only store a discrete number of integrated object representations (see Fig. 6 ). This model was proposed by a highly influential study conducted by Luck and Vogel ( 1997 ), who used a change-detection task to quantify VWM capacity. In the task, participants were instructed to remember an array consisting of items of a single or a conjunction of features (color, orientation, etc.). After a brief delay (900 ms), a test array was presented that was either identical to the previous array or differed in terms of a single feature. Participants were instructed to indicate whether a change had occurred. Accuracy was assessed as a function of the number of items in the stimulus array in order to determine how many items could be accurately maintained in VWM.
Illustration of VWM models. According to the fixed slot model, VWM can only store a discrete number of integrated objects. The continuous resource model proposes that VWM has a finite resource that becomes more thinly distributed as the number of items in a display increases. And the flexible slot model is an integration of the latter two, stating VWM has a discrete number of slots, but that a finite resource can be flexibly allocated to each slot
In a series of experiments, Luck and Vogel ( 1997 ) gave participants change-detection tasks that varied the number of colored squares presented in an array (one to 12). They observed that performance was at ceiling for arrays of one to three items, and then declined systematically as set size increased from four to12 items. Overall, the average K value (estimations of VWM capacity) among participants was around three or four items. This finding led to the foundation of the slot model, which posits that individuals can only store between three and four objects in VWM.
In addition to experiments consisting of arrays of single features, Luck and Vogel ( 1997 ) also presented participants with arrays consisting of a conjunction of features (i.e., lines differing in orientation and color). Participants completed a change-detection task, but the researchers varied whether participants had to remember a single feature or a conjunction of features. For example, participants would see an array consisting of lines of different orientations and colors. In the color condition, only a color change could occur, and participants were instructed to look for a color change. In the orientation condition, only an orientation change could occur, and participants were instructed to look for an orientation change. And in the conjunction condition, either a color or orientation change could occur, and participants were instructed to remember both features of each item. Thus, in the conjunction condition, participants had to remember eight features but only four integrated objects. If VWM storage capacity is limited by individual features (e.g., color, orientation), then performance should decline at lower set sizes in the conjunction compared to the single feature conditions. However, if VWM storage capacity is limited by integrated objects (e.g., one red, horizontal line), then the same pattern of results should be observed throughout all three conditions.
Consistent with the latter case, they observed that VWM capacities were the same for single feature and conjunction items. Altogether, this provided the basis for the fixed slot model that VWM capacity was constrained by slots of ~3–4 integrated objects. While further research has expanded upon these initial findings, it is also important to note that several experiments have failed to replicate the critical conjunction experiments. These follow-up studies have observed that VWM capacity is, in fact, reduced as feature load increases, irrespective of the number of objects (Fougnie, Asplund, & Marois, 2010 ; Olson & Jiang, 2002 ; Wheeler & Treisman, 2002 ). To explain performance for conjunction conditions, researchers must consider both feature load (i.e., number of features to be remembered) and object load (i.e., number of objects in the display; Hardman & Cowan, 2015 ). Taken together, the current consensus in the field is that there is a cost to maintaining integrated features in VWM.
A key component of this model is that these VWM slots are considered all or nothing—an observer either remembers every object with the same fidelity (i.e., amount of information) within the capacity limit or fails to remember the object completely. This all-or-nothing component is potentially problematic, as it suggests that an observer has the same amount of information per item whether they viewed single or multiple items. What if an observer sees two encoding displays in a typical change-detection task: one with a single image of an apple and another with four images of very similar looking apples? In each test array, there is either no change, or one of the objects is replaced with another very similar looking image of an apple. According to the fixed slot model, the precision of information available to the observer is the same in both conditions. It does not account for the potential interference four very similar objects may have in terms of their representations in VWM, or how they may affect the decision-making process when an observer decides whether a change has occurred.
Continuous resource model
Another model used to explain apparent limitations in VWM is referred to as the continuous resource model. Unlike the fixed slot model, which defines capacity as being constrained by all-or-nothing slots of integrated objects, the continuous resource model conceptualizes VWM capacity as information based and limited by a finite resource (see Fig. 6 ). Furthermore, this finite resource can vary unevenly across different items in a display. This unequal division of resources across representations can differ due to a variety of factors, such as top-down goals (e.g., attention) or the total information load of the display (i.e., set size; Bays & Husain, 2008 ; Wilken & Ma, 2004 ).
Support for the continuous resource model came from Wilken and Ma ( 2004 ), who developed the continuous report method as a way of measuring the fidelity, or amount of information, contained in VWM representations. In the continuous report paradigm, participants are instructed to remember an array consisting of items of a single feature (color, orientation, etc.). After a brief delay (1.5 seconds), a square cue appeared centered at the location of one of the previously presented items. At the same time, a test probe is displayed in the center of the screen, which allows for continuous report of the probed feature. For example, in the color condition, a color wheel containing all possible color values appears in the center of the screen, and participants indicate the color of the probed item by clicking a color on the wheel, using a mouse. Responses are then reported as the degree error from the true color value, and a distribution can be made based on a participant’s responses. The standard deviation ( SD ) of this distribution can then be used to estimate a participant’s precision of color information in VWM.
In a series of experiments, Wilken and Ma ( 2004 ) varied the set size of displays as well as the type of feature being probed in memory. Regardless of the type of feature probed, they observed that as set size increased, the precision of VWM representations decreased. However, even at large set sizes, the distribution of responses was still centered around the true value of an item, and the precision (i.e., SD ) was large but still well above chance. This led the researchers to conclude that individuals could store a continuous amount of information in VWM, but the precision with which an individual item was represented varied as a function of the total information load of the display (i.e., set size). When set size is greater than four items, observers are able to store more than four items in memory. However, fewer resources are available to allocate to each item. Given the constraints of a typical change-detection task, an item may still be represented in VWM, but without the necessary amount of information to make a successful comparison. This suggests that the ~3–4 object limit proposed by the fixed slot model may simply be a behavioral artifact of the tasks being used to assess such limits.
A potential limitation of the research used to support the continuous resource model is that variable precision has not been shown for holistic representations of objects. Studies in support of this model typically probe memory along a single feature dimension (i.e., color), even if observers are viewing images of real-world objects. It remains possible that representations for objects in VWM rely on different kinds of information, some of which may be variable and others that may not. For example, when an observer sees a single image of a real-world object, their representation of that object may contain categorical knowledge (e.g., teddy bear) in addition to other knowledge such as color (e.g., brown). The information of color available to the observer may be variable as a function of set size, but it is likely that categorical knowledge is not—observers either know the categorical identity of an object or they do not.
Work by Schurgin and Flombaum ( 2015 , 2018 ) provides evidence this may be the case. In their task, participants saw two images of real-world objects in a display, that were then briefly masked, and participants then had to make a 2AFC judgment containing a previously seen object and a completely new object (indicating which of the two was the old object). Critically, they added image noise to stimuli at test by randomly scrambling up to 75% of the pixels in each image. They observed that VWM performance was unaffected by noise at test, with 0% noise and 75% noise demonstrating the same level of performance. The continuous resource model predicts that noise at test should make comparisons in memory harder, as observers would be comparing a noisy internal representation to a noisy external representation (i.e., test stimuli). Thus, when noise is added at test, performance should decrease. In contrast, the fixed slot model would predict no performance decrease. Observers would have no noise in their internal representation, which would allow them to manage noise at test when making a comparison. Altogether, this work suggests that while resolution for memory could vary along a continuous resource for a single feature, such as color, the same may not be true along all dimensions, such as holistic object representations.
Flexible slot model
Contrasting evidence exists that may support either the fixed slot or continuous resource models. The flexible slot model provides a middle ground between the two, proposing that VWM is constrained to a maximum of ~3–4 representations, but that this capacity may also be limited by the amount of information load in the display. In short, VWM is constrained by slots, but there is flexibility within the system for distributing limited resources across these slots.
One study interpreted by some to support the flexible slot model was conducted by Alvarez and Cavanagh ( 2004 ). They utilized a typical change-detection task but varied the information load of displays by changing the type of stimuli presented. On each trial, one to 15 objects were presented for 500 ms, followed by a brief delay (900 ms), and then a test array. On half the trials, one of the objects changed identity, and on the other half, the displays were identical. Participants were instructed to indicate whether one of the objects had changed. Critically, trials could contain stimuli pertaining to a single stimulus class that each differed in their visual complexity: line drawings, shaded cubes, random polygons, Chinese characters, and colored squares.
If VWM is limited by a fixed number of representations (i.e., slots), then performance should be equivalent across all stimulus categories, but if VWM capacity is limited by information load, then these estimates should vary across stimulus categories. After converting responses to K estimates of VWM capacity, Alvarez and Cavanagh ( 2004 ) observed there was varying capacity estimates across different stimulus classes, ranging from 1.6 for shaded cubes to 4.4 for colored squares. This provided support that VWM is limited in its number of representations (~4 objects), but also limited by the amount of information (i.e., stimulus complexity) of what is being remembered. However, while some may interpret these results in support of the flexible slot model, a limited number of representations in VWM is not necessarily the same as objects being stored in a slot-like way.
There is disagreement as to whether these differences reflect storage limitations, therefore supporting a flexible slot model, or rather reflect comparison errors made during the decision-making process. It could be that items with higher visual complexity also have higher similarity to one another, and this will lead to greater errors at test even though overall memory capacity for items is the same. Awh et al. ( 2007 ) investigated this possibility using Alvarez and Cavangh’s ( 2004 ) method and stimuli, but with one critical modification: categorical change. When a change occurred at test, they could either be within-category changes (as in Alvarez & Cavanagh; i.e., a Chinese character replaced with a different Chinese character), or they could be between-category changes (i.e., a Chinese character replaced with a line drawing). They observed that for within-category changes, performance varied across stimulus categories, consistent with Alvarez and Cavanagh. Conversely, for between-category changes, they observed no difference in performance relative to stimulus category. This was taken to suggest that variable performance across different kinds of stimuli may be due to differences in similarity, and not information load (consistent with the fixed slot model). However, recent research has found these between-category changes are primarily driven by the use of global ensemble or texture representations. When objects are clustered in a display by type, individuals can discriminate based on a change in clustering (i.e., using an ensemble or texture representation) rather than one based on individual item memory (Brady & Alvarez, 2015 ). These results suggest the need for more flexible models of VWM that integrate a role for spatial ensemble representations.
Considerations affecting VWM beyond capacity
An important consideration to our theoretical understanding of working memory is that there are large individual differences in performance, specifically in relation to capacity. Previous research has found capacity estimates of VWM vary substantially across individuals, ranging from 1.5 to 5.0 objects (Vogel et al., 2001 ; Brady et al., 2011 ), and that these individual differences in capacity correlate strongly with broad measures of cognitive function, such as academic performance (Alloway & Alloway, 2010 ) and fluid intelligence (Fukuda, Vogel, Mayr, & Awh, 2010 ). These individual differences in capacity and relationships with other cognitive functions are likely because VWM is the combination of multiple processes, including short-term memory and executive control mechanisms, many of which vary in performance across individuals (Baddeley, 2003 ; Conway, Kane, & Engle, 2003 ).
In the context of the models discussed above, each could be easily amended to account for individual differences. Whether VWM capacity is limited by a fixed or continuous resource, either could conceivably vary across individuals. However, understanding and explaining these individual differences remains key in theoretical discussions of working memory, as they could be the result of potentially different sources. For example, it could be that individual differences in capacity are the result of individual differences across a resource (whether fixed or continuous). Alternatively, these capacity limitations could arise from other components contributing to working memory performance, such as executive function or attention (Baddeley, 2003 ; Conway et al., 2003 ). Indeed, research has found potential limitations or biases in VWM can arise from the combination of different sources of information, such as the memory of an item in combination with categorical information (Huttenlocher, Hedges, & Duncan, 1991 ), nonuniformities in attention (Schurgin & Flombaum, 2014 ), or ensemble information (Brady & Alvarez, 2011 ). As a result, understanding the source of individual differences in working memory capacity remains critical to our overall understanding of how capacity limitations arise.
In an effort to explain the capacity limitations of VWM, all the models discussed above also make an implicit assumption that the fidelity of VWM representations is directly affected by the number of objects to be remembered. However, recent research has demonstrated that even with a fixed number of items in a display there is variability across trials in the precision of VWM (Bae, Olkkonen, Allred, Wilson, & Flombaum, 2014 ; Fougnie, Suchow, & Alvarez, 2012 ; Van den Berg, Shin, Chou, George, & Ma, 2012 ). This suggests there are not only differences in visual working memory limits across individuals but that the quality of working memory representations varies within an individual as well. Moreover, this variability cannot be explained by fluctuations in attention or capacity resources (i.e., slots, continuous resource, etc.; Fougnie et al., 2012 ).
To better account for these results, researchers have proposed different kinds of variable precision models. These models account for random fluctuations in encoding precision that tend to occur from trial to trial and have been shown to fit data better than traditional fixed slot or continuous resource models (Fougnie et al., 2012 ; Van den Berg et al., 2012 ). However, it is important to note that variable precision does not necessarily favor either a slot or continuous resource account of working memory capacity (Van den Berg & Ma, 2017 ). As a result, the role of variability affecting VWM fidelity should be treated as separate from the source of capacity limitations described by the models above.
Common methods in VWM
Change-detection task.
In order to understand the capacity of VWM, many experiments have utilized a change-detection task. In this paradigm, observers first see an array of items (whether a single feature, conjunction of features, or images of real-world objects) and are told to remember what they saw. The array then disappears and after a brief delay reappears with either all the items exactly as before or with a single item changed. Observers are asked to identify whether a change occurred or not (see Fig. 7 ). The general goal of this paradigm is that by varying the number of items in a display (two, four, eight, etc.), researchers can investigate what kinds of constraints may be limiting VWM capacity (Awh et al., 2007 ; Luck & Vogel, 1997 ; Vogel et al., 2001 , 2006 ; Xu, 2002 ).
Illustration of typical VWM methods. In a change-detection task, participants are briefly shown an initial array of items (colors, objects, etc.). After a delay, they are then shown a test display. Half of the time the display is exactly the same, whereas in the other half one of the items in the display has changed. Participants are told to indicate whether a change occurred. In the single probe report task, at test participants are only shown one item instead of the entire display (with or without a change). In the continuous report task, at test one of the previous items is probed and participants indicate their memory for a single feature in a continuous space (i.e., report the previous color of the item using a color wheel). (Color figure on line)
A variation of the change-detection task uses a single probe report, showing only one item at test instead of the entire display (with or without a change). A potential advantage of single probe change-detection tasks is that it may be harder for observers to use relational or summary statistical information (i.e., how much the overall display was “blueish” and whether the test display is different in the overall “blueishness”) to inform their judgments. A potential disadvantage of this procedure is that if visual memory for certain stimuli (objects, scenes, etc.) relies on relational information (i.e., position, layout, etc.), then probing a single item may remove important information and erroneously reduce performance. It may also be the case that when participants are given the entire display at test, this encourages them to remember the entire display. It is worth nothing that VWM capacity estimates have been found to be comparable whether they were based on the whole-display or a single-probe procedure (Luck & Vogel, 1997 ; Jiang, Olson, & Chun, 2000 ).
Time course of consolidation in VWM
Vogel et al. ( 2006 ) were interested in understanding the time course of consolidation in VWM using typical change-detection methods. By consolidation, they meant the process whereby VWM becomes durable enough that it is not disrupted by new sensory inputs. The goal of the study was two-fold: to measure the time course of VWM consolidation and whether the rate of consolidation varied as a function of the number of items being consolidated. In the primary experiment, participants viewed arrays of between one and four colored squares. In order to interrupt consolidation, pattern masks at the location of the colored squares were introduced between 117 and 484 ms from the initial onset. After a brief delay (controlled to be the same across different mask interruption timings), a test array was presented that either contained a change or no change. Participants were instructed to judge whether a change occurred. Across Set Sizes 2–4, performance declined when the delay between the initial encoding array and the onset of the mask was shorter, and this effect became larger as set size increased. Overall, the rate of consolidation was estimated to be ~50 ms per item (although it may be faster, ~10 ms per item; see Sperling, 1963 ). These results suggest that consolidation is limited in capacity (increasing in time as set size increases) but occurs quite rapidly.
How object based is VWM?
Change-detection tasks have also been used to evaluate how object-based encoding in VWM may be affected by remembering objects with two features within the same dimension (i.e., two colors per item) or different dimensions (i.e., color and orientation per item). In one experiment, participants viewed arrays of mushroom-like objects that had two distinct parts: a cap and a stem. In the conjunction condition, five mushroom-like objects were shown, with each cap and stem in an object being a distinct color from one another. In the feature condition, 10 separate parts (five caps and five stems) of varying colors were displayed. Across both conditions, participants were instructed to only monitor cap colors for a possible change, stem colors for a possible change, or both cap and stem colors for a possible change. Participants viewed an array, followed by a brief delay, and then a test array appeared with a possible change present. It was found there was no object-based advantage (i.e., conjunction trials) for remembering one or both colors (Xu, 2002 ). This suggests that VWM does not always encode visual arrays as integrated objects.
A follow-up experiment investigated this finding further, exploring whether potential object-based advantages existed for remembering two features along different dimensions. The experiment was similar to the first, but all the mushroom stems were a uniform green color. Instead of varying by color, they varied by orientation (either 45°, 90°, or 135° relative to horizontal). Again, participants viewed conjunction or feature displays, and had to monitor either cap changes (color), stem changes (orientation), or both. Interestingly, participants demonstrated better performance across all conditions in the conjunction relative to the feature display. These results indicate an object-based encoding advantage may exist, but, if so, only for when objects contain two features along different dimensions (Xu, 2002 ).
Olson and Jiang ( 2002 ) used a single probe change-detection task to investigate a similar question of whether VWM was limited by the number of objects or features in a display. Participants first saw a display of three or six color items. After a brief delay, a single item reappeared that was either the same from the previous display or different. Participants indicated whether a change had occurred. All color stimuli were simple objects of the same type (inner square or the outer frame of a square). Crucially, there were two types of stimuli: feature (a single color, either inner square or outer frame) and conjunction (two colors, one in the inner square of the object and another in the outer frame of the object). This created three types of trials. A feature-feature trial consisted only of feature stimuli, and at test a feature item was probed. A conjunction-conjunction condition consisted of both feature and conjunction stimuli, and at test a conjunction item was probed. And a conjunction-feature condition contained both feature and conjunction stimuli, and at test a feature item was probed. It was observed that as set size increased, performance declined across all three trial types. However, performance was higher overall for the feature-feature condition than either of the two conjunction conditions, suggesting there was a cost to maintaining more colors in VWM, even when those colors were bound to a single object. Additional experiments involving color and orientation did observe that integrated objects may improve VWM capacity under certain conditions (Olson & Jiang, 2002 ).
More recently, Hardman and Cowan ( 2015 ) conducted a series of change-detection experiments to investigate whether VWM is limited solely by the number of objects that can be held, or if it is also limited by the complexity of those objects (i.e., number of features to be remembered). Most strikingly, in a direct replication of the four-feature experiment conducted by Luck and Vogel ( 1997 ), they found a cost for maintaining more features in VWM in the conjunction condition. Across a variety of experiments (using different encoding times, single probe report, etc.), they consistently observed an effect of feature load, regardless of whether two or four features were contained per object (each along different dimensions; i.e., color, orientation, gap, and length). These results provide strong evidence that VWM capacity is limited both by the number of objects and number of relevant features of those objects and is inconsistent with the prediction of the fixed slot model that VWM operates in units of integrated objects (Hardman & Cowan, 2015 ).
Continuous report
In order to measure fidelity of VWM, experiments have used a paradigm often referred to as continuous report. Generally, observers are presented an array of items of a single feature (i.e., color, orientation). After a delay, a single item position is probed (typically highlighted with a square), and participants indicate the specific feature of that item in a continuous space. So if the participant saw four colored squares, after a delay a single item was probed, and participants indicated the color of the object using a continuous color wheel. This allows researchers to create a distribution of how precise the responses for items are (by taking the difference between responses and the true value), and to see how these distributions vary as a function of different features or set sizes. For example, as set size increases a normal distribution with a larger standard deviation ( SD ) would indicate a less precise VWM representation of that feature. A limitation of change-detection tasks is that they provide little information as to how well each individual object is remembered. However, continuous report allows for a measure that quantifies the amount of information available in memory for an item.
How precise are VWM representations?
Zhang and Luck ( 2008 ) were interested in evaluating the role continuous resources and/or slots may play in explaining VWM capacity limitations using a continuous report method. In their experiment, participants were presented with one to six color objects in an array. After a brief delay, a single item was probed (using a highlighted square), and participants reported the color of the probed item using a continuous color wheel surrounding the display. In order to calculate performance, they took the error of each response to create a distribution around the true color value, and through a mixture model analysis obtained an estimate of the quantity (i.e. guess rate) and precision (i.e. SD ) of memory. They observed that as set size increased from one to three items, SD increased, but then remained constant as set size increased to six. Further studies have collaborated these results (Brady et al., 2013 ).
Such results are not compatible with a pure resource model of VWM (which would predict SD s to increase across all set sizes), and instead suggest some variation of a “slot” model. Through a follow-up change-detection experiment, Zhang and Luck ( 2008 ) proposed that a slots and averaging model (an amended version of the fixed slot model) best fit the observed data in the continuous report experiment. The logic of this model is that an observer has a fixed amount of “slots” in VWM, able to store information in an all-or-nothing process with a given precision. The key difference between this model and the fixed slot model is that these slots can be flexibly allocated across different items. So if an observer has a capacity of four slots and a display only contains one item, the observer can allocate all these slots to that single stimulus. By then averaging the slots together, the observer can obtain more precise estimates.
Bays, Catalao, and Husain ( 2009 ) also used continuous report to build upon this work, but while also taking into account both errors in color and location. Their experiment was similar to Zhang and Luck’s ( 2008 )—participants saw an array of one to six colored items, and after a brief delay a single item from the display was probed. Participants reported the color of the probed item by clicking on a color wheel. When modeling distributions of the responses to the targets, however, they also took into account possible responses to nontargets (i.e., location errors). They observed that responses to nontargets were not uniform (which would have reflected guessing), suggesting participants were making location errors during the task. When this error was taken into account (modeling distributions for both the target and nontarget), they observed that precision decreased as the number of items in the display increased. This pattern of data is best explained by a common continuous resource distributed dynamically across the entire display, and not by a slots and averaging model that constrains the number of items that can be represented.
Can VWM strategically trade off between precision and capacity?
To assess the flexibility of VWM, Fougnie, Cormiea, Kanabar, and Alvarez ( 2016 ) conducted a VWM experiment where during each trial participants were briefly shown five colors to remember, and after a brief delay they were then asked to report the color of the items using continuous report. Critically, trials were blocked into two conditions. In the standard task, a single random location was cued, and participants were asked to report the color of that item as precisely as possible (with a monetary reward for more precise responses). In the get-them-all task, participants were asked to report the color of all five items and received a monetary reward only if all five responses were on the correct half of the color wheel. Data were analyzed using the same method as Zhang and Luck ( 2008 ) to estimate the quantity (i.e., guess rate) and quality (i.e., precision) of memory performance. Interestingly, they observed a trade-off between precision and guess rate across conditions. Specifically, in the standard task, participants displayed significantly more precise representations, but at the cost of reduced capacity (i.e., increased guess rate). In contrast, in the get-them-all task participants displayed significantly more capacity, but at a cost to the precision of those representations (Fougnie et al., 2016 ). These results demonstrate that observers can strategically control the content of VWM, suggesting greater insight may be possible by studying flexibility in VWM in addition to capacity limitations.
Synthesizing dichotomies in visual memory
When looking at the memory literature, what one notices very clearly is within a particular paradigm, memory system, or stimulus type we often talk about memory in dichotomies. We think of what memory is through the lens of a specific system, method, or type of stimulus. When one zooms out, it becomes natural to ask to what extent are these dichotomies related to each other?
In fact, many of these dichotomies may be intimately related or may even be the same. For instance, the idea that people simply fail to represent information altogether is a component of some fixed capacity theories of VWM and some theories of recall in VLTM. In a typical change-detection task, if an observer fails to detect a change both the fixed slot and flexible slot model suggest the observer did not represent the changed item in memory. The failure at test is not due to an observer not having enough information to make the correct judgment but rather that they had no information in order to make the correct judgment.
Along similar lines, if during a VLTM test an observer fails to identify a previously seen item, according to the DPSD model this may be the result of a recollection failure. When the observer saw the previously seen image at test, recollection failed to occur, so the observer had no information to inform their decision. Without any information related to the previously seen item, they incorrectly classified the image as new. In both cases, fixed capacity theories of VWM and the DPSD model of VLTM say failures of memory are the result of the observer having no information available at test.
A natural question arises as to what extent are the fixed/flexible slot model and recollection describing the same kinds of phenomena but using distinct methods and theories? By utilizing methods across these fields, it may be possible to inform both perspectives. For instance, many VWM researchers studying the fixed slot model (and in VWM research generally) use some variation of a change-detection task. What if classical constructs studied in the broader episodic memory literature, such as familiarity versus recollection, were measured in the context of VWM? For example, participants could be given a working memory display to remember and at subsequent test asked to classify a test object as either old or new, as well as make a source localization judgment. Would it be possible for an observer to incorrectly classify an old object, but make a correct source judgment? What would the implications of such results be for our understanding of VWM capacity? Typically, these results would be interpreted in the context of swap errors, perhaps through misbinding features of items or incorrectly reporting another memory item present in the display (Fougnie & Alvarez, 2011 ; Ma et al., 2014 ). However, what if such results were interpreted under the framework guiding long-term memory? If observers are able to make a correct source but incorrect identity judgment, this might suggest they have different kinds of information available at test, consistent with the long-term memory literature. Such data would be inconsistent with a fixed slot model, as an observer would have all or none of the information about an item available at test. It would also have potentially far-reaching implications for continuous resource models of VWM (continuous resource or flexible slot), as it would suggest that not only the amount of information about an item can vary, but the kinds of information available about an item can vary as well.
There is also the idea that failures during memory tests are not the result of failing to represent information, but rather that the information available to observers to make decisions declines or becomes less precise under certain conditions. This is a notion shared by both the continuous resource model of VWM and theories of familiarity in VLTM. In a typical change-detection task, it may not be the case that observers fail to represent an item if they do not detect a change occurred. The continuous resource model explains such performance failures as the result of a finite continuous resource that becomes more thinly distributed as the number of items in VWM increases. An observer may incorrectly report no change occurred, but they may still have some information of representation of that item. It was simply that they did not have enough information about that item in order to make the required judgment (i.e., change or no change).
In VLTM, both the DSPD and CDP models of familiarity suggest that sometimes an observer may fail to correctly recognize a previously seen item but still have some information in memory about that item. An observer may have a familiarity signal related to a previously seen item. Familiarity operates via a signal-detection-based process, so even if the signal is in memory, an observer may still fail to recognize a previously seen item depending on a variety of factors. The memory signal used in the comparison process could be weak, or the observer may have a particularly conservative decision criterion (avoiding all potential false alarms at the cost of recognizing some previously seen items). Regardless, this means an observer may fail to recognize an object, but still have some information about that object in memory.
Again, it becomes natural to ask to what extent are such theories describing the same kinds of phenomena? Are certain kinds of memory performance classified as “familiarity” in VLTM simply measuring memories with more thinly distributed resources? What happens in VLTM tasks as set size during encoding increases? Do observers transition from “recollection” based responses to “familiarity” responses? At high set sizes, can observers still demonstrate responses typically associated with recollection for certain items (consistent with the fixed slot model of VWM), or does performance reliably conform to familiarity-based responses (consistent with the continuous resource model)? Interestingly, variable performance at high set sizes for recollection-based and familiarity-based responses in VLTM would be analogous to the flexible slots model, and entirely consistent with DPSD and CDP models of familiarity and recollection.
Pattern separation and pattern completion bear obvious resemblance to tolerance and discrimination. Tolerance refers to our ability to recognize something despite considerable changes in inputs across encounters. Pattern completion, which is the process by which varying inputs are recognized as belonging to the same source, seems to be one such process through which tolerance may be achieved. Discrimination refers to our ability to distinguish similar but distinct inputs from one another, and pattern separation is defined as the process of recognizing overlapping inputs as distinct. Given these definitions, it becomes abundantly clear that researchers studying VLTM in terms of pattern separation and pattern completion, and vision researchers studying object recognition in terms of tolerance and discrimination are investigating the same processes. But these two fields of research have operated largely independently of one another, devising their own methods and terminology to investigate the same phenomena. How, then, might methods from vision scientists studying object recognition inform episodic long-term memory researchers studying pattern separation/completion? Do rules of perception that guide object identity also affect visual episodic long-term memory? Recent research suggests this is the case, as expectations about spatiotemporal continuity (broadly known to support object perception; see Scholl, 2001 , for a review) appear to be critical for the construction of stronger, more tolerant long-term memories (Schurgin & Flombaum, 2017 ). Broadly, beginning to answer these questions may provide further insight to our understanding of memory, in addition to bridging sometimes disparate but related fields of study across vision science, psychology, and neuroscience.
Despite generally being studied separately, VWM, VLTM, and even object recognition research are clearly intimately related to one another. Indeed, many dichotomies established across these fields may be describing the same kinds of processes or behaviors. Here, I have provided a review of the major theories and methods guiding VWM and VLTM research, with the goal to provide background of one field to researchers primarily operating in the other. While tremendous advances in our understanding of visual memory have been made operating separately of one another, each of these fields may greatly benefit through increased interaction and application with one another. By more closely relating how aspects already identified in visual working memory and long-term memory may interact and support one another, we can better address the major unresolved questions of visual memory. Namely, what are the representational structure and contents of our memories, and how do we acquire them?
Author note
I thank Jonathan Flombaum for the thoughtful discussions and comments that led to the creation of this review. Howard Egeth and Timothy Brady also provided useful suggestions and feedback. In memoriam of Dr. Jack Schurgin.
It is important to note that discrimination is sometimes referred to as “explicitness,” and tolerance is sometimes referred to as “invariance” in the object recognition literature. For the purposes of this review, the terms are interchangeable.
Another common way of conceptualizing 2AFC performance is that participants compute a difference score between the two signals and base their decision on that variable (Macmillan & Creelman, 2004 ).
Alloway, T. P., & Alloway, R. G. (2010). Investigating the predictive roles of working memory and IQ in academic attainment. Journal of Experimental Child Psychology, 106 (1), 20–29.
Article PubMed Google Scholar
Andreopoulos, A., & Tsotsos, J. K. (2013). 50 years of object recognition: Directions forward. Computer Vision and Image Understanding , 117 (8), 827–891.
Article Google Scholar
Alvarez, G. A., & Cavanagh, P. (2004). The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological science, 15 (2), 106–111.
Awh, E., Barton, B., & Vogel, E. K. (2007). Visual working memory represents a fixed number of items regardless of complexity. Psychological Science , 18 (7), 622–628.
Baddeley, A. (2003). Working memory: Looking back and looking forward. Nature Reviews Neuroscience, 4 (10), 829–839.
Baddeley, A. D., & Hitch, G. (1974). Working memory. Psychology of Learning and Motivation , 8 , 47–89.
Bae, G. Y., Olkkonen, M., Allred, S. R., Wilson, C., & Flombaum, J. I. (2014). Stimulus-specific variability in color working memory with delayed estimation. Journal of Vision , 14 (4), 7. https://doi.org/10.1167/14.4.7 .
Bays, P. M., Catalao, R. F., & Husain, M. (2009). The precision of visual working memory is set by allocation of a shared resource. Journal of Vision , 9 (10), 7.1–7.11. https://doi.org/10.1167/9.10.7 .
Bays, P. M., & Husain, M. (2008). Dynamic shifts of limited working memory resources in human vision. Science , 321 (5890), 851–854. https://doi.org/10.1126/science.1158023 .
Article PubMed PubMed Central Google Scholar
Biederman, I., & Ju, G. (1988). Surface versus edge-based determinants of visual recognition. Cognitive Psychology , 20 (1), 38–64.
Bowles, B., Crupi, C., Pigott, S., Parrent, A., Wiebe, S., Janzen, L., & Köhler, S. (2010). Double dissociation of selective recollection and familiarity impairments following two different surgical treatments for temporal-lobe epilepsy. Neuropsychologia , 48 (9), 2640–2647.
Brady, T. F., & Alvarez, G. A. (2011). Hierarchical encoding in visual working memory: Ensemble statistics bias memory for individual items. Psychological Science, 22 (3), 384–392.
Brady, T. F., & Alvarez, G. A. (2015). No evidence for a fixed object limit in working memory: Ensemble representations inflate estimates of working memory capacity for complex objects. Journal of Experimental Psychology: Learning, Memory and Cognition , 41(3), 921–929.
Google Scholar
Brady, T. F., Konkle, T., & Alvarez, G. A. (2011). A review of visual memory capacity: Beyond individual items and toward structured representations. Journal of Vision , 11 (5). https://doi.org/10.1167/11.5.4 .
Brady, T. F., Konkle, T., Alvarez, G. A., & Oliva, A. (2008). Visual long-term memory has a massive storage capacity for object details. Proceedings of the National Academy of Sciences , 105 (38), 14325–14329.
Brady, T. F., Konkle, T., Gill, J., Oliva, A., & Alvarez, G. A. (2013). Visual long-term memory has the same limit on fidelity as visual working memory. Psychological Science , 24 (6), 981–990.
Brady, T. F., Konkle, T., Oliva, A., & Alvarez, G. A. (2009). Detecting changes in real-world objects: The relationship between visual long-term memory and change blindness. Communicative & Integrative Biology, 2 (1), 1–3.
Cansino, S., Maquet, P., Dolan, R. J., & Rugg, M. D. (2002). Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex , 12 (10), 1048–1056.
Conway, A. R., Kane, M. J., & Engle, R. W. (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences, 7 (12), 547–552.
Cowan, N. (2008). What are the differences between long-term, short-term, and working memory? Progress in Brain Research , 169 , 323–338.
Cox, D. D., & DiCarlo, J. J. (2008). Does learned shape selectivity in inferior temporal cortex automatically generalize across retinal position? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience , 28 (40), 10045–10055.
Cox, D. D., Meier, P., Oertelt, N., & DiCarlo, J. J. (2005). “Breaking” position-invariant object recognition. Nature Neuroscience , 8 (9), 1145–1147.
Dede, A. J., Squire, L. R., & Wixted, J. T. (2014). A novel approach to an old problem: Analysis of systematic errors in two models of recognition memory. Neuropsychologia , 52 , 51–56.
Diana, R. A., Yonelinas, A. P., & Ranganath, C. (2007). Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences , 11 (9), 379–386.
DiCarlo, J. J., & Cox, D. D. (2007). Untangling invariant object recognition. Trends in Cognitive Sciences, 11 (8), 333–341.
DiCarlo, J. J., Zoccolan, D., & Rust, N. C. (2012). How does the brain solve visual object recognition? Neuron , 73 (3), 415–434.
Dunn, J. C. (2004). Remember-know: a matter of confidence. Psychological review, 111 (2), 524.
Fan, J. E., Hutchinson, J. B., & Turk-Browne, N. B. (2016). When past is present: Substitutions of long-term memory for sensory evidence in perceptual judgments. Journal of Vision , 16 (8), 1–12.
Fortin, N. J., Wright, S. P., & Eichenbaum, H. (2004). Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature , 431 (7005), 188–191.
Fougnie, D., & Alvarez, G. A. (2011). Object features fail independently in visual working memory: Evidence for a probabilistic feature-store model. Journal of Vision, 11 (12), 3–3. https://doi.org/10.1167/11.12.33 .
Fougnie, D., Asplund, C. L., & Marois, R. (2010). What are the units of storage in visual working memory? Journal of Vision, 10 (12), 27–27. https://doi.org/10.1167/10.12.27 .
Fougnie, D., Cormiea, S. M., Kanabar, A., & Alvarez, G. A. (2016). Strategic trade-offs between quantity and quality in working memory. Journal of Experimental Psychology: Human Perception and Performance, 42 (8), 1231. https://doi.org/10.1037/xhp0000211 .
PubMed Google Scholar
Fougnie, D., Suchow, J. W., & Alvarez, G. A. (2012). Variability in the quality of visual working memory. Nature Communications , 3 , 1229. https://doi.org/10.1038/ncomms2237 .
Fukuda, K., Vogel, E., Mayr, U., & Awh, E. (2010). Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review, 17 (5), 673–679.
Gabrieli, J. D., Fleischman, D. A., Keane, M. M., Reminger, S. L., & Morrell, F. (1995). Double dissociation between memory systems underlying explicit and implicit memory in the human brain. Psychological Science , 6 (2), 76–82.
Green, D. M., & Swets, J. A. (1966). Signal detection theory and psychophysics (Vol. 1). New York: Wiley.
Guerin, S. A., Robbins, C. A., Gilmore, A. W., & Schacter, D. L. (2012). Retrieval failure contributes to gist-based false recognition. Journal of Memory and Language , 66 (1), 68–78.
Hardman, K. O., & Cowan, N. (2015). Remembering complex objects in visual working memory: Do capacity limits restrict objects or features? Journal of Experimental Psychology: Learning, Memory, and Cognition, 41 (2), 325–347. https://doi.org/10.1037/xlm0000031 .
Harrison, S. A., & Tong, F. (2009). Decoding reveals the contents of visual working memory in early visual areas. Nature, 458 (7238), 632–635. https://doi.org/10.1038/nature07832 .
Holdstock, J. S., Mayes, A. R., Roberts, N., Cezayirli, E., Isaac, C. L., O’Reilly, R. C., & Norman, K. A. (2002). Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus , 12 (3), 341–351.
Hollingworth, A., Matsukura, M., & Luck, S. J. (2013). Visual working memory modulates rapid eye movements to simple onset targets. Psychological Science , 24 (5), 790–796.
Hollingworth, A., Richard, A. M., & Luck, S. J. (2008). Understanding the function of visual short-term memory: Transsaccadic memory, object correspondence, and gaze correction. Journal of Experimental Psychology: General , 137 (1), 163–181. https://doi.org/10.1037/0096-3445.137.1.163 .
Huttenlocher, J., Hedges, L. V., & Duncan, S. (1991). Categories and particulars: Prototype effects in estimating spatial location. Psychological Review, 98 (3), 352–376.
Ingram, K. M., Mickes, L., & Wixted, J. T. (2012). Recollection can be weak and familiarity can be strong. Journal of Experimental Psychology: Learning, Memory, and Cognition , 38(2), 325–339. https://doi.org/10.1037/a0025483 .
Jiang, Y., Olson, I. R., & Chun, M. M. (2000). Organization of visual short-term memory. Journal of Experimental Psychology: Learning, memory, and cognition, 26 (3), 683.
Kahneman, D., Treisman, A., & Gibbs, B. J. (1992). The reviewing of object files: Object-specific integration of information. Cognitive Psychology, 24 , 175–219.
Kensinger, E. A., Garoff-Eaton, R. J., & Schacter, D. L. (2006). Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language , 54 (1), 99–112.
Kim, J., & Yassa, M. A. (2013). Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus , 23 (4), 287–294.
Konkle, T., Brady, T. F., Alvarez, G. A., & Oliva, A. (2010a). Conceptual distinctiveness supports detailed visual long-term memory for real-world objects. Journal of Experimental Psychology: General , 139 (3), 558–578. https://doi.org/10.1037/a0019165 .
Konkle, T., Brady, T. F., Alvarez, G. A., & Oliva, A. (2010b). Scene memory is more detailed than you think: The role of categories in visual long-term memory. Psychological Science, 21 (11), 1551–1556.
Loiotile, R. E., & Courtney, S. M. (2015). A signal detection theory analysis of behavioral pattern separation paradigms. Learning & Memory , 22 (8), 364–369.
Luck, S. J., & Vogel, E. K. (1997). The capacity of visual working memory for features and conjunctions. Nature , 390 (6657), 279–281.
Ma, W. J., Husain, M., & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience , 17 (3), 347–356.
Macmillan, N. A., & Creelman, C. D. (2004). Detection theory: A user’s guide. New York: Psychology Press.
Miyamoto, K., Adachi, Y., Osada, T., Watanabe, T., Kimura, H. M., Setsuie, R., & Miyashita, Y. (2014). Dissociable memory traces within the macaque medial temporal lobe predict subsequent recognition performance. The Journal of Neuroscience , 34 (5), 1988–1997.
Neunuebel, J. P., & Knierim, J. J. (2014). CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron , 81 (2), 416–427.
Olson, I. R., & Jiang, Y. (2002). Is visual short-term memory object based? Rejection of the “strong-object” hypothesis. Perception & Psychophysics , 64 (7), 1055–1067.
Pessoa, L., Gutierrez, E., Bandettini, P. A., & Ungerleider, L. G. (2002). Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron, 35 (5), 975–987.
Pinto, N., Cox, D. D., & DiCarlo, J. J. (2008). Why is real-world visual object recognition hard? PLOS Computational Biology , 4 (1), e27. https://doi.org/10.1371/journal.pcbi.0040027 .
Potter, M. C. (1976). Short-term conceptual memory for pictures. Journal of Experimental Psychology: Human Learning and Memory , 2 (5), 509–522.
Potter, M. C., & Levy, E. I. (1969). Recognition memory for a rapid sequence of pictures. Journal of Experimental Psychology , 81 (1), 10–15.
Reagh, Z. M., & Yassa, M. A. (2014). Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proceedings of the National Academy of Sciences , 111 (40), E4264-E4273.
Rust, N. C., & Stocker, A. A. (2010). Ambiguity and invariance: two fundamental challenges for visual processing. Current Opinion in Neurobiology , 20 (3), 382–388.
Schapiro, A. C., Turk-Browne, N. B., Botvinick, M. M., & Norman, K. A. (2017). Complementary learning systems within the hippocampus: A neural network modelling approach to reconciling episodic memory with statistical learning. Philosophical Transactions of the Royal Society B , 372 (1711). https://doi.org/10.1098/rstb.2016.0049 .
Scholl, B. J. (2001). Objects and attention: The state of the art. Cognition, 80 (1/2), 1–46.
Scholl, B. J., & Flombaum, J. I. (2010). Object persistence. In B. Goldstein (Ed.), Encyclopedia of perception (Vol. 2, pp. 653– 657). Thousand Oaks: SAGE.
Schurgin, M. W., & Flombaum, J. I. (2014). How undistorted spatial memories can produce distorted responses. Attention, Perception, & Psychophysics, 76 (5), 1371–1380.
Schurgin, M. W., & Flombaum, J. I. (2015). Visual long-term memory has weaker fidelity than working memory. Visual Cognition , 23 (7), 859–862.
Schurgin, M. W., & Flombaum, J. I. (2017). Exploiting core knowledge for visual object recognition. Journal of Experimental Psychology: General, 146 (3), 362–375.
Schurgin, M. W., & Flombaum, J. I. (2018). Visual working memory is more tolerant than visual long-term memory. Journal of Experimental Psychology: Human Perception and Performance.
Schurgin, M. W., Reagh, Z. M., Yassa, M. A., & Flombaum, J. I. (2013). Spatiotemporal continuity alters long-term memory representation of objects. Visual Cognition , 21 (6), 715–718.
Shepard, R. N. (1967). Recognition memory for words, sentences, and pictures. Journal of Verbal Learning and Verbal Behavior, 6, 156–163.
Sperling, G. (1963). A model for visual memory tasks. Human factors, 5 (1), 19–31.
Squire, L. R. (2004). Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory , 82 (3), 171–177.
Standing, L. (1973). Learning 10000 pictures. Quarterly Journal of Experimental Psychology, 25 (2), 207–222.
Stark, S. M., Yassa, M. A., Lacy, J. W., & Stark, C. E. (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia , 51 (12), 2442–2449.
Todd, J. J., & Marois, R. (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature , 428 (6984), 751–754.
Van den Berg, R., & Ma, W. J. (2017). A rational theory of the limitations of working memory and attention. bioRxiv . https://doi.org/10.1101/151365 .
Van den Berg, R., Shin, H., Chou, W. C., George, R., & Ma, W. J. (2012). Variability in encoding precision accounts for visual short-term memory limitations. Proceedings of the National Academy of Sciences , 109 (22), 8780–8785.
Vogel, E. K., Woodman, G. F., & Luck, S. J. (2001). Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance , 27 (1), 92–114.
Vogel, E. K., Woodman, G. F., & Luck, S. J. (2006). The time course of consolidation in visual working memory. Journal of Experimental Psychology. Human Perception and Performance , 32 (6), 1436–51.
Wallis, G., & Bülthoff, H. (1999). Learning to recognize objects. Trends in Cognitive Sciences , 3 (1), 22–31.
Wallis, G., & Bülthoff, H. H. (2001). Effects of temporal association on recognition memory. Proceedings of the National Academy of Sciences , 98 (8), 4800–4804.
Wheeler, M. E., & Treisman, A. M. (2002). Binding in short-term visual memory. Journal of Experimental Psychology: General, 131 (1), 48-64.
Wilken, P., & Ma, W. J. (2004). A detection theory account of change detection. Journal of Vision , 4 (12), 1120–1125.
Wixted, J. T., & Stretch, V. (2004). In defense of the signal detection interpretation of remember/know judgments. Psychonomic Bulletin & Review, 11 (4), 616–641.
Xu, Y. (2002). Limitations of object-based feature encoding in visual short-term memory. Journal of Experimental Psychology: Human Perception and Performance , 28 (2), 458–468.
Yassa, M. A., Lacy, J. W., Stark, S. M., Albert, M. S., Gallagher, M., & Stark, C. E. (2011). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, 21 (9), 968–979.
Yassa, M. A., & Stark, C. E. (2011). Pattern separation in the hippocampus. Trends in Neurosciences , 34 (10), 515–525.
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of memory and language, 46 (3), 441–517.
Yonelinas, A. P., Aly, M., Wang, W. C., & Koen, J. D. (2010). Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus , 20 (11), 1178–1194.
Zhang, W., & Luck, S. J. (2008). Discrete fixed-resolution representations in visual working memory. Nature , 453 (7192), 233–235.
Download references
Author information
Authors and affiliations.
Department of Psychology, University of California, San Diego, McGill Hall 5217, 9500 Gilman Drive #0109, La Jolla, CA, 92093, USA
Mark W. Schurgin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mark W. Schurgin .
Rights and permissions
Reprints and permissions
About this article
Schurgin, M.W. Visual memory, the long and the short of it: A review of visual working memory and long-term memory. Atten Percept Psychophys 80 , 1035–1056 (2018). https://doi.org/10.3758/s13414-018-1522-y
Download citation
Published : 23 April 2018
Issue Date : July 2018
DOI : https://doi.org/10.3758/s13414-018-1522-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Visual working memory
- Memory: Long-term memory
- Memory: Visual working and short-term memory
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

The recall of information from working memory: insights from behavioural and chronometric perspectives
John n. towse.
Department of Psychology, Lancaster University
Nelson Cowan
Department of Psychological Sciences, University of Missouri
Graham J. Hitch
Department of Psychology, University of York
Neil J Horton
In four experiments we test a recall reconstruction hypothesis for working memory, according to which reading span items can be recovered or specified from multiple memory representations. Each reading span experiment involves memoranda either embedded within or unrelated to the sentence content. This manipulation affected the timing of recall, with longer pauses accompanying items that are linked to processing. Levels of recall accuracy vary between these task formats, dependent on the orienting task for processing. Experiment 1 compares the chronometry of spoken recall for word span and reading span, in which participants complete an unfinished sentence. Experiment 2 and 3 confirm recall time differences without using word generation requirements, while Experiments 4 used an item and order response choice paradigm with nonspoken responses. We argue that verbal and manual recall timing offers an informative measure for understanding working memory.
Introduction
Working memory reflects the ability to hold in mind transient representations while simultaneously processing and assimilating ongoing events ( Baddeley & Hitch, 1974 ). There are a wide variety of circumstances in which we are required to carry out mental operations and remember intermediate information (for instance, retain a carry item in mental arithmetic or a referent for an anaphoric pronoun) as well as situations in which current mental activities need to draw on past episodic knowledge (e.g., mapping the problem space for a current task using knowledge of related situations). They emphasise the importance of understanding active maintenance and transformation processes. Consequently, the concept of working memory has been the focus of considerable research.
Although theories of working memory differ considerably (see Miyake & Shah, 1999 ), the most common method for assessing its capacity is to draw upon at least one of a family of tasks known as working memory (WM) span. Reading span was the first such task to be reported in adults ( Daneman & Carpenter, 1980 ). Participants read a series of unconnected sentences, and the final word in each sentence yields a memorandum to be reported afterwards in serial order. In essence, an individual’s reading span score reflects how many end-of-sentence words can be remembered whilst reading. Daneman and Carpenter (1980) showed reading span to be a very good measure of reading skill (see also Daneman & Merikle, 1986). The predictive prowess of WM span tasks (including alternatives such as operation span where the processing task involves arithmetic operations, Turner & Engle, 1989 ) provides empirical support for the conceptual idea that the processing-plus-memory requirements tap an important skill in complex cognition.
Several theories suggest, in different ways and to different degrees, that a competitive relationship between processing and memory activities is critical to measuring WM capacity. In other words, that the maintenance of information takes place in the face of distraction or interference from concurrent processing. For example, Case (1985) proposed that limited-capacity general-purpose cognitive resources were allocated to either processing or memory demands. Jarrold and Bayliss (2007) discuss evidence that combining or coordinating processing with memory places demands on WM, above and beyond those imposed by each requirement per se . Towse, Hitch and Hutton (1998) argued that processing activity produces informational degradation because memories are not actively or continuously maintained (in this respect, see also Barrouillet, Bernadin, & Camos, 2004 ). Kane and Engle (2003) suggested that controlled attention is important to preserve memory representations at the same time as the concurrent processing requirements.
We can see important insights to be gained from each of these accounts, and we do not intend here to arbitrate between them. Rather, our focus is directed towards the concept that links them; processing and memory are thought to be separable, even exclusive events. We certainly accept that processing can interfere with retention. Nonetheless, we present data that lead us to conclude that this is not the whole story; processing may also complement or support memory (for an early and seminal version of this perspective, see Craik & Lockhart, 1972 ).
Our core proposal that processing and memory need not always be thought of as completely separate events. Using behavioural and chronometric evidence, we propose that psychological models can be enhanced by considering a broader view of the WM representations that are present at the point of recall.
Chronometric analysis of memory recall – the timing of correct output sequences – has generally focused on short-term memory (STM) tasks such as word span, where a sequence of unrelated items are presented at a regular rate and then reproduced in their original order (e.g., Dosher & Ma, 1998 ; for an overview, Towse & Cowan, 2005 ). Whilst such research has been undoubtedly productive, given the body of evidence to distinguish STM from WM (e.g., Daneman & Carpenter, 1980 ; Engle, Kane & Tuholski, 1999 ), there is a clear motivation to investigate recall timing in WM. Cowan et al. (2003) did just this. They found children’s reading span recall times were dramatically longer than has been obtained in STM studies, and that for both children and adults (but especially the former) response durations for listening span exceeded those of counting span and digit span. Cowan et al. also reported that WM response durations predicted children’s word reading skills over and above the contribution of span scores per se : recall evidently incorporates processes relevant to children’s cognitive development and attainment.
The particularly long interword pauses in reading span and listening span led Cowan et al. (2003) to two related conclusions. First, memory representations may not always be maintained in a highly accessible state during processing. If they had been, one would expect their rapid production during recall. Second, participants sometimes draw on memory of the sentence, in terms of thematic and semantic context, for the elicitation of the target items. Cowan et al. found that recall in counting span was less protracted than listening span, and attributed this to the lack of distinctive processing in the enumeration of visual displays, and thus the absence of a similar scaffolding process. Thus, reading span and listening span recall can involve the consideration of a much richer ensemble of (perhaps loosely encoded) memories of the trial episode than is the case for other tasks.
To capture these ideas we advance here a ‘recall reconstruction’ hypothesis for WM performance. The central proposal is that participants may bring to recall more than just representations of experimentally-assigned memoranda (i.e. target memory words). In the specific case of reading span, this can involve sentential information, which is combined with other representations over time. As a consequence, we propose that the memory sequence may not be continuously and actively maintained and consequently recall involves the resuscitation of degraded information.
According to this recall reconstruction perspective, WM potentially involves the intertwined and integrated aspects of processing and memory. Processing and memory activities need not inherently be in complete opposition to each other, dependent on the specific WM task. Consider an example sentence from Daneman and Carpenter (1980) : “ The lumbermen worked long hours in order to obtain the necessary amount of wood .” According to the position just outlined, later recall of “wood” might be facilitated by gist or episodic memory about lumbermen, or indeed associations made during reading to the implicit concept “trees”. An individual need not commit a sentence to memory verbatim, but relevant linguistic information could nonetheless be accessible, either to help reconstruct the word “wood” or to discount sentence-terminal words appropriate to other serial positions.
This recall reconstruction hypothesis is quite compatible with the evidence in children that recall pauses predict cognitive ability since item reconstruction using self-generated cues may be a source of skilled performance. Moreover, Saito & Miyake (2004) have pointed to a relationship between processing activity and memory within their representation-based interference account of WM. While they concentrated on how processing events can hinder memory, via overlapping representations that interfere, they similarly argue that the content of processing can be relevant to memory performance.
So far as we are aware, no studies have directly investigated the relationship between processing content and memory requirements in WM. However, Osaka, Nishizaki, Komori & Osaka (2002) investigated reading span, and for language-specific reasons underlined the word that was to be remembered. They either underlined a “focus” word –the most important word for sentence comprehension-or a non-focus word, which was less central. Recall was substantially and significantly better for focus words. While all their memoranda were thematically connected with sentence material, their data support a link between processing material and what is remembered.
Other indirect evidence can be marshalled in support of this idea. Copeland & Radvansky (2001) reported a reversed phonemic similarity effect in reading span, but a standard effect for operation span. They suggested that phonemically similar lists were at an advantage because recall was facilitated by the sentence context. Hitch, Towse & Hutton (2001 : Fig. 2 ) reported that among children, the rate of forgetting on an operation span task (as a function of arithmetic processing time) was faster than the rate of forgetting on a reading span task (as a function of sentence processing time). Distinctive sentences may help to retard forgetting because they can be used in the reconstruction of target memoranda, in a way that less distinct arithmetic problems cannot.

Duration and standard error bars of correct sequences for reading span in Experiment 2, as a function of the phase of recall. PI = preparatory interval. Words = average duration of recalled items. Pause= interword pause duration (averaged at list length 3).
In a series of experiments, we test the recall reconstruction hypothesis directly. Its validity is important because it addresses the widespread assumption that processing and memory are necessarily competitive components of WM tasks. Yet it also broadens the conceptual focus, encouraging theoretical models of WM to incorporate recall and not just maintenance processes (see also Unsworth & Engle, 2006 ). We therefore attempt to replicate the long reading span pauses found by Cowan et al. (2003) and test accounts of what underlies this phenomenon. The experiments substantially extend the analysis of Cowan et al. by providing converging paradigms to investigate recall timing, using spoken recall as well as non-spoken responses. This latter approach opens up new opportunities for studying the chronometry of recall and the nature of memory representations.
Experiment 1
To examine why reading span performance is characterised by long response durations, we manipulated the relevance of sentential processing for the memory items. For example, a participant might read the sentence “The rocket went into outer ___” and we would expect them to suggest “space” as the completion word. The memorandum could be either “space” or an unrelated word, such as “bridge”. In the former case, participants can use representations about the sentence (knowledge that it referred to a rocket for example) to inform their recall choice but drawing upon this additional information will slow down recall. In the latter case, with independent memory material participants might use alternative – and effective – maintenance processes, but they cannot easily draw on the processing content, and so correct recall should be more rapid. If the alternative maintenance strategies in the independent condition are less effective, then lower levels of recall will also be found. We also collected data from a STM task to form a point of comparison.
Participants
Twenty-four Lancaster University students (22 women and 2 men) volunteered via departmental recruitment procedures and were paid £3. They were randomly assigned to the integrated and independent condition, as described below.
A corpus of 88 sentences (based on medium-length stimuli described in Towse, Hamilton, Hitch & Hutton, 2000 ) were randomly divided into two sets, “set A” and “set B”. Participants were assigned at random to receive the reading span sentences from either set A or set B, with the alternative end-of-sentence items used for word span stimuli. Sentences typically contained 8–10 words and had been formulated to elicit target completion words with a high probability among children (for example, "While I was sleeping I had a strange" typically leads to the completion response "dream").
Computer events were driven by an Apple Macintosh ibook G4 (programmed using the “Revolution” language running under OS X) with response latencies measured in (1/60 s) ticks. Visual displays used the 14 inch laptop screen. Audio recordings were captured directly to a minidisk player (Sony MZ-N710, with a Sony ECM-DS70P microphone).
All participants completed a STM (word span) test, and either the integrated or independent word WM (reading span) test in counterbalanced order. Each task was explained prior to administration of experimental trials.
Reading span trials
On each trial, a set of (between 2 and 5) incomplete sentences appeared on screen, and participants were to read these sentences and generate a suitable word to complete them. Afterwards, they attempted to recall the memoranda in the correct serial order. An incremental-order test procedure was employed. Thus, trials commenced with 3 sets of 2 sentences. Provided at least one memory sequence was recalled correctly, an additional sentence was added to the series and 3 further trials were administered, up the maximum 5-sentence sets. Participants knew the list length prior to each trial.
In the integrated word condition, the completion words formed the memoranda (if a participant produced a non-expected completion word, this was adopted as the memory target). Once the participant completed the sentence, the experimenter immediately tapped a computer key to initiate the next experimental event, which occurred after a 1 second interval. Participants were instructed not to rehearse words during the reading phase and to begin reading each sentence immediately on its presentation.
In the independent word condition, participants also read sentences and supplied a completion word. This was followed (after .25 sec) by a .5 sec presentation centre-screen of a separate, unconnected word, which was the memorandum, and which participants read aloud. There was a subsequent .25 sec pause before the next experimental event commenced. The independent words for “set A” were taken from the “set B” pool, and vice versa.
Word span trials
Participants watched the visual presentation of a sequence of unconnected words. Each word was shown centre-screen for 0.5 sec, with a 0.5 second ISI. Initially there were 3 trials containing 2 memoranda, and sequence length increased by a single item, provided that at least one trial was successfully recalled, up to a maximum list length of 5 words.
Instructions asked participants to recall the word sequence to a trial as soon as (but not before) the computer produced the auditory recall signal. Participants were asked to limit their spoken response to the recall words only (to avoid other words such as “I think”, “then it was” or “and”) and were reminded of this if necessary during test administration. The experimenter recorded answers onto computer after the output sequence was complete.
We examined the effect of task administration order (whether word span or reading span was administered first, for each stimulus pool set and task configuration) and found no significant effects on global timing measurements. We therefore collapse across order in subsequent analyses.
Participants undertook reading span trials where either the sentence interpretation led directly to the memoranda (the integrated word condition), or was unrelated (the independent word condition). Sentence reading times for these formats ( M =3.39, SD =.31 and M =3.29, SD =.64 respectively) were equivalent, t (22)=.47, p =.647, η 2 =.009. The number of correctly recalled words is reported in Table 1 . Analysis confirmed memory accuracy was substantially greater for integrated words compared to independent words, t (22)=5.01, p <.001, η 2 =.533 1 .
Memory performance reported as the number of correctly recalled words (range 0–42, except in Expt 3 where it is 0–45). Standard deviations in parentheses. Short-term memory (STM) trials in Experiment 1 differ only with respect to the working memory (WM) task that was also completed.
| Expt.1(STM) | Expt.1(WM) | Expt.2 | Expt.3 | Expt.4(dis.) | Expt.4(rmn..) | |
|---|---|---|---|---|---|---|
| Integrated WM | 38.3 (4.92) | 27.2 (8.98) | 31.3 (4.56) | 25.9(5.91) | 33.5 (6.25) | 33.8 (5.21) |
| Independent WM | 37.8 (3.32) | 12.6 (4.56) | 28.1 (5.40) | 21.9(8.38) | 24.6 (6.84) | 24.2 (7.73) |
To extract recall times, the portion of the sound files relating to each correct response sequence was selected and examined. In some cases, recall times were ignored because the participant restarted their list (e.g. “yellow… dream…. no, wait, door…yellow…dream”) or in some way gave an ambiguous recall with respect to timing issues.
The computer produced both an auditory and visual recall cue. Auditory recall was segmented into three contiguous phases (see, for example, Cowan et al., 1998 ); the time between the recall signal and the start of recall (the preparatory interval); the time to articulate the relevant words (each word duration), and the gaps between words (interword pauses). A single trained researcher extracted timing values, for whom blind timings both correlate and correspond with those made by an independent coder (for a sample of 99 word and interval measurements, r (97)=.993). Specific recall time segments were screened for outliers by examining z-score distributions of each time measurement; where z >2.58, that trial value was not used in the compilation of recall times. Measures of stability are reported in Table 2 . To make analysis easier to present, we focus on the three recall phases, combining individual values (e.g., the first and second word in two-item sequences).
Stability of recall timing: correlations between recall durations of list-length 2 & 3 in Experiment 1, 2, and 3 (asterisks represent significant correlations, at least p <.05).
| Expt.1 STM | Expt.1 WM | Expt.2 WM | Expt.3 WM | Expt.4 WM | |
|---|---|---|---|---|---|
| Preparatory Intervals | r(22)=.661* | r(14)=.062, | r(24)=.535* | r(24)=.137 | |
| Interword Pauses | r(22)=.571* | r(14)=.168, | r(24)=.484* | r(24)=.609* | |
| Word durations | r(22)=.766* | r(14)=.694* | r(24)=.626* | r(24)=.051 | |
| Recall intervals | r(22)=.465* |
Figure 1 shows the profile of recall durations. At list length 2, the mean pause between integrated words was longer than that between independent words, although this difference was only marginally significant, t (22)=2.00, p =.058, η 2 =.154. At list length 3, the effect was in the same direction, but not significant, t (14)=.57, p =.580, η 2 =.023, while at list length 4 there were too few data points in the independent condition for analysis. Combining data across list length 2 and 3, pauses in the integrated word condition were twice the length of the independent word condition, t (22)=2.13, p =.045, η 2 =.171.

Duration and standard error bars of correct sequences for reading span trials in Experiment 1, as a function of the phase of recall. PI = preparatory interval. Words = average duration of recalled items. Pause= interword pause duration (averaged at list length 3).
Preparatory intervals and word durations were longer before integrated words but comparisons at list length 2 and 3 were non significant ( t s<1.36, p s>.187, η 2 <.078). This dissociation in sensitivity is consistent with the notion that the separate recall segments can be differentiated ( Cowan et al., 1998 ). We carried out additional analysis on STM recall, but since these are less relevant to the main experimental issue, they are reported in Appendix 1 .
Cowan et al. (2003) noted that WM recall could be differentiated from STM recall in terms of pause length. Furthermore, the longer recall was most evident for WM tasks that involved linguistic-based processing. The recall reconstruction hypothesis explains this finding by proposing that memory search processes might incorporate representations persisting from the sentences, and this takes time.
The present study experimentally evaluated this hypothesis by manipulating the link between sentential processing and memory items. When processing events can scaffold recall, pauses should be extended as a richer set of representations are evaluated. Indeed, gaps between words were longer when processing and memory were linked and more sequences were correctly recalled.
All other things being equal, one might expect that a difference in memory accuracy would work against the recall time difference that we found because with weaker memory representations, accessing the correct item should be more difficult. Thus, the evidence for more rapid correct recall in the independent condition is all the more telling.
To forestall possible mis-interpretation, we do not suggest that the processing event in reading span provides only a supportive environment for recall. Memory for the sentence ideas, or sentence words, may well degrade access to the designated memory item, offering alternative recall candidates and adding to the problem of discriminating between memoranda and activated non target representations ( Saito & Miyake, 2004 ; see also below). Our argument is that the elicitation of recall words can be affected by memory for the processing material, and that this emergent property of the way reading span trials are often constructed is one contributory factor to the protracted recall of items reported here and elsewhere ( Cowan et al., 2003 ).
Experiment 2
In the previous study, the processing task was to read aloud an incomplete sentence and generate a (constrained and thus predictable) word. This task format has been used among adults ( Towse, Hitch & Hutton, 2000 ) but especially children (e.g. Leather & Henry, 1994 ; Towse et al., 1998 ), since the completion requirement helps to ensure that they adequately process the sentences. Yet, participants in the integrated condition remember a self-generated item, while those in the independent condition do not. Slamecka and Graf (1978) have demonstrated superior memory for generated items relative to read items, and so the procedure may influence recall.
In fact, self-generation of memoranda may affect recall by elaborating the processing that takes place and enriching the consequent representation, very much analogous to the sentence content. Therefore, any self-generation effect may overlap with recall reconstruction processes outlined here. Nonetheless, we removed the generation requirement in the next experiment, to determine whether it accounts for the findings.
Twenty-eight Lancaster University students (22 women and 6 men) were paid £3 to complete reading span trials and ancillary tasks (not described here). They were randomly assigned to the integrated and independent condition.
Stimuli and Apparatus
We used the same apparatus as in Experiment 1. The sentence stimuli were the same too, except that they were presented in completed form; there were no missing words.
Participants completed either the integrated or independent word task. In the former, each sentence appeared in black type with the final word in purple. In the latter, the completed sentence appeared in black type with the subsequent memory word shown in purple type. Rather than being required to read and complete each sentence, instructions asked participants to “read the sentences aloud and think about the sentence meaning as you do so.”
Sentence reading times for integrated and independent conditions ( M =2.95, SD =.50 and M =2.84, SD =.33 respectively) were equivalent, t (26)=.71, p =.488, η 2 =.019, and just slightly quicker than in Experiment 1 (sampling differences and the absence of sentence completion requirements could explain the discrepancy). In terms of the number of correctly recalled words, shown in Table 1 , the integrated format enjoyed an advantage, but in this dataset it was not significant, t (26)=1.23, p =.231, η 2 =.055. Notably, the difference from the previous study is that without the generation requirements, performance in the independent condition has improved whilst that in the integrated condition is similar.
As in Experiment 1, recall time outliers were screened prior to compilation of trial data 2 . Recall times are illustrated in Figure 2 ( Table 2 reports stability measures). At list length 2, the pauses between integrated words were significantly longer than between independent words, t (26)=2.24, p =.034, η 2 =.162. The difference for word durations was in the same direction but marginally significant, t (26)=1.96, p =.061, η 2 =.129. The average list length 3 pause was significantly longer in the integrated condition, t (24)=2.54, p =.020, η 2 =.212, and difference in the average pause across list lengths 2 and 3 was likewise significant, t (26)=3.08, p =.005, η 2 =.267. At list length 4 there were few data points for meaningful analysis. There was no reliable difference in the word durations at list length 3, t (24)=.22, p =.828, η 2 =.002. Preparatory intervals did not differ between the independent and integrated conditions (e.g. for the averaged preparatory intervals, t (26)=.32, p =.751, η 2 =.004).
The data offer further support for the recall reconstruction hypothesis. We again found evidence that recall pauses are longer when there is a connection between the memoranda and the processing context associated with them. There was also a trend for superior levels of recall in the integrated condition but this was not significant in the current dataset.
Thus, removing the word generation requirement did not eliminate the slow but accurate levels of recall in the integrated condition. The obvious point of change across experiments lies in the level of recall in the independent condition. Generating a word to complete a sentence, as opposed to just reading a sentence, makes it harder to then recall a separate word that follows. This could be because the generated, irrelevant word affects the encoding of the subsequent item or maintenance of items already encoded. Alternatively, just reading a sentence may be insufficiently demanding, permitting separate memorial operations to take place, though it is not clear why this would not also be true for the integrated condition. Regardless, data demonstrate that longer pauses are due to the processing-memory connection, not the processing task per se .
Experiment 3
Participants take reliably longer to produce words that are semantically linked to the sentence. However, whereas the difference in recall accuracy was significant in Experiment 1, it was not significant in Experiment 2. We therefore consider additional data. The set of sentences was taken from the corpus used by Friedman & Miyake (2004) . Design configurations meant that the number of trials at each sequence length was also larger.
Thirty-three Lancaster University students (27 women and 6 men) formed a subset of a larger experiment. They were paid £4 to complete the reading span trials and additional tasks (not described here), and were randomly assigned to the integrated and independent condition.
Apparatus and Procedure
The same apparatus as in Experiment 2 was employed. The core stimulus pool comprised 90 sentences selected from the Friedman & Miyake (2004) corpus. This was divided into two subsets (A & B) as before.
Participants completed either the integrated or independent reading span task. In the former, each sentence appeared in black type with the final word in purple and there was a 0.5 sec interval between sentences. In the latter, the entire sentence appeared in black type with the subsequent memory word shown in purple type for 0.5 sec. Participants were administered a total of 15 trials; 5 for each list length 2–4, in ascending sequence length order. Verbal instructions were the same as Experiment 2.
Sentence reading times for integrated and independent conditions ( M =5.22, SD =.69 and M =4.86, SD =.56 respectively) did not differ significantly, t (29)=1.58, p =.125, η 2 =.079. The sentences were longer than those used in previous experiments, leading to extended reading times, but the pattern of performance is the same. In terms of the number of correctly recalled words, shown in Table 1 , the integrated format again enjoyed an advantage, but in this dataset it was not significant, t (31)=1.82, p =.078, ηs=.097.
We then combined the number of correctly recalled words with the data from Experiment 2. The trial structure changed across experiments, and so the maximum possible number of recallable items differed, and indeed the number of words actually recalled reflected this, F (1,57)=13.4, p =.001, η p 2 =.191, Nonetheless it is the difference between sentence formats that is relevant here, and the analysis showed that more words were recalled from the integrated format, F (1,57)=5.19, p =.027, η p 2 =.083. There was no interaction between experiment and task format, F <1, p =.804, η p 2 =.001. Thus, the integrated format does lead to greater levels of recall, even without a word generation requirement in processing, though the effect is significant only with data aggregated across the two studies.
One judge (who contributed to measurements in Experiment 2) extracted timing measurements from auditory records of each correct reading span sequence, in the same manner as for previous studies. Once again, outliers were screened prior to compilation of recall times from individual trials.
Recall times are illustrated in Figure 3 . At list length 2, the pauses between integrated words were significantly longer than between independent words, t (31)=2.88, p =.010, η 2 =.211. Moreover, the word duration was significantly longer in the integrated condition, t (31)=2.65, p =.012, η 2 =.185. At list length 3, the pause was also significantly longer in the integrated condition, t (24)=2.37, p =.029, η 2 =.190 while the word durations did not differ, t (24)=.079, p =.938, η 2 <.001. The difference in the pause averaged across list lengths 2 and 3 was significant, t (24)=2.68, p =.017, η 2 =.230.

Duration and standard error bars of correct sequences for reading span in Experiment 3, as a function of the phase of recall. PI = preparatory interval. Words = average duration of recalled items. Pause= interword pause duration (averaged at list length 3).
The preparatory intervals did not differ between the independent and integrated conditions for list length 2, t (31)=.81, p =.425, η 2 =.021, but were longer in the independent condition for list length 3, t (24)=2.19, p =.038, η 2 =.167. Although participants were asked to read aloud the independent word as soon as it appeared on screen, some were still articulating this item whilst the recall cues occurred, and consequently their ‘preparatory interval’ included additional reading, which can account for the longer intervals obtained.
This study confirms and extends the results from Experiments 1 and 2. We again found that participants produced consistently longer pauses between recall words when these words were semantically related to the sentences that had been read, compared to when the words were unrelated to the sentences preceding them.
The difference in recall accuracy between the integrated and independent reading span conditions was clearly larger in Experiment 1 than in Experiment 2 and Experiment 3. Recall accuracy was roughly comparable in the integrated condition, regardless of whether participants either generated a sentence completion and final word. However, accuracy was relatively poor in Experiment 1 when participants generated a word to complete the sentence, and then remembered a separated item, rather than reading an entire sentence and then remembering a separate item. We conclude that the memory for the independent words must be fragile, and thus retention can be disrupted by competing representations such as a generated item. Yet this reinforces a central contention of the present paper: in reading span, participants arrive at recall with more than just the experimentally-defined memoranda in mind. ‘Processing’ and ‘memory’ may represent separate phases of the working memory span trial, but memory is not a neatly segregated and separate activity.
Experiment 4
We next undertook a conceptual replication of the preceding studies but chose not to use spoken output. Instead, participants compiled a recall sequence from a visually-presented set of choices, using a touch-screen device.
The use of manual responding offers a potentially complementary source of evidence about recall timing, avoiding the requirement that words be assembled into articulatory programs (see Chuah & Maybery, 1999 ; Maybery, Parmentier & Jones, 2002 , for analysis of the timing of recall involving spatial stimuli). The approach yields a set of inter-response intervals, rather than word and pause times.
The experimental procedure returns to the ‘read-and-complete’ sentence processing procedure used in Experiment 1. However, the nature of the recall display represents an intermediate step between Experiment 1 and 2/3. In the integrated condition, the computer displays the sentence-terminal words (but in no particular order). The recall display also includes two types of error lure; a word from the processing sentence, since it is known that participants sometimes produce words from elsewhere in the sentence ( Chiappe, Hasher & Segal, 2000 ; Friedman & Miyake, 2004 ), and a target answer from the preceding trial. Thus, the participant must overcome the impact of proactive interference from earlier trials ( Lustig, Hasher & May, 2001 ), or at least make source-information judgements about current and past memories ( Hedden & Park, 2003 ).
In the independent condition, then, the computer displayed the correct, (sententially irrelevant) words plus words from the sentences and from the previous trial. Notably, then, the recall display did not offer the sentence terminal word as a response candidate in the independent condition, which previous experiments suggest is source of difficulty arising from the sentence completion task. We used configurations in which chosen responses either disappeared from the recall screen or remained on screen, to ascertain the role of screen complexity on performance.
Twenty-seven University of Missouri students (16 women and 11 men) participated for partial fulfilment of course credit requirements. One participant was subsequently excluded because they consciously ignored serial recall instructions.
Computer events were driven by an Apple Macintosh ibook (running “Revolution” programming language under OS X) with response latencies measured in (1/60 s) ticks. A Liyama touchscreen monitor (model INTH380-BS plus Keyspan RS232-USB Adaptor) displayed the experimental screen and recorded participants’ responses. Since this study draws on North American participants, some sentences were adjusted for idiomatic phrases as necessary (whereas English students are familiar with reference to eating “fish and chips” or playing with a “skipping rope”, American students are more attuned to the concept of eating “hamburger and fries” and playing with a “jump rope” respectively). We added to the set of memory word stimuli such that memoranda could be selected without replacement throughout the entire experiment.
Except in the following respects, the procedure for the delivery of processing and memory followed Experiment 1. The experiment employed an incremental-order test procedure but administered all list lengths (i.e. length 2 – 5). Independent memoranda were presented for .75 sec before and after .125 sec interval. If a participant produced a non-expected completion word (completing “Food and water makes plants” with “live” instead of “grow”) the experimenter would volunteer the target word (in the case given, say “ or grow ”) before proceeding to the next experimental event 3 . Participants completed both a ‘remain’ and ‘disappear’ response selection condition, which were presented in counterbalanced order, with a minimal break between each.
Following the completion of the processing events, the computer presented a recall screen that comprised the target memoranda as well as incorrect words. For each correct word, there was also a ‘ processing-phase ’ lure; a word carrying semantic content that had appeared in the sentence (or very occasionally, when no suitable candidate was available, an associated prime word). There were two ‘ protrusion ’ lures, correct answers from the previous trial (the first trial employed two randomly selected words instead). Each of the ((list length * 2) + 2) response choices was assigned at random to one of 16 pre-specified locations across the screen. Participants identified their chosen recall sequence by tapping the word locations on-screen in the appropriate order. The computer recorded response latencies as well as recall selections.
In the ‘disappear’ condition, the chosen response was removed from the screen upon selection. In the ‘remain’ condition, responses continued to be visible after they had been chosen. In either case there was an auditory signal to confirm the computer’s registration of the response selection. Participants were informed about the recall configuration at the start of the condition.
Inspection of Table 1 indicates that participants were again able to recall more words from the integrated word condition (in both the disappear and remain condition) compared with the independent word condition. Analysis of variance confirmed a significant recall advantage in the integrated condition, F (1,24)=16.2, p =.001, η p 2 =.402, but no difference between the recall formats, F <1, η p 2 <.001, and no interaction, F <1, η p 2 =.004.
We did not anticipate reading time differences between the disappear and remain condition, since they differ only in recall screen dynamics, and they were equivalent ( M =3.50 vs. M =3.60 respectively), t (25)=.75, η 2 =.022. There were also no reliable reading time differences for integrated and independent words, for either the disappear or remain conditions ( t s(24)<.97, η 2 <.038).
Recall times were screened for outliers in the same manner as previously. Figure 4 describes pauses for correct sequences for each list length and response format. Graphs suggest list position effects (i.e. a speeding up in recall through the list) in both the disappear and remain conditions. Comparisons of the two response configurations indicated significantly quicker recall in the disappear condition at list length 3 only and no interactions – we therefore simply the following results by collapsing across this variable.

Duration of recall delays between the correct selection of responses in Experiment 4, as a function of output position. Graph includes standard error bars. Top panel=data from integrated word condition (when response choices disappear after selection or remain after selection on the left and right respectively). Bottom panel=data from independent word condition (when response choices disappear after selection or remain after selection on the left and right respectively).
Analysis of the average inter-item recall durations for each list length broadly confirmed analysis from previous experiments. Although list length 2 and 3 differences were not significant [ t (24)=.26, p =.796, η 2 =.003 and t (22)=.31, p =.756, η 2 =.004 respectively], at list length 4 the integrated word response pauses were significantly longer than independent response pauses, t (20)=2.56, p =.029, η 2 =.247. There was also a difference at list length 5 but this was marginal, t (14)=1.82, p =.090, η 2 =.191. We then combined the correct recall times for all available list lengths, and this confirmed the longer pauses in the integrated condition, t (24)=2.74, p =.021, η 2 =.238. In the round, analyses show that the independent condition involves quicker recall, but with a response choice paradigm this paradigm is most salient at longer list lengths.
Analysis of recall errors
With the present paradigm, there are clear constraints on the nature of recall errors. Correct answers were present of course, but serial order errors could occur. In addition, participants could select a processing-phase word that had been part of the original processing, or a protrusion word taken from a previous trial. However, error probabilities are not constant across trials; at list length 2 for example, there is only one order error possible (the string A-B recalled as B-A), but at list length 3, there are 5 order error permutations. Furthermore, the number of protrusion error lures was a constant two items across list length (necessary to minimise recall screen complexity) and thus protrusions are less likely to occur through random selection at larger list lengths. For clarity and brevity, we present analysis of errors after combining data for the disappear and remain conditions.
In what follows we consider data based on performance up to and including the span-terminal level 4 .
Table 3 reports the distribution of response choices. Errors are not randomly distributed: there are more order errors than processing-phase errors in both the independent condition, t (12)=3.60, p =.004, η 2 =.519, and integrated condition, t (12)=5.50, p <.001, η 2 =.716. Furthermore, although there are more opportunities for processing-phase lures than protrusion lures, error proportions for these two categories were not significantly different, either for the independent or integrated conditions, t s(12)=.48 & −.03 respectively, η 2 <.019.
Proportion of recall choices falling into different response categories in Experiment 4, for all trials up to and including span length. Standard deviations in parentheses.
| Response choice | ||||
|---|---|---|---|---|
| Correct | Order error | Proc.-phase error | Protrusion error | |
| Integrated | .802 (.112) | .134 (.070) | .032 (.021) | .032 (.045) |
| Independent | .626 (.118) | .212 (.129) | .076 (.034) | .086 (.058) |
The type of errors that were committed varied with experimental condition. The proportion of protrusion errors was higher in the independent condition than integrated condition, t (24)=2.63, p =.015, η 2 =.224, while the proportion of order errors was marginally higher in the independent condition, t (24)=1.93, p =.069, η 2 =.134.
In the independent condition, there is no link between the processing material and the target memory word, and thus there is nothing to bind the processing episode to activation levels of words. Answers to previous trials are likely to retain activation, and thus may be chosen by the participant instead of the correct item. In contrast, when processing and memory are linked, the processing context may help rule out these protrusion lures, making source-monitoring decisions more accurate. In other words, an important function of the integration between sentences and words to be recalled is to make these words more distinctly tied to the present trial as opposed to previous trials.
Finally, we note that participants were tempted by the presence of processing-phase lures. All 13 participants in the independent condition selected this lure type at least once, and 12 / 13 participants in the integrated condition did so.
This experiment addresses several issues. First, it offers data on recall timing on a complex memory task using motoric responses (to on-screen options) rather than spoken recall. Spoken recall measures have been highly important in increasing our understanding of memory phenomena (e.g., Cowan et al., 1992 ; Haberlandt, Lawrence, Krohn, Bowe & Thomas, 2005 ; Tehan & Lalor, 2000 ) but of course it is possible that some phenomena are properties of the specific methods of responding rather than general characteristics of recall. Spoken recall generally requires item and order information, yet the present methodology offers the possibility that item and order information can be teased apart (by varying the choices at recall). From a pragmatic standpoint, measuring spoken recall is a highly intensive process. Consequently, it is valuable to have convergent evidence from different, more easily registered, methods of recall to permit a more informed assessment of recall phenomena.
In these terms, the experiment has been a success. Recall processes are consistent and systematic. Moreover, participants are prone to confuse items in terms of their sequence order, and confuse them with no-longer-relevant items as well as items that they were exposed to but not asked to remember (see also Caretti, Cornoldi, De Beni & Romano 2005 ; Friedman & Miyake, 2004 ). There are both item and order constraints in WM.
General Discussion
We argue that sentence processing in reading span can contribute to recall performance because memory for the sentence persists into the recall period. Several theoretical views consider the processing-memory relationship in competitive terms, even where inherently they may not need to do so. For example, according to Daneman and Carpenter (1980) who first developed the reading span paradigm, the processing activity within a working memory span trial used general cognitive resources that were consequently denied to retention activities. Working memory span therefore reflected the balance of resources between processing and memory. The present data show instead that recall is partly a function of the compatibility between processing and memory, and that processing activity produces representations that affect recall, providing a source of both recall facilitation and interference. As a result, we argue that processing and retention in reading span are not as functionally distinct as considered hitherto.
The recall reconstruction hypothesis – long pauses in the recall of information from working memory that derive from memory search through the processing episodes – has resonances with performance on conceptual span task ( Haarmann, Davelaar & Usher, 2003 ). Conceptual span tests involve the partial recall of presented information via category cues. Haarmann et al. (2003) report some overlap between reading span and conceptual span, even though the latter does not require the conventional ‘processing plus memory’ combination. The present data - which go beyond the more typical analysis of recall accuracy and errors – promotes the conclusion that both paradigms overlap in terms of recall processes, and may incorporate a link between encoding context and recall.
Support for the recall reconstruction hypothesis is drawn from data in four experiments that involve both spoken recall and manual item selection. These present convergent and complementary evidence in the form of recall timing together with information about recall accuracy and error types. They suggest that the correct recall of items embedded within processing operations is more time consuming than the recall of items unrelated to preceding processing events, even though the integrated format allows more items to be remembered than the independent format (so that, other things being equal, one might expect more rapid and highly accessible item production). That recall time differences are not significant at every list length (and some accuracy differences emerge only with a larger sample size) emphasises that (a) recall timing can be variable and recall reconstruction may not be required on every trial; (b) participants may be able to draw on other strategies to retain the memoranda that can in some situations be effective.
The response choice paradigm supports this basic finding that pauses are shorter in the independent condition although significant effects were not consistently found at each sequence length. We suspect that this technique places less stringent requirements on the participant to maintain the fidelity of item information (as opposed to order information), because the correct items are always present at recall. Items must be retained, because various types of lures are also present at recall. Nonetheless, perhaps imperfect representations are sufficient for successful reconstruction.
Conway et al. (2005) have reviewed the use of WM span tasks and point out that some reading span tasks in the literature have used the independent format while others have used the integrated format. While we are not aware of studies that have compared these configurations, it is important to recognise that we are not making commitments about which is the theoretically preferred form of the task, especially in the context of individual differences. One might wish to minimise the reconstructive element in recall, or one might wish to emphasise it. Nonetheless it is important to appreciate the implications of adopting either format.
We suggest that, during recall of information in reading span, reconstructive processes can operate on what are often degraded representations. These processes may include memory search and decision-making about target words that draw on ‘contextual’ information from a variety of domains or content. Contextual memories are complicated and multifaceted, and can offer support for target memories as well as generating interfering representations ( Haarmann et al., 2003 ; Saito & Miyake, 2004 ). The present research also suggests that while the distinction between processing and memory in working memory span tests may be a useful research heuristic, these task components may functionally overlap for participants, so that processing activities and target memory words may actually become intertwined.
The present data show that WM theories can benefit from the incorporation of formal hypotheses about recall activities. Of course, when individuals produce a sequence of experimentally-defined items in WM tasks, they must arrive at the point of recall with some representations of these items. Yet we have shown that there is far more to response production than that. Before and during itself, they assemble or construct a memory list using some form of search process. They also strive to avoid non-target items that have some active representations (from preceding trials, and from the processing context). Finally, we argue that the present data reinforce the view that recall timing has an important place in advancing our understanding of immediate memory processes, especially but not only in an experimental context and when harnessed to information from recall accuracy.
Acknowledgements
We acknowledge the support of the ESRC (grant RES000220452 and RES000230859). The paper was prepared in part while the first author was a research visitor at the University of Colorado at Boulder. Scott Saults undertook blind recall timings on some trials of Experiment 1 and allowed derivation of consistency measurements. An overview of the procedures for timing spoken responses can be found at the URL: http://www.psych.lancs.ac.uk/research/TowseWM/
Appendix 1: Additional analysis of data from Experiment 1
The STM task, measured as the number of correctly recalled words, was within the capacity of some participants (7 participants remembered all 42 words) while everyone remembered at least half of the words – see Table 1 for a breakdown. Since we focus on the timing of correct recalls, this high level of performance is advantageous in maximising the data density for analysis.
Several recall phenomena are evident from Figure 5 . First, mean word articulation at approximately .4 sec, is similar to reading span data. Second, pauses between words are much shorter, but non-negligible, at less than .2 sec. Third, the preparatory intervals are (approximately three times) longer than interword pauses, consistent with the interpretation that they reflect a different mental activity. Data contrast with recall times for reading span trials that were noticeably longer (i.e. a different scale is used across Figures), and there are large, almost qualitative changes in the pattern of responding across list lengths.

Duration and standard error bars of correct sequences for word span trials in Experiment 1, as a function of the phase of recall and the sequence length. PI = preparatory interval. Words = average duration of recalled items. Pause= interword pause duration (averaged at list length 3 and beyond).
1 Where appropriate as here, probabilities have been corrected after adjusting degrees of freedom because of non-equal variances. In the case of analysis of variance, we report Greenhouse-Geisser values where warranted.
2 Two independent judges extracted timing measurements, using the same procedures as Expt. 1. Independent t-tests on all list-length 2 and list-length 3 segments indicated comparable judgements between coders (all ps>.10)
3 This was necessary because recall items here were fixed prior to presentation, although it was used very rarely.
4 In the ‘remain’ condition, it is possible to produce a ‘repeat’ error; many of these reflected registration issues for touch screen responses (e.g. immediate repetitions with an interval <0.5 sec). For simplicity, they were coded here as order errors.
Contributor Information
John N. Towse, Department of Psychology, Lancaster University.
Nelson Cowan, Department of Psychological Sciences, University of Missouri.
Graham J. Hitch, Department of Psychology, University of York.
Neil J Horton, Department of Psychology, Lancaster University.
- Baddeley AD, Hitch GJ. Working Memory. In: Bower G, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [ Google Scholar ]
- Barrouillet P, Bernadin S, Camos V. Time constraints and resource sharing in adults' working memory span. Journal of Experimental Psychology: General. 2004; 133 (1):83–100. [ PubMed ] [ Google Scholar ]
- Caretti B, Cornoldi C, De Beni R, Romano M. Updating in working memory: A comparison of good and poor comprehenders. Journal of Experimental Child Psychology. 2005; 91 (1):45–66. [ PubMed ] [ Google Scholar ]
- Case R. Intellectual development: birth to adulthood. New York: Academic Press; 1985. [ Google Scholar ]
- Chiappe P, Hasher L, Siegel LA. Working memory, inhibitory control and reading ability. Memory & Cognition. 2000; 28 (1):8–17. [ PubMed ] [ Google Scholar ]
- Chuah YML, Maybery MT. Verbal and spatial short-term memory: Two sources of developmental evidence consistent with common underlying processes. International Journal of Psychology. 1999; 34 :374–377. [ Google Scholar ]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review. 2005; 12 (5):769–786. [ PubMed ] [ Google Scholar ]
- Copeland DE, Radvansky GA. Phonological similarity in working memory. Memory & Cognition. 2001; 29 (5):774–776. [ PubMed ] [ Google Scholar ]
- Cowan N, Day L, Saults JS, Keller TA, Johnson T, Flores L. The role of verbal output time in the effects of word length on immediate memory. Journal of Memory and Language. 1992; 31 :1–17. [ Google Scholar ]
- Cowan N, Towse JN, Hamilton Z, Saults JS, Elliott EM, Lacey JF, et al. Children's working memory processes: A response-timing analysis. Journal of Experimental Psychology: General. 2003; 132 (1):113–132. [ PubMed ] [ Google Scholar ]
- Cowan N, Wood NL, Wood PK, Keller TA, Nugent LD, Keller CV. Two separate verbal processing rates contributing to short-term memory span. Journal of Experimental Psychology: General. 1998; 127 (2):141–160. [ PubMed ] [ Google Scholar ]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal Verbal Learning and Verbal Behavior. 1972; 11 :671–684. [ Google Scholar ]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980; 19 :450–466. [ Google Scholar ]
- Daneman M, Merikle PM. Working memory and language comprehension: A meta-analysis. Psychonomic Bulletin & Review. 1996; 3 :422–433. [ PubMed ] [ Google Scholar ]
- Dosher BA, Ma J. Output loss or rehearsal loop? Output-time vs. pronunciation time limits in immediate recall for forgetting matched materials. Journal of Experimental Psychology: Learning, Memory and Cognition. 1998; 24 (2):316–335. [ PubMed ] [ Google Scholar ]
- Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of working memory. New York: Cambridge University Press; 1999. pp. 102–134. [ Google Scholar ]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent variable analysis. Journal of Experimental Psychology: General. 2004; 133 :101–135. [ PubMed ] [ Google Scholar ]
- Haberlandt K, Lawrence H, Krohn T, Bowe K, Thomas JG. Pauses and durations exhibit a serial position effect. Psychonomic Bulletin & Review. 2005; 12 (1):152–158. [ PubMed ] [ Google Scholar ]
- Haarmann HJ, Davelaar EJ, Usher M. Individual differences in semantic short-term memory capacity and reading comprehension. Journal of Memory and Language. 2003; 48 :320–345. [ Google Scholar ]
- Hedden T, Park D. Contributions of source and inhibitory mechanisms to age-related retroactive interference in verbal working memory. Journal of Experimental Psychology: General. 2003; 132 (1):93–112. [ PubMed ] [ Google Scholar ]
- Hitch GJ, Towse JN, Hutton UMZ. What limits working memory span? Theoretical accounts and applications for scholastic development. Journal of Experimental Psychology: General. 2001; 130 (2):184–198. [ PubMed ] [ Google Scholar ]
- Jarrold C, Bayliss D. Variation in working memory due to typical and atypical development. In: Conway ARA, Jarrold C, Kane M, Miyake A, Towse JN, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 134–161. [ Google Scholar ]
- Kane MJ, Engle RW. Working memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop Interference. Journal of Experimental Psychology: General. 2003; 132 (1):47–70. [ PubMed ] [ Google Scholar ]
- Leather CV, Henry LA. Working memory span and phonological awareness tasks as predictors of early reading ability. Journal of Experimental Child Psychology. 1994; 58 :88–111. [ PubMed ] [ Google Scholar ]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001; 130 (2):199–207. [ PubMed ] [ Google Scholar ]
- Maybery M, Parmentier FBR, Jones DM. Grouping of list items reflected in the timing of recall: implications for models of serial verbal memory. Journal of Memory and Language. 2002; 47 :360–385. [ Google Scholar ]
- Miyake A, Shah P, editors. Models of working memory. Cambridge University Press: New York; 1999. [ Google Scholar ]
- Osaka M, Nishizaki Y, Komori M, Osaka N. Effect of focus on verbal working memory: Critical role of the focus word in reading. Memory & Cognition. 2002; 30 (4):562–571. [ PubMed ] [ Google Scholar ]
- Saito S, Miyake A. On the nature of forgetting and the processing-storage relationship in reading span performance. Journal of Memory and Language. 2004; 50 :425–443. [ Google Scholar ]
- Slamecka NJ, Graf P. The generation effect; Delineation of a phenomenon. Journal of Experimental Psychology: Human Learning & Memory. 1978; 4 :592–604. [ Google Scholar ]
- Tehan G, Lalor DM. Individual differences in memory span: The contribution of rehearsal, access to lexical memory, and output speed. Quarterly Journal of Experimental Psychology. 2000; 53A (4):1012–1038. [ PubMed ] [ Google Scholar ]
- Towse JN, Cowan N. Working memory and its relevance for cognitive development. In: Schneider W, Schumann-Hengsteler R, Sodian B, editors. Young Children’s Cognitive Development: Interrelationships among Executive Functioning, Working Memory, Verbal Ability, and Theory of Mind. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 9–37. [ Google Scholar ]
- Towse JN, Hamilton Z, Hitch GJ, Hutton U. Sentence completion norms among adults: A corpus of sentences differing in length (Technical Report No. CDRG 7) Royal Holloway: University of London; 2000.
- Towse JN, Hitch GJ, Hutton U. A reevaluation of working memory capacity in children. Journal of Memory and Language. 1998; 39 (2):195–217. [ Google Scholar ]
- Towse JN, Hitch GJ, Hutton U. On the interpretation of working memory span in adults. Memory & Cognition. 2000; 28 (3):341–348. [ PubMed ] [ Google Scholar ]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory and Language. 1989; 28 :127–154. [ Google Scholar ]
- Unsworth N, Engle RW. Simple and complex memory spans and their relation to fluid abilities: Evidence from list-length effects. Journal of Memory and Language. 2006; 54 :68–80. [ Google Scholar ]

IMAGES
COMMENTS
Introduction. Working memory has fascinated scholars since its inception in the 1960's (Baddeley, 2010; D'Esposito and Postle, 2015).Indeed, more than a century of scientific studies revolving around memory in the fields of psychology, biology, or neuroscience have not completely agreed upon a unified categorization of memory, especially in terms of its functions and mechanisms (Cowan ...
Both working memory and attention can be conceptualized in different ways, resulting in a broad array of theoretical options for linking them. The purpose of this review is to propose a map for organizing these theoretical options, delineate their implications, and to evaluate the evidence for each of them. The meaning of the concept working ...
Working memory. Working memory is primarily associated with the prefrontal and posterior parietal cortex (Sarnthein et al., 1998; Todd and Marois, 2005).Working memory is not localized to a single brain region, and research suggests that it is an emergent property arising from functional interactions between the prefrontal cortex (PFC) and the rest of the brain (D'Esposito, 2007).
Fig. 1. Simulations of a dynamic field model showing an increase in working memory (WM) capacity over development from infancy (left column) through childhood (middle column) and into adulthood (right column) as the strength of neural interactions is increased. The graphs in the top row (a, d, g) show how activation ( z -axis) evolves through ...
The Diseased Brain and Working Memory. Age is not the only factor influencing working memory. In recent studies, working memory deficits in populations with mental or neurological disorders were also being investigated (see Table 3).Having identified that the working memory circuitry involves the fronto-parietal region, especially the prefrontal and parietal cortices, in a healthy functioning ...
I present an account of the origins and development of the multicomponent approach to working memory, making a distinction between the overall theoretical framework, which has remained relatively stable, and the attempts to build more specific models within this framework. I follow this with a brief discussion of alternative models and their relationship to the framework. I conclude with ...
Working memory (WM) — the ability to maintain and manipulate information over a period of seconds — is a key cognitive skill. Constantinidis and Klingberg discuss non-human-primate ...
Working memory, or the ability to temporarily hold information in mind, underlies many everyday behaviours. In this Review, Naveh-Benjamin and Cowan discuss age-related changes in working memory ...
Main. The theoretical concept of working memory assumes that a limited capacity system, which temporarily maintains and stores information, supports human thought processes by providing an ...
For more than 50 years, psychologists and neuroscientists have recognized the importance of a working memory to coordinate processing when multiple goals are active and to guide behavior with information that is not present in the immediate environment. In recent years, psychological theory and cognitive neuroscience data have converged on the idea that information is encoded into working ...
Literature Reviews Why Is Working Memory Performance Unstable? A Review of 21 Factors Rachael N. Blasiman* a, Christopher A. Was a [a] Kent State University, Kent, OH, USA. Abstract In this paper, we systematically reviewed twenty-one factors that have been shown to either vary with or influence performance on working memory (WM) tasks. ...
In the working memory literature, the presentation of stimulus automatically activates perceptual and related long-term representations, creating a transient sensory memory trace ... The goal of this review was to outline working memory consolidation and long-term memory consolidation, in order to determine how these processes relate to each ...
What is Working Memory? An Introduction and Review. Working memory is the small amount of information that can be held in mind and used in the execution of cognitive tasks, in contrast with long-term memory, the vast amount of information saved in one's life. Working memory is one of the most widely-used terms in psychology. It has often been connected or related to intelligence, information ...
The role of working memory as a processing resource for other cognitive abilities (Salthouse, 1990; Chai et al., 2018) implies that working memory improvements after targeted working memory training (WMT) might naturally lead to positive transfer effects to other cognitive functions and even fluid intelligence (Au et al., 2015).
Finally, working memory (WM) involves maintaining and using information appropriately even if it is not perceptually present (Baddeley & Hitch, 1994; Kent, 2016). That is, it is the cognitive ...
The purpose of this review is to propose a map for organizing these theoretical options, delineate their implications, and to evaluate the evidence for each of them. The meaning of the concept working memory (WM) depends on the theory in which the concept figures. The definitions reviewed by Cowan (. 2017.
A literature search using PsychNet Gold was conducted using the terms working memory. In addition, the writer reviewed recommendations from a sampling of recent neuropsychology texts in regard to the assessment of attention and working memory, as well as the two most recent editions of the Wechsler Memory Scale.
This systematic review sheds light on the crucial role of working memory in L2 oral production and provides valuable insights for language educators and researchers. View Show abstract
The majority of research on visual memory has taken a compartmentalized approach, focusing exclusively on memory over shorter or longer durations, that is, visual working memory (VWM) or visual episodic long-term memory (VLTM), respectively. This tutorial provides a review spanning the two areas, with readers in mind who may only be familiar with one or the other. The review is divided into ...
Working memory (WM) supports a wide range of complex behaviours, including reading comprehension, following instructions, and problem-solving (Feldman Barrett et al., Citation 2004; Holmes et al., Citation 2021; Jaroslawska et al., Citation 2018; Peng & Kievit, Citation 2019).WM varies between individuals and can be measured using a variety of paradigms (e.g., backward recall, complex span or ...
This manuscript aims to provide a review of the interaction between language and Working Memory (WM) by reviewing the functional MRI studies published between 2000 and 2020. Language is the most essential and prominent mode of communication in humans, and WM is considered one of the main functions of the cognitive domain showing the most ...
A literature review of working memory for those with ASD was conducted. •. Lower scores in verbal working memory were associated with greater problems in adaptive behavior and more restrictive and repetitive behavior. •. Additional results, implications for educational practice and future research are discussed.
Introduction. Working memory reflects the ability to hold in mind transient representations while simultaneously processing and assimilating ongoing events (Baddeley & Hitch, 1974).There are a wide variety of circumstances in which we are required to carry out mental operations and remember intermediate information (for instance, retain a carry item in mental arithmetic or a referent for an ...